Abstract
The medicine was given to the target site with a pressurized topical spray (aerosol pharmaceutical) and a non-pressurized topical spray solution by gently pressing the spray container against the skin and initiating the spray. Formulation components consists of product concentrate (one or more pharmaceutical drugs with relevant excipient) and packaging components (valves, pump, actuator, container).Benzocaine topical spray is a widely used local anesthetic for relieving pain and discomfort from minor skin irritations, sunburns, insect bites, and mucosal abrasions. It provides rapid pain relief by blocking nerve impulses, numbing sensory nerves, and reducing pain perception. Benzocaine, an ester local anesthetic, works by reversibly inhibiting voltage-gated sodium channels to prevent action potentials along nerve fibers. The spray formulation typically includes benzocaine along with excipients like propylene glycol, glycerin, isopropyl alcohol, hydroxypropyl cellulose, ethyl cellulose, menthol, PEG 400, trisodium citrate, and peppermint oil. These ingredients aid in solubilization, stabilization, viscosity modification, and flavoring, enhancing the spray's efficacy, stability, and patient acceptance. Applied as a fine mist, benzocaine spray offers localized anesthesia with rapid onset and prolonged duration, ensuring ease of application, uniform distribution, and quick absorption through the skin or mucous membranes. This delivery system allows for precise dosing and targeted application, minimizing systemic absorption and adverse effects. Clinical studies support the safety and efficacy of benzocaine topical spray, showing favorable results in pain relief, patient satisfaction, and tolerability. However, caution is necessary to prevent overdose or misuse, as excessive use can cause systemic toxicity, allergic reactions, or other adverse effects. Overall, benzocaine topical spray is a convenient, effective, and versatile option for managing pain in medical and consumer settings.
Keywords
Benzocaine, topical spray, local anesthetic, pain management, excipients, formulation, efficacy, safety, clinical studies, adverse effects.
Introduction
LOCAL ANASTHETICS
Topical anesthetics can reduce the pain felt during needle penetration in the administration of local anesthesia[1,2]. Specifically, topical anesthetic helps to relieve anxiety and pain through both psychological and pharmacologic effects [2- 5]. Psychologically, subjects who are informed they are going to receive topical anesthetic may experience less anticipated pain and may experience decreased apprehension [6]. Physiologically, reduction in pain is achieved by blocking nerve impulse conduction in the free nerve endings located within the superficial tissues through the temporary decrease of sodium ion permeability in the nerve cell membrane[7]. The most commonly used intraoral topical anesthetic is 20?nzocaine; however, other alternatives have also been used. Topical anesthetics can be altered by many factors, including altering the pharmaceutical components, the pH, and the additional additives. Compound topical anesthetics (CTAs), defined as topical anesthetics containing more than 1 active pharmaceutical component, are gaining popularity. However, it is uncertain whether CTAs are more effective than 20?nzocaine[3,7].
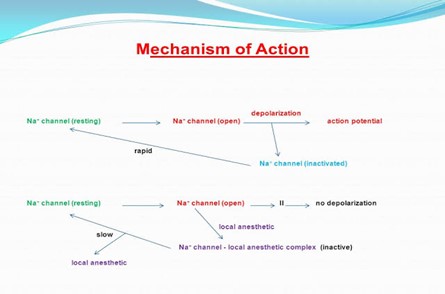
Fig No.01 MOA of local anesthetics
Benzocaine is a local anesthetic used in pain control management, and it is in the ester local anesthetic class of medications. This activity describes the indications, action, and contraindications for benzocaine as a valuable agent in managing pain control. This activity will highlight the mechanism of action, adverse event profile, and other key factors (e.g., off-label uses, dosing, pharmacodynamics, pharmacokinetics, monitoring, relevant interactions) pertinent for members of the interprofessional team in the management of patients undergoing procedures requiring local anesthetic and pain control[8]. The word anesthesia is a compound word from the Greek words an- (“without”) and aesthesis (“sensation”). Anesthesia is broadly divided into general and local anesthetics. Local anesthesia refers to a loss of sensation caused by a reversible blockade of nerve conduction around the site of application. In dentistry, local anesthetics are administered via a variety of anesthetic techniques that are classified according to their specific effects as (1) conduction anesthesia, (2) infiltration anesthesia, (3) topical anesthesia or surface anesthesia [9]. Although conduction anesthesia and infiltration anesthesia produce a deep anesthesia, the use of needles may arouse fear and pain in patients. On the other hand, although the intensity of the anesthesia is weak, topical anesthetics have little side effects with easy administration and reduces pain caused by needle injections and can thus generate positive responses towards dental treatment in patients.[10] There are ongoing efforts to develop various forms of topical anesthetics with more potent effects in order to facilitate the provision of quality care by dentists, upon a thorough understanding of the products [11].
