Abstract
HPTLC is a perfect analytical tool which has been used for qualitative and quantitative estimation of active markers. In recent years, tremendous work is going on for isolating active principles from plant sources their pharmacological and biochemical estimations and their preclinical, clinical studies for new drug development. For this we studied extraction process of the crude drug as well as we studied different evaluation parameters including structure elucidation using IR and NMR, HPTLC etc., ash value and extractive value determination, phytochemical evaluation of the same was performed while antibacterial activity studied using Gram +ve bacteria. The data could be used as a QC standard. The method used in this work resulted in good peak shape and enabled good resolution of Quercetin from other constituents of the hydroalcoholic, ethanolic and Aqueous extract of leaves of Cassia senna sophera L.
Keywords
HPTLC, Cassia senna, Extraction, Screening, Method Development, Phytochemical.
Introduction
HPTLC is a perfect analytical tool which has been used for qualitative and quantitative estimation of active markers. No any reports are also available on HPTLC method development of C. senna Sophera L. by fingerprint analysis of quantitative estimation of this crude drug. One can get availability of the particular crude drug in the finished product. Quercetin is the important target molecules of C.senna Sophera L. Presence or absence of these active markers can be analyzed by HPTLC method development. By this way availability or chemical degradation of active constituents in final product can be traced out. HPTLC fingerprint is the applicable and critical parameter associated with the standardization of crude drugs. Here, we used hydroalcoholic extract because maximum chemical constituents soluble in hydroalcoholic solvent. However, HPTLC method for quantitation of Quercetin from Cassia senna sophera linn, has not been reported. Densitometric HPTLC has been widely used for the phytochemical evaluation of the herbal drugs, due to its simplicity and minimum sample clean up requirement. Hence, a densitometric HPTLC method has been developed in the present work for quantitation of gallic acid from hydroalcoholic extract of dried leaves of Cassia senna sophera linn.
OBJECTIVES
In recent years, tremendous work is going on for isolating active principles from plant sources their pharmacological and biochemical estimations and their preclinical, clinical studies for new drug development.
The crude drug was subjected for following studies:
Collection and authentication of leaves of Cassia senna sophera linn.

Hindi Cassia Species
A medicinal plant those substances which can be used for therapeutic purposes or which are precursors for the synthesis of useful drugs. Traditional or indigenous drugs used by different ethnic groups of the world for the treatment of diseases. They are relatively safe, easily available and affordable to masses. For the past two decades, here has been an increasing interest in the investigation of different extracts obtained from traditional medicinal plants as potential sources of new therapeutic agents. Traditional drugs have given important lead in drug search, resulting in the discovery of novel molecules. Fingerprinting of Ayurvedic drugs and herbals are done by HPLC, HPTLC, 1H NMR. Herbal medicines are prepared from materials of plant origin which are prone to contamination, deterioration and variation in composition. Therefore, quality control of herbal medicines offers a host of problems. A marker can be defined as a chemical entity, in the plant material which may or may not be chemically defined and serves as a characteristics fingerprint for that plant. In other ways through various analytical techniques like TLC, HPLC and HPTLC, we can visualize the presence of this compound in the plant and also quantify it to ascertain the limits. Biomarkers on the other hand are a group of chemical compounds which are in addition to being unique for that plant material also correlate with biological efficiency. There is batch to batch variation depending on time of collection of material and multiply during storage and further processing. So, the need arises to lay standards by which the right material is selected and incorporated in to the formulation.
Synonyms
Sanskrit-
Ksamarda
Telagu- Paidi Tangaedu
Bengali-Kalakasunda English- Senna sophera
Tamil- Poria Takarai - Bas-ki-kasondi
Maylalam-Ponnantakara Marathi- Ran Takala
Taxonomical classification of the genus Cassia
KINGDOM PLANTAE
Division Magnoliophyta
Class Magnoliopsida
Subclass (Unranked) Rosidae
Order Eurosids I
Family Fabales Leguminosae
Subfamily Caesalpinioideae
Tribe Cassieae
Subtribe Cassinae Cassia L.
