Natural dyes have a lesser harmful effect on living bodies. Cotton is used as a fiber due to its higher ability of dye intake. To determine the darkness percentage of henna with different mordants. Henna powder is used for dyeing cotton with different mordants like water, lemon, sugar water, salt water and oil. Fastness Properties of washing, rubbing and light of all the samples were determined by grayscale. For henna tattoo, henna was applied with different solvents like water, oil, lemon and salt to determine the darkness percentage. Body temperature of all three persons were recorded. It showed different results with different people due to the biochemistry of different bodies.
Henna dye, henna tattoo, fastness properties, mordants and solvents.
Various industries, in recent times, use dyes for printing textile, paper, leather and different materials for coloring their products[16]. This brings customers interest towards colorful shades of the product. Among all industries, textile is one of the main industries which use dye for coloring their fabric.[16] According to estimations, more than 100000 commercial dyes are known, with annual production of more than 700000 tonnes in a year[16]. However, Dyeing fabric with synthetic dyes has led to several worse effects not only to the water bodies through effluents but also to human health.[16] Major Synthetic dyes used are azo dyes about 60% - 70% for dyeing. [1],[3] These azo dyes about 10% - 15% are released into the environment through effluent, as they don't bind to the fibre.[3] Synthetic dyes like cationic dyes, releases effluent with dye in it; which contains harmful heavy metals like Copper (Cu), Cobalt (Co), Zinc (Zn), Lead (Pb), Chromium (Cr) in aqueous form. Effluent of Anionic dyes have hydroxyl anions in it, which are soluble in water; hence it's difficult to remove anions from the effluent. [16] In most studies, they are considered carcinogenic to health [2],[3] and can cause several health hazards to the environment.[3],[7] They are toxic, mutagenic and carcinogenic.[3] Though many of the dyes are banned, some of them are still allowed, only when used within a certain limit. [16] Whereas natural dyes are a safer choice as it has minimal impact on the environment.[16] They are derived from animals, plants, insects and minerals.[16] They are non - substantive and can be used for dyeing only along with mordant.[16] The substantive natural dye is turmeric which does not require any mordant and can be applied directly. [16] Lawsonia inermis L. Commonly called henna, is a large, evergreen shrub or small tree with a densely branched habit.[4] The staining property of henna is mainly attributed to a 2-hydroxy-1,4-naphthoquinone compound named as lawsone also known as hennotannic acid which is seen in abundance in the dried leaves. [4],[17] Henna dyeing when done without using any harmful mordants, leaves no hazard behind to the living bodies of the environment.[17] Hence the dyeing interest is now shifting towards the naturally occuring dyeing agents.[14],[17] Henna has lawsone pigment which gives red-orange color.[15] Henna is substantive dye for protein fiber and textile fibres. [17] Similarly, carrot, beetroot, blueberry, papaya, leaves, etc. Also have carotenoid, betalin, anthoxanthin, chlorophyll pigments which helps in different shades of colouration. There have been many researches on the dyeing ability of henna on cotton fabric. Which showed that the darkness of henna depends on temperature [7] and different percentage dilution with water, [8],[9] the type of solvent used like Hot and cold water and ethanol solvents, [10] different types of fiber, [10] the pH of the dye. [11] Different mordants used [10] with henna also gave different results. Another property that, the coloring compound of henna exhibit is, ‘Lawsone’ strongly absorbs UV light.[6] Hence, henna irradiated with UV radiation can show difference in color intensity.[7] For this, studies showed that the dye extracted from the henna at an optimum temperature 65°C, with radiated henna depicted good staining on fabric.[7] The implications of henna dye have been shown to have poor to moderate dyeing capability towards cotton fabrics. [9] However, cotton has a higher rate of dye intake than other fiber, [10] hence, we used cotton in this study. The color fastness with respect to light exposure, washing and rubbing have been found satisfactory in previous papers. [12] Fastness properties of dyed woolen yarn samples were also found to be considerably good. [13] Mordants are the fixing compounds which are used to lock dyes to the fabric.[17] Those mordants are mostly metallic salt, which are considered to be hazardous to certain extent.[17] Hence, studies are focussing more on dyeing without mordant.[17] However, in this study, we used lemon, oil, salt water, sugar water as natural mordant to check the intensity of dyeing. Whether these natural mordants will have the dye to fabric binding capacity. For this, cotton was the fabric selected, as its better binding capacity with the dye.[10] Fastness properties like washing, light and rubbing of the samples were measured using Colors grab software application. Henna tattoo - Henna is used as a decorative material applied on hands, feet, nails, hair, etc.[19] Henna powder is extracted by drying, milling and sifting the leaves.[19] Traditionally, oil and sugar is used for extracting dye and deepening the color.[19] Sometimes, henna is adulterated with jagua (genipin and geniposide are the coloring components) and PPD (p-phenylenediamine) which intensifies the color.[19] Some studies showed that PPD is used with henna for blackening the color and has been found to be allergic.[20] Genipin was the main component that can cause allergic effect.[19]During ancient period, henna was used as medicine externally or internally for jaundice, leprosy, smallpox, and skin complaints.[18],[19] Lawsone coloring compound in henna reacts chemically with the protein keratin in skin resulting in a strong permanent stain that lasts until the skin is shed. [5] This could be because henna pierces the outer dead skin cells.[6] During review of literature, it was observed to lack studies in the field of henna tattoo. We are focusing on filling those gaps as there is little information regarding skin tattoos. Here, we used different solvents to identify the difference in intensities of three people. The darkness percentage was recorded by software application - color grab upto 96 hours.
METHODS AND PREPARATION
Preparation of henna samples-
- Blank sample - 2gm henna Powder + 10ml warm water about 40-60°C
- Lemon sample - 2 gm Henna Powder + 5ml lemon extract + 5 ml warm water
- Sugar sample - 2 gm Henna Powder + ml sugar in warm water
- Salt sample - 2 gm Henna Powder + ml salt in warm water
- Oil sample - 2 gm Henna Powder + 5 ml coconut oil + 5 ml warm water
Methods used-
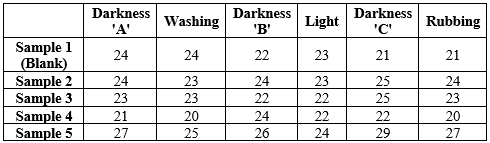
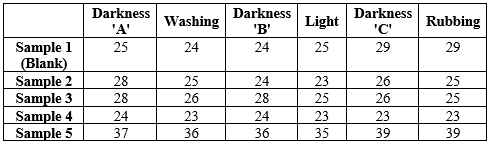
Cotton has a high affinity to dye. The white cotton cloth was scoured with salt (NaCl) and detergent supplied by Procter and gamble company and bleached using Sodium hypochlorite (NaClO) to remove undesirable or unwanted compounds attached to it. The cotton was then dipped into the evaporating dish containing the sample. Each sample had three cotton pieces labeled as A,B,C. First batch was washed after 30 minutes. Another batch was washed after 31 hours. The cloth pieces were washed thoroughly with detergent. The measurements of its darkness % from grayscale was taken down as reading from colors grab, a software application. Washing Fastness property was recorded after washing with detergent and drying, pieces labeled as 'A'. Their darkness % from grayscale was recorded. Light fastness property was obtained keeping the pieces labeled as 'B' in sunlight for 3-4 hours. Their darkness % from grayscale was recorded. Rubbing fastness property was obtained by rubbing the pieces labeled as 'C'. Darkness % from grayscale was recorded.