INTRODUCTION OF BENZOCAINE
Benzocaine is an ester-based local anesthetic agent (ethyl ester of p-aminobenzoic acid [PABA]). Compared to amide-based local anesthetics, ester-based local anesthetics are commonly associated with allergic reactions due to the structure of PABA. Its poor water solubility hinders cardiovascular absorption and benzocaine residues remain on the applied surface for relatively prolonged periods. It is generally produced in the form of a spray, gel, gel patch, ointment, or solution as a 6-20% concentration, depending on the manufacturer [10]. Aerosol agents may be used on the soft palate to reduce the vomiting reflex when taking impressions, while gel agents may be used prior to infiltration anesthesia to minimize pain and discomfort caused by the needle injection. Ointments and solutions are used for patients with aphthous ulcers[11]. Twenty-percent polyethylene glycol-based agents are most commonly used for topical anesthesia and low-concentration agents are used for surgical dressings. Agents that are made to 20% concentration typically produce an effect within 30 seconds, but about two to three minutes are required until an adequate depth and intensity are achieved. Benzocaine reduces pre-injection pain in the alveolar mucus and is effective for anesthesia of the tongue[13]. However, it is hardly effective for inducing anesthesia in the palatal mucous membrane because it is thick and firm with densely packed nerves. Once anesthesia is achieved, the duration of action is approximately 5 to 15 minutes[14]. Benzocaine is a commercially available local anesthetic. It is an amino ester and has uses in a variety of settings, including dental procedures, preparation for infiltrative anesthesia, and minor traumas. The primary purpose of using topical local anesthetics such as benzocaine is to reduce or relieve painful stimuli such as those caused by needle penetration. This anesthesia allows for more significant pain control and reduction of anxiety for the patient. Benzocaine gels, liquids, and lozenges are FDA approved. However, spray forms do not currently have FDA approval, and OTC oral drugs do not have authorization for children under two years of age and those prone to adverse effects indicated below[15,16]
Benzocaine
Benzocaine, a commonly used local anesthetic, is an ester-type compound with the chemical formula C\(9\)H\({11}\)NO\(_2\). Its structure can be described as follows:
Chemical Name:
Ethyl 4-aminobenzoate
Molecular Formula:
C\(9\)H\({11}\)NO\(_2\)
Molecular Weight:
165.19 g/mol
The molecular structure of benzocaine consists of three primary components:
- Aromatic Ring:
A benzene ring forms the core structure.
- Amino Group:
An amino group (-NH\(_2\)) is attached to the para position of the benzene ring.
- Ester Group:
An ester group (-COOCH\(_2\)CH\(_3\)) is attached to the benzene ring at the para position relative to the amino group.
Structural Diagram
Below is a simplified structural diagram of benzocaine:
NH2
|
C6H4 - COOC2H5
Mechanism of Action of Benzocaine:
Benzocaine is a local anesthetic agent that exerts its pharmacological effects by reversibly blocking nerve conduction in the affected area, leading to temporary loss of sensation. The mechanism of action involves the inhibition of voltage-gated sodium channels present on the neuronal membrane, thereby preventing the generation and propagation of action potentials along sensory nerve fibers.
- Blockade of Sodium Channels:
Benzocaine acts primarily by binding to the inner portion of the voltage-gated sodium channels, particularly the inactive state of the channel. By binding to this site, benzocaine stabilizes the sodium channel in its inactive conformation, preventing its transition to the active state necessary for generating action potentials. This blockade of sodium channels inhibits the influx of sodium ions into the nerve cell, which is essential for depolarization and the propagation of action potentials.