Distribution
Throughout India and most in tropical countries.
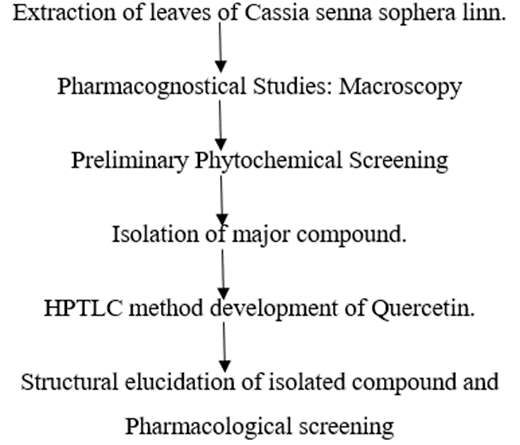
Table 1: Reported Phytoconstituents
METHODOLOGY
1. Plant Material Procurement
Leaves of Cassia senna sophera linn. (CS) were collected from Sangli (M.S).
2. Identification and Authentication
The plant was identified and authenticated from Vivekananda College, Kolhapur.
3. Macroscopic study of plant material
Morphological study was carried out for organoleptic evaluation. The color, structure, shape and size were visually observed.
4. Drying of plant material
The crude drug was shade dried for 72 hrs and powdered by using industrial grinder.
5. Determination of Physicochemical Constants of Powdered Plant Material
EVALUATION
1. Ash values
The total ash, acid insoluble ash and water-soluble ash were determined by using procedures given in the Ayurvedic Pharmacopoeia.
Determination of total Ash value Procedure:
- A crucible dish was weighed and ignited.
- About 2 gm of powder was weighed and added to crucible.
- The crucible was kept about 7 cm away from flame till the vapours cease.
- Then it is heated strongly to burn off all the carbon, and crucible was cooled in Desiccators.
- The ash was weighed and percentage of total ash was calculated with reference to air dried powder.
Determination of acid-insoluble ash value
Procedure:
- The ash obtained from above procedure and it was washed by 25 ml of dilute Hydrochloric acid in 100 ml beaker.
- The above solution was boiled for 5 minutes.
- The solution was filtered through ashless filter paper and residue was washed twice with water, residue was taken into pre-ignited, cooled and weighed crucible.
- The filter paper with residue was heated gently until vapours cease and then heated strongly until whole carbon was removed. Crucible was cooled in a desiccator.
- The ash was weighed and percentage of acid insoluble ash was calculated with reference of air-dried powder.
Extractive value
Using procedures given in the Ayurvedic Pharmacopoeia, determination of alcohol soluble extractive value and water-soluble extractive value were carried out.
Procedure:
5 gm of powder was dissolved in 20 mL ethanol and water separately and sonicated for 15 minutes. The solution was filtered through Whatman No. 41 filter paper. The residue was dried and weighed. The percentage yield of residue was calculated.
Determination of leaf constant
The leaf constants like vein islet no, stomatal index, stomatal no and palisade ratio was calculated as per procedure by using Camera Lucida.
6. Extraction of plant material
The powder of plant material obtained were passed through sieve no. 85, weighed & then used for extraction. Drug extracts preparations obtained by extracting herbal drug at certain particle size with suitable extraction medium.
Ethanolic extract:
In the present study, the plant was carefully selected and shade dried. The dried material was reduced to powder in the mechanical grinder and passed through a sieve no. 40 to obtain powder of desired size. About 125 gms of powdered material was subjected to exhaustive extraction with 95% alcohol in a Soxhlet extractor at a temperature of 45-50 0C. The extraction was continued until the solvent in the thimble became clear, then few drops of solvent were collected in the test tube during the completion of the cycle and chemical test of the solvent was performed. After each extraction the solvent was distilled off and the extract was concentrated at low temperature. Some part of the total extract was reserved for phytochemical investigation and rest of the extract was used for evaluation of various activities.