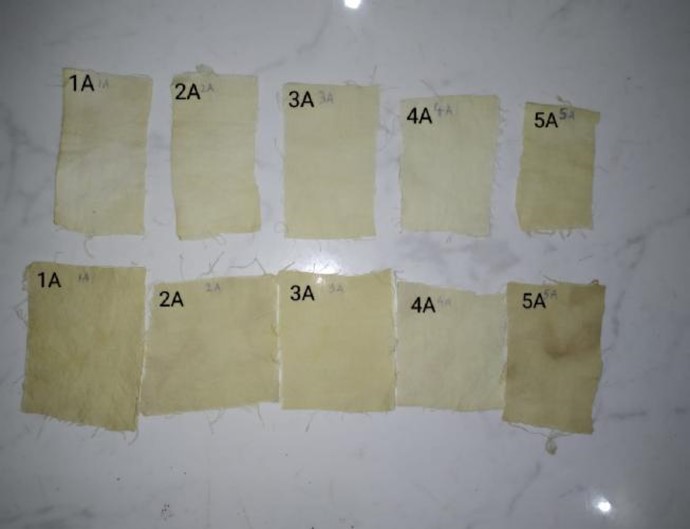
Fig. No. 1. Prepared samples for dyeing
For henna tattoo –
Henna extract was supplied by ASHTANG healthcare pvt. Ltd.
4 samples were used
- Blank - Henna Powder + water
- Oil - Henna Powder + oil
- Lemon - Henna Powder + lemon extract
- Salt - Henna Powder + salt water
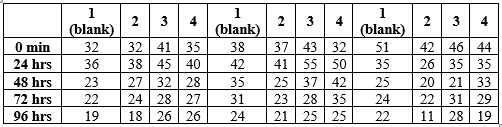
The water used here was lukewarm between 40°C - 60°C. Before applying henna, their body temperatures were recorded. Later, these henna samples were applied on skin thrice for 1 hour. The measurements were recorded from colors grab, a software application for consecutive 4 days after each 24 hrs including after removal reading.
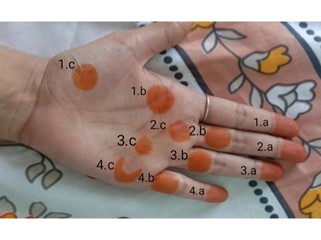
Fig. No. 2. Henna tattoo just after removal. (1. Blank, 2. Oil, 3. Lemon, 4. Salt)

Fig. No. 3. Samples after 30 mins. dyeing and washing

Fig. No. 4. Samples after 31 hrs dyeing and washing
Standardization of readings -
For fabric dyeing, external factors like light intensity, reflection, time difference, contact with water, different locations were the main cause of error in reading. Hence, for standardizing these conditions, pictures were clicked and analysed at night under the same light, time and location; to avoid error due to the difference in light intensity of sunlight. For henna tattoo, biochemical factors like hormones, body temperature, contact with water, light intensity, reflection and different locations caused error in reading. To avoid this, three tests for the 4 samples were simultaneously perrformed on one hand, which is in less contact with water. And to avoid hormonal changes and body temperature changes between two consecutive tests. Pictures were clicked and analysed at night under the same light, time and location to avoid light intensity differences between consecutive readings
OBSERVATIONS


 Vibha S. Bhagat*
Vibha S. Bhagat*







 10.5281/zenodo.12761219
10.5281/zenodo.12761219