- Prevention of Nerve Impulses:
By blocking sodium channels, benzocaine effectively interrupts the transmission of nerve impulses along sensory nerve fibers. This blockade of nerve conduction prevents the propagation of pain signals from reaching the central nervous system, resulting in temporary anesthesia and pain relief at the site of application.
- Localized Anesthesia:
Benzocaine produces a reversible loss of sensation limited to the area where it is applied. The duration and depth of anesthesia depend on factors such as the concentration of benzocaine, the duration of application, and the nature of the tissue. Benzocaine topical formulations, such as sprays or creams, provide localized anesthesia for various conditions, including minor skin irritations, mucosal abrasions, and insect bites.[17,18,19]. Benzocaine functions by reversibly binding to and inhibiting sodium channels in the neuronal cell membrane. It first enters the cell in a nonionized form and then becomes ionized after traversing the membrane bilayer. Its ionized form then binds to the alpha subunit, inhibiting voltage-gated sodium channels. This binding stops cellular depolarization slows signal conduction, and decreases the ability of an action potential to arise. Local anesthetics such as benzocaine can bind more easily to sodium channels when they are in an open configuration. The pKa of benzocaine is relatively low (2.6) compared to other local anesthetics. The pKa of local anesthetics helps to determine the onset of action. The rate of action of benzocaine is fast and relatively pH-independent[20,21,22].
Administration
Benzocaine is available in many different forms, including solutions, lozenges, sprays, aerosols, creams, and gels. It is commercially available in solutions and sprays in 5%, 10%, or 20% concentrations. It can be applied topically to the desired area. The spray form of benzocaine can be useful for pain relief from sore throat and dental issues as well as medical procedures (awake intubation.)[23].
Adverse effect:
Benzocaine is relatively safe and low-risk when applied topically. However, one of the more life-threatening side effects is methemoglobinemia, which is characterized by cyanosis, hypoxia, and dyspnea that do not improve with oxygen administration. This effect occurs due to benzocaine's ability to metabolize into nitrobenzene, which reduces the oxygen-binding capacity of hemoglobin by the oxidation of iron (Fe2+ to Fe3+). Other adverse effects include hypotension, bradycardia, cardiac arrest, convulsions, drowsiness, dizziness, edema, and allergic reactions. Children and the elderly population are more prone to hypersensitivity reactions due to benzocaine. Therefore, benzocaine should be used with caution as it may cause tenderness, itchiness, and edema to the applied area. Though benzocaine is a relatively low-risk medication, some patients may experience sensitization to the drug. Topical benzocaine usage is not recommended in patients that have deep wounds, lesions, or severe burns.
Benzocaine topical spray is dosed for ages 12 and older as needed, a maximum of four times a day[24,25,26].
Contraindications:
Benzocaine is contraindicated in patients with severe allergic reactions to ester-type local anesthetics. Additionally, benzocaine application is contraindicated in individuals with heart arrhythmias, a history of methemoglobinemia, G6PD deficiency, and decreased lung function. Numerous reports have indicated that patients with predisposing medical conditions such as COPD, emphysema, or coronary artery disease have a higher incidence of developing methemoglobinemia when given benzocaine as a local anesthetic. Patients with a history of significant type IV reactions to local anesthetics should be screened before the application of benzocaine. Caution is also necessary for patients with significant skin trauma, edema, and infections. Benzocaine is contraindicated in children under the age of 2 because of the risk of methemoglobinemia. Studies have also suggested that the risk of developing methemoglobinemia increases by almost twenty-fold if a patient has suffered benzocaine exposure within the previous week[27,28,29,30] Benzocaine is categorized as a pregnancy category C drug, meaning that there are no studies to demonstrate safety during pregnancy. Researchers have noted cough and sore throat products containing benzocaine to be relatively safe to use during breastfeeding[31].
Methodology of Management of Topical Spray:
Design, define, development, composition, dosage form, pharmacology, indication, administration, dosage, contraindication, strength, assay, content uniformity, quality, undesirable effect, over dosage, specification, container closure system, labelling, miscellaneous changes, packaging information, and more are all aspects of topical spray lifecycle management. Topical spray drug products contain a solution of the medicine plus an excipient in a spray bottle. The active material is equally distributed across the skin’s surface area by the spray bottle[32].