Aqueous Extraction:
About 500 gm of fresh powder was used. It was then subjected to cold maceration with chloroform: water (1.0 %) I. P. in a 2 liters round bottom flask for about 7 days at room temperature. The flask was securely plugged with absorbent cotton and was shaken periodically till complete maceration. After maceration, the marc was pressed in a muslin cloth and the filtrate was concentrated to residue at low temperature.
PHYTOCHEMICAL SCREENING
Preliminary Phytochemical Tests
1. Test for Alkaloids
- Dragendorff's test
- Mayer’s test
- Hager’s test
- Wagner’s test:
2. Test for Amino acids
a. Ninhydrin test
3. Test for Anthraquinone glycosides
a. Borntrager's test
4. Test for Cardiac glycoside:
- Keller killani test
- Legal’s test
5. Test for Cyanogenic glycoside
Grignard reagent or sodium picrate test
6. Test for Coumarin
7. Test for Flavonoids
8. Test for Mucilage
9. Test for Proteins
- Biuret test
- Million’s test
Steps involved in High Performance thin Layer Chromatography
Schematic procedure for HPTLC
RESULT AND DISCUSSION
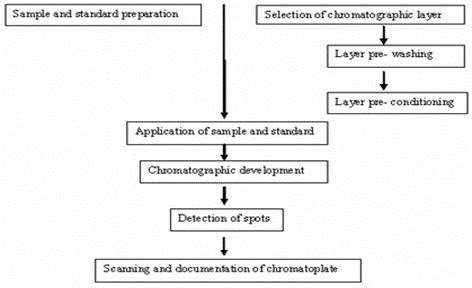
- Selection of TLC/ HPTLC plates and sorbent
Handmade plates:
The amount of each sorbent indicated is sufficient to coat five plates of 20 × 20 cm each. Following are some examples of handmade plates-
- Cellulose (native)
- Cellulose with starch as binder
- Cellulose (microcrystalline)
- Cellulose (microcrystalline) with fluorescent indicator
- Acetylated cellulose + CaSO4. . H2O
- Silica gel or Silica gel G
Silica gel with starch
Pre-coated plates: Usually plates with sorbent thickness of 100– 250 ?m are used for qualitative and quantitative analysis, however for preparative TLC work, plate with sorbent thickness of 1-2 mm are available in addition to chemically modified layers.
Glass support:
It is resistant to heat and chemicals, easy to handle and always offers superior flat and smooth surface for chromatographic work; disadvantage being fragility, relativity high weight (plate is usually 1.2 mm thickness), additional Packing material and higher production cost.
Method of Extraction of Flavonoids
Process of Extraction of Flavonoids:
Extraction was done in Soxhlet apparatus; 930 gm of powder was used for extraction. Petroleum ether was used for removal of fatty material from powder material. After treatment of petroleum ether marc was treated with chloroform, followed by ethanol, Water, Hydro Alcohol. Fractions of ethanol water and Hydro Alcohol extracts were kept for seven days. The presences of flavonoids were confirmed by taking chemical tests.
Isolation and Development of Quercetin
Preparation of Sample Solution
An accurately weighed 100 mg of flavonoids fraction was transferred to 20 ml of volumetric flask and about 15 ml of methanol was added to the flask. The flasks was sonicated for 20 minutes in an ultrasonic water bath and diluted up to the mark with methanol. The solution was filtered through Whatman no. 41 filter paper and used for further chromatographic analysis. The TLC plate was allowed to run up to 80 mm from the point of application. TLC plate was dried in hot air oven at 600C. Densitometric scanning was performed using CAMAG TLC scanner 3 in the absorbance mode at 280 nm and operated by win CATS software (V 1.4.3.6336). The slit dimension was 5.0 × 0.45 mm with the scanning speed of 20 mm s-1. Evaluation was done via peak area with linear regression.