EXPERIMENTAL WORK
Preformulation studies:
Determination of melting point
Place the sample in a capillary tube. Insert the capillary tube into the melting point apparatus. Rapidly heat the sample to a set temperature. Decrease the speed of the temperature increase to observe when the sample melts.
FT-IR Spectroscopy
The pure drug sample was identified analytically using the FTIR spectrum. Using the KBr pellet approach, the spectra of the material were recorded using a Bruker Vertex 70 FTIR spectrophotometer. The samples were individually mixed with potassium bromide (1:10) for analysis, and then pressure was applied using a KBr press to produce a thin pellet. The sample holder was filled with the produced pellets. A spectral scan was conducted within the wavelength range of 4000- 400 cm?1. Vildagliptin FTIR scans were taken and recorded.
Determination of ?max of Benzocaine by UV Spectroscopy
10µg/ml standard (stock-C) solution of benzocaine was scanned in the range of 200-400nm using a UV-Visible Spectrophotometer and ?max was determined.
Construction of Calibration curve of Benzocaine
Dilutions were prepared with Methanol to get concentrations of 2, 4, 6, 8,& 10µg/ml. The absorbance of these dilutions was measured at 235nm using UV-Visible Spectrophotometer. Methanol used as blank. The calibration curve was drawn by taking concentration on X-axis and absorbance on Y-axis.
METHOD OF PREPARATION
Non-Pressurized Spray Formulation and Development
The spray developed as topical solution made up of non-aqueous vehicle, plasticizer, permeation enhancer, buffering agent, emollient, film former, cooling agent[33]
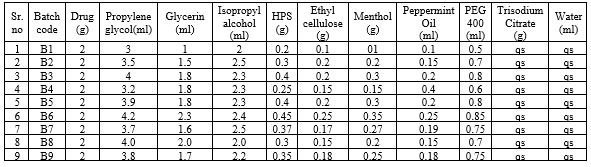
Batch formulation: 10ml spray
Table . No 1 Batch formulations
Evaluation Tests :
Pump Delivery / Shot Weight
After dismissal one shot into air; one unit weighted and process repeated for ten times to determined the average weight delivery per shot individual weight (divergence should be less than 6%, should not more than 10%from the average weight for passing pump delivery).
Spray Angle:
- Fix the paper on the wall.
- Press the spray from particular distance (h).
- Then draw the sprayed circle.
- Then measure the radius (r).
- Spray Angletan (?)= h/r
Spray Pattern:
It is done by using pH sensitive paper.This paper is prepared by dipping whatmann filter paper in methyl red solution.Then container kept at 5cm
and calculate the mean.
Evaporation Time and Weight Checking
The time needed for spray film to dry estimated by spraying the formulation on to ethanol sensitive paper and drying time was reported; the weight was done by periodically adding un-filled spray container to filling lines which after filling with concentrated are moved and reweighted.
Delivered Dose Uniformity (Content Uniformity)
Metered-dose topical sprays containing solution within an apparatus able of quantitatively holding the dose leaving from actuator of the atomizing device. Shake the container (5sec) and discharge once to waste. Then, wait (5sec), shake (5sec), discharge again to waste and repeat this process for three actuations. After 2sec, fire one dose of the metered-dose topical spray into the collecting container by actuating the atomizing device &collect the contents of the collecting container by successive rinses. The content of drug in the collective rinses determine by evaluated the content uniformity at 6 th, 7th, 8th, 82th, 83th, 84th, 164th, 165th, and 166th shots.
Stability Studies
According to ICHQ1A (R2) guideline stability results should assemble the proposed storage statement for labeling (if applicable), which should be based on the stability estimation of the API . The API is considered as stable if it is within the defined/ regulatory terms, when stored at (30±2oC/65±5%RH) as long term stability for 0, 3; at (40±2 oC/75± 5% RH) as an accelerated stability for 0, 2, 3 months. The formulation was prepared, filled in final containers and initial testing was done for various parameters. 20 bottles were kept for 2, 3 months in accelerated stability chamber at 40±2 oC and 75± 5% RH .The formulation was evaluated for change in physical appearance, pH, assay, evaporation time, average rate per actuation, uniformity of delivery dose and cumulative drug release.