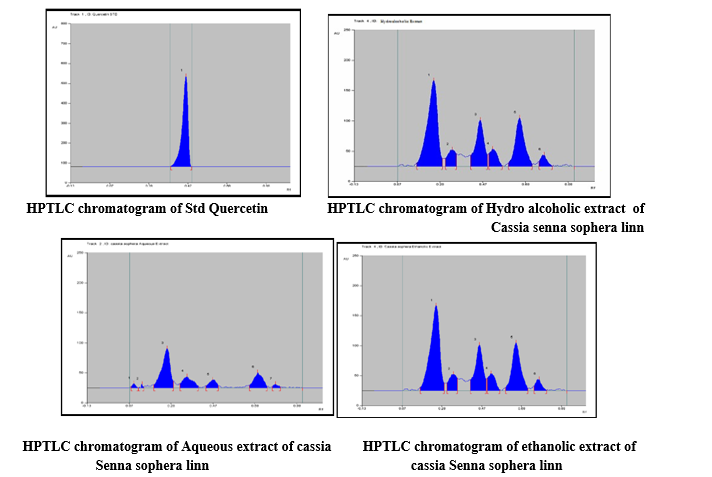
Development of the optimum mobile phase
The TLC procedure was optimized with a view to quantify the herbal extract. Initially chloroform: ethyl acetate: formic acid in varying ratios was tried. The mobile phase Toluene : Ethyl Acetate : Acetonitrile : Formic Acid (10:1:1:1, v/v/v/v) gave good resolution with Rf = 0.40 for gallic acid but peak shape was missing. Finally, the mobile phase consisting of Toluene : Ethyl Acetate : Acetonitrile : Formic Acid (10:2.5:1:1, v/v/v/v) gave a sharp and well-defined peak at Rf = 0.45 in fig. Well Defined spots were obtained after the chamber was saturated with mobile phase for 15 min at room temperature. The TLC plate was visualized under UV light at 282 nm, without derivatization. A photograph of a TLC plate after chromatography of Quercetin standard and a hydroalcoholic extract of the leaves of Cassia senna sophera linn. The identity of the Quercetin bands in sample chromatograms was confirmed by comparison of the chromatogram obtained from the sample with that obtained from the reference standard solution and by comparing retention factors of Quercetin from sample and standard solutions. The peak corresponding to Quercetin from the sample solution had same retention factor as that of the retention factor from the Quercetin standard (Rf 0.45).
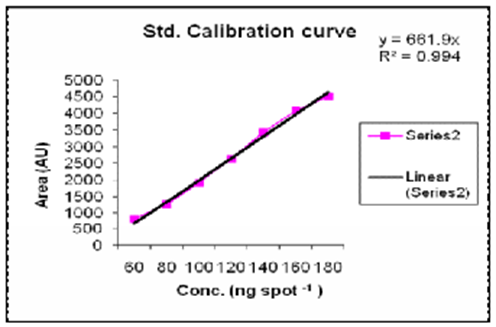
Preparation of Quercetin standard solution and Calibration curve
A stock solution of standard Quercetin (40 ?g/mL) was prepared by transferring 4 mg of Quercetin, accurately weighed, into a 100 mL volumetric flask, dissolving in 50 mL methanol. It was then sonicated for 15 minutes and the final volume of the solutions was made up to 100 mL with methanol to get stock solutions containing 40 ?g/mL. The calibration curve from 60-180 ng/ spot was prepared and checked for Reproducibility.
Preparation of sample solution
Accurately weighed 100 mg of dried hydroalcoholic extract of C. Sophera L. was transferred to a 100 mL volumetric flask dissolving in 80 mL of methanol. It was then sonicated for 15 minutes and the contents of the flask were filtered through Whatman No. 1 paper (Merck, Mumbai, India). The final volume of the solution was made up to 100 mL with methanol to get stock solution containing 10 ?g/ mL.