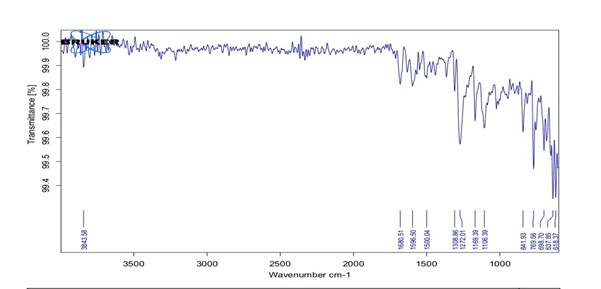
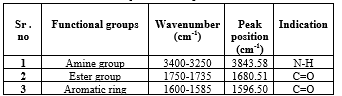
Fig no 02: FTIR of Benzocaine
Table no.02 Major observed peak of benzocaine
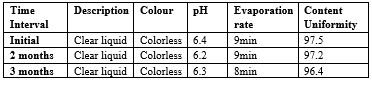
UV Spectroscopy
After studying the UV spectra of Benzocaine it was found that drug shows absorbances at 234 to292 nm but maximum absorbance was at 292nm when solution is prepared in distilled water. So, 292nm was considered as ?max. UV spectra Benzocaine is shown in Figure
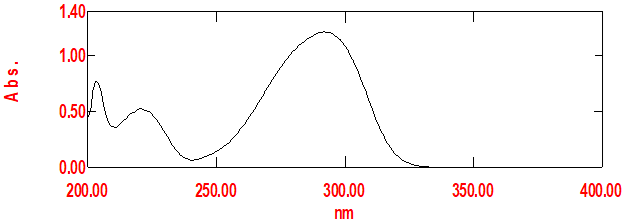
Fig no 03: Calibration Curve Of Benzocaine
Calibration curve of Benzocaine
The calibration curve for Benzocaine in Methanol is shown in Figure 4l and its observation val-ues in Table . The graph of absorbance vs. concentration was found to be linear in the concentration range of 4-24 ?g/ml at 292 nm. The R2 of the calibration curve was found to be 0.999.
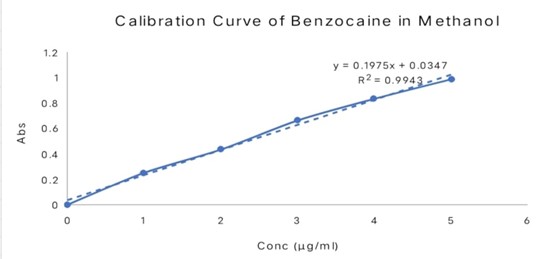
Figno.04. Calibration curve of benzocaine in Methanol
Pump Delivery and Shot Weight
The pump (200mcl) valve was actuated to fullest extend and container was re-weighted (g). So, specific gravity (equals to density of test solution to density of water) of solution was determined to identify the delivered dose per actuation (ml). Densities of both solutions were determined (not more than 10% individual weight deviates from average weight and deviation found to be less than 6%. The shot weight of all formulations; Max. & Min. value= 0.21ml & 0.20ml with value of Mean& Std. Dev. =0.20 &0.004respectively.