Chromatographic conditions
HPTLC was performed on 20 cm × 10 cm aluminium backed plates coated with silica gel 60 F 254. Standard solution of gallic acid and sample solution were applied to the plates as bands 6.0 mm wide, 30.0 mm apart, and 10.0 mm from the bottom edge of the same chromatographic plate by use of a Camag Linomat V sample applicator equipped with a 100-?L Hamilton syringe. Ascending development to a distance of 80 mm was performed at room temperature (28°C), with Toluene: Ethyl Acetate: Acetonitrile: Formic Acid (10:1:1:1, v/v/v/v), as mobile phase, in a Camag glass twin-trough chamber previously saturated with mobile phase vapor for 15 min. After development, the plates were dried and then scanned at 280 nm with a Camag TLC Scanner with WINCAT software, using the deuterium lamp.
- Structure Elucidation of Isolated Compound
Spectroscopy:
UV Visible spectra of isolated compound were monitored by UV Visible Spectroscopy (Jasco dual beam UV Visible spectrophotometer, Model V-570, Japan)
Infrared spectroscopy:
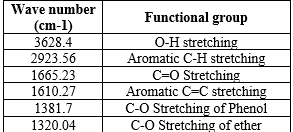
The isolated compound was characterized by using infrared spectroscopy (Jasco Japan). The isolated compound was grounded with KBr powder and pressed into pellets for IR spectra measurement in the frequency range of 400-4000 cm-1.
Nuclear Magnetic Resonance of isolated compound.
1 H NMR was performed in CDCl3 solvent at Department of chemistry, Shivaji University, Kolhapur.
- Pharmacological Evaluation
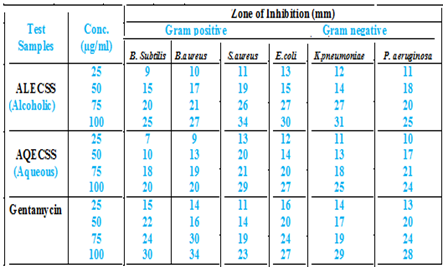
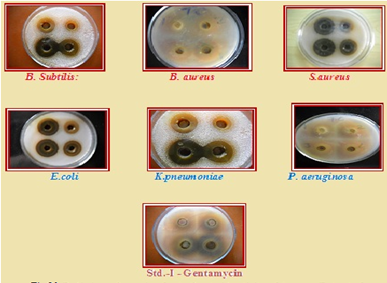
Antibacterial Activity
Organism
Bacillus subtilis, Bacillus aureus and Staphylococcus aureus (Gram positive), Escherichia coli, K pneumoniae and Pseudomonas aeruginosa (Gram negative)
Culture Media
The media used in Pour Plate Method was sterile nutrient agar.
Materials used:
- Nutrient agar
- Distilled water
- Beef extract
- Peptone
- Sodium chloride
Procedure for Autoclaving:
- Load the autoclave with the freshly prepared culture media.
- Close and lock the autoclave door.
- Set the autoclave time for 15 minutes or longer and select a slow rate of exhaust.
- Make certain that the autoclave temperature is set to 121°C & 15 p.s.i. for 15 min.
- Start the autoclave by pushing the start button or twisting the knob to the start position.
- When the period of sterilization is completed and the pressure in the chamber reads 0, carefully open the door and remove the containers, using heat-proof gloves.
Preparation of Bacterial Suspension:
The bacterial suspension was prepared by transferring a loopful of inoculum into 1ml sterile saline solution from the stock culture maintained at 40C in 10 ml nutrient broth.
Preparations of Plates:
Nutrient agar medium was sterilized at 15 lb/cm2 pressure for 20 min in an autoclave about 15 ml of medium was poured in each Petri plates under sterile conditions and keep for solidification in freeze for 20 mins.
Preparation of test Samples:
Alcoholic and aqueous bark extracts of Pongamia pinnata was prepared in sterile distilled water (1mg/ml). Further test dilutions were made ranging from 10 µg/ml to 100µg/ml in sterile distilled water Test Bacteria: The Bacterial culture employed in this study are Bacillus subtilis, Bacillus aureus, Staphylococcus aureus, Escherichia coli, K pneumoniae and Pseudomonas aeruginosa obtained from the department of Microbiology, Shivaji University, Kolhapur.
- Macroscopic Study
Determination of Physical Constituent/ Proximate analysis
Determination of total ash value Calculation:
Wt. of the empty crucible = 62 gm
Wt. of drug taken =0.2 gm
Wt. of crucible + ash = 62.2 gm
Wt. of ash = 0.2 gm
Total ash value of sample = 100 x 0.244/2 = 12.2% The total ash value found to be 12.2% of drug.
- Acid-insoluble ash value
Calculation:
Wt. of ash = 0.2 gm
Wt. of residue = 0.09 gm
% of acid insoluble ash = 100 x 0.054/2 = 2.7%
The % of acid insoluble ash was found to be 2.7%.
Extractive values

Leaf constants
EXTRACTION
- The leaves were dried in shadow and powered. The powders obtained were passed through sieve no. 85, weighed and then used for extraction.
- The weighted powder was placed in thimble made up of filter paper and was continuously extracted for 18-20 hours using ethanol (95%) as a solvent.
- The resulting extracts were concentrated under reduced pressure using rotary vacuum evaporator to get the semisolid mass. This mass was transferred in petri dish and allowed to dry in an oven for about 2 to 3 hours.
- This ethanol extract was subjected for further sequential fractionation.
- The ethanol extract was adsorbed on silica gel (60-120), dried and placed in thimble and then used for sequential fractionation with chloroform and ethyl acetate by using soxhlet extraction method.
- The remaining residue was washed with the ethanol and considered to be ethanol fraction. (Table 5)

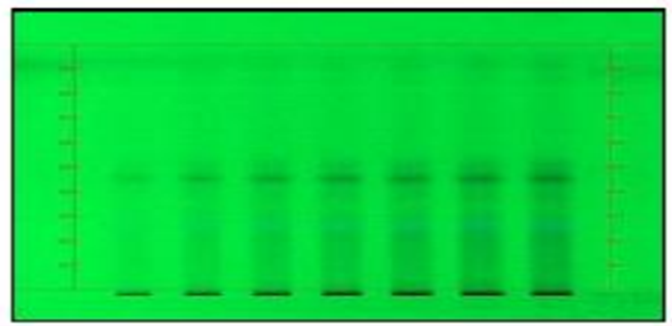
HPTLC of Calibration of Standard Quercetin
Calibration curve of standard Quercetin
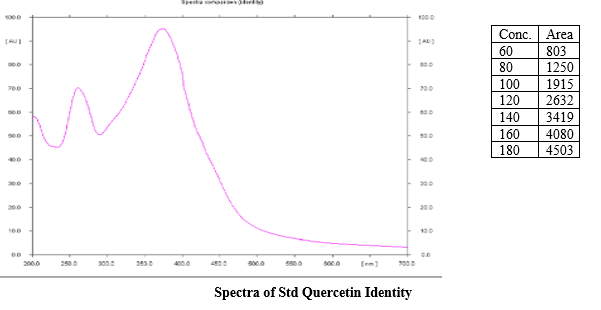
Spectra of Std Quercetin I
IR spectra of isolated compound
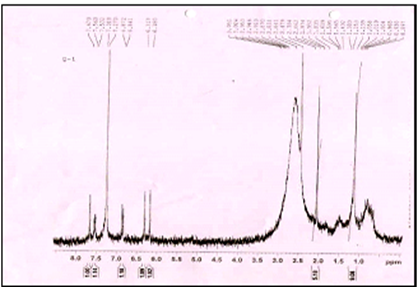
HNMR spectra of isolated compound
Preliminary screening for antibacterial activity of leaves extract of cassia senna sophera linn

CONCLUSION
In the present study, the phytochemical screening suggests the presence of flavonoids in the Hydro alcoholic, aqueous and ethanolic extracts. A rapid, simple, accurate and specific HPTLC method for quantitative estimation of Quercetin present in the extract of leaves of Cassia senna sophera L. has been developed. The data could be used as a QC standard. The method used in this work resulted in good peak shape and enabled good resolution of Quercetin from other constituents of the hydroalcoholic, ethanolic and Aqueous extract of leaves of Cassia senna sophera L. There were no interferences with the Queretin peak from other constituents present in the extract of leaves of Cassia senna sophera L.The aqueous bark extract of Cassia senna sophera shown less significant activity against six organisms tested but the aqueous extract of Cassia senna sophera less activity against B. Subtilis organism as compared to the standards drugs. The aqueous bark extract of Cassia senna sophera shown most significant against S.aureus as compared to standards drug. The aqueous extract at 100 ?g/ml concentration was significant as compared to the standard drugs 25 ?g/ml Gentamycin and 25?g/ml against all organisms. The alcoholic bark extract of Cassia senna sophera shown most significant activity against six organisms tested but the alcoholic extract of Cassia senna sophera low activity against B. Subtilis organism as compared to the standards drugs. The alcoholic bark extract of Cassia senna sophera shown most significant against S.aureus as compared to standards drug and it has been shown in Fig-21. The alcoholic extract at 100 ?g/ml concentration was significant as compared to the standard drugs 25 ?g/ml Gentamycin and 25?g/ml against all organisms.
REFERENCES
- Chopra RN, Nayar SL, ChopraI C. Glossary of Indian Medicinal Plants, CSIR, New Delhi:1956:55
- Rangari VD. Pharmacognosy and Phytochemistry. Part 1st ed. Nashik: Carrer Publication 2006: pp.129¬-150.
- Smith RM. Nomenclature for supercritical chromatography and extraction. Pure Appl Chem 1993; 65:2397-2403.
- Nadkarni, K. M. Indian plants and drugs with their medicinal properties and uses. 2005; p. 95-96
- Nagore DH. Determination of Phenolic content of Cassia Sophera Linn. And the potential anti-ulcer activity in experimentally induced ulceration. Journal of Complimentary and Integrative Medicine 2009;6(1):1-20.
- Mustafa M, Ahmed M, Jahana IA, Choudhury JU. Composition of oil from the seeds of Cassia Sophera Linn. Bangladesh J Sci Ind Res 2007; 42(1): 7578.
- Kokate CK. Practical Pharmacognosy Vallabh Prakashan New Delhi; 1994;4th ed. :107-111.
- Dr. Sethi PD. High Performance Thin Layer Chromatography Qualitative Analysis of Pharmaceutical Formulations, 1st ed. 1996;1-72.
- Remington, 21st edition, Vol-I:773.
- Indian Pharmacopoeia (I.P.), 1996, A-157, Appendix 13.2
- Crompton DWT. How much human helminthiasis is there in the world? The Journal of Parasitology 1999; 85:397-403.
- Tortora, Microbiology an Introduction, Pearson Publisher, India, 2009.
- Kaur HA, Instrumental Method of Chemical Analysis Pragati, Ed 4th revised edition, pp. 1078-1079.
- http://www.who.int/wormcontrol/statistics/.
- Malhotra S, Mishra K, A New Antraquinone from Cassia sophera heartwood. Planta Medica 1982; 46:247-438.


 Nilambari Rudragonda Patil* 2
Nilambari Rudragonda Patil* 2
 Siddarth S. Desai 1
Siddarth S. Desai 1


















 10.5281/zenodo.11613574
10.5281/zenodo.11613574