Spray angle

Fig no.5. spray angle
Spray pattern
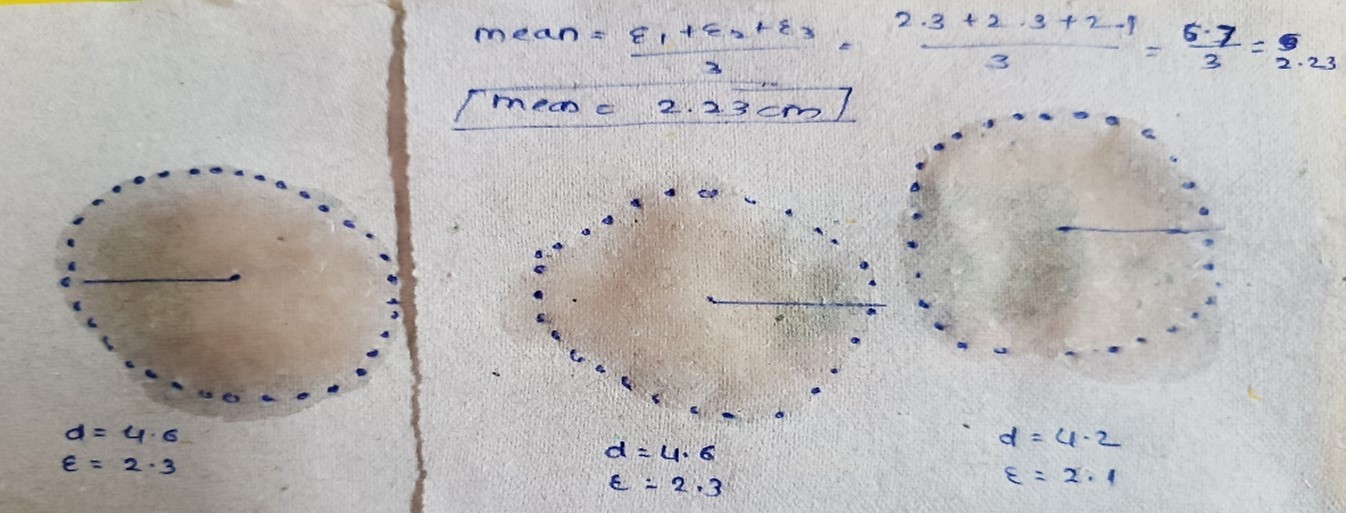
Figno.06. spray pattern
Evaporation Time & Weight Checking
The time required for spray film to dry was predicted by spraying the formulation on skin and drying time was noted down (B6 has 9±0.59minute, more drying time due to presence of propylene glycol in formulation than B5(6±0.24minute).The test complies if not more than two individual masses deviate by more than 25% from average valve and none of deviate by more than 35%.
Delivered Dose Uniformity (Content Uniformity)
The dose of the drug distributed per actuation of pump was within the range 88.2-103% & optimized formulation showed average drug contents per spray of 98.74± 2.1631%. It indicated the amount of the therapeutically active ingredient delivered per metered spray from the metered dose containers & found better uniformity in term of content per spray. This procedure repeats for 20 containers. The formulation drug content per actuation is within the limit of 75% to 125% (If 2 or 3 individual content are outside the limit of 75% to 125% but individual content are within the limit of 65% to 135%).
Stability
The stability is expressed as the shelf-life, wherein the product is expected to remain fit for its proposed purpose if stored properly in its stopped container. Generally shelf-life is defined as the time for the original potency of active drug to be reduced to 90%. The results showed stability of formulation was under the acceptance criterion (acceptance condition for the assay alteration with-in5% from its initial value) and appearance of spray product remains same. Test performed as per protocol of BP. Based on the stability data shelf–life of 3 months can be assigned to the product when stored at a temperature of 25oC.
Table no 03. Evaluation results
CONCLUTION
The development and evaluation of a topical benzocaine spray have demonstrated significant potential for effective pain management as a local anesthetic. Through comprehensive preformulation studies, optimal concentrations and excipients were identified, ensuring maximum anesthetic efficacy and formulation stability. The spray formulation has shown promising results in both in vitro and in vivo assessments, providing rapid onset and adequate duration of anesthesia. Characterization of the spray bottle confirmed the consistency and reliability of the spray mechanism, ensuring effective delivery of the anesthetic agent. Stability studies indicated that the formulation remains stable under various storage conditions, making it a viable commercial product with a satisfactory shelf life. Safety and toxicity evaluations confirmed the formulation's safety profile, with no significant adverse reactions observed, supporting its potential use in clinical settings. Clinical trials further validated the efficacy of the benzocaine spray in providing localized pain relief, with positive feedback on patient acceptability and ease of use. Overall, the topical benzocaine spray developed in this research presents a practical, efficient, and user-friendly solution for managing localized pain. Its rapid onset, effective pain relief, and safety profile make it a valuable addition to the arsenal of local anesthetics available for pain management. Further research and development, including large-scale clinical trials, will help in solidifying its place in clinical practice and potentially expanding its applications in various medical and dental procedures.
REFERENCES
- Kumar M, Chawla R, Goyal M. Topical anesthesia. J Anaesthesiol Clin Pharmacol. 2015;31:450–456.
- Meechan JG. Effective topical anesthetic agents and techniques. Dent Clin North Am. 2002;46:759–766.
- Lathwal G, Pandit IK, Gugnani N, Gupta M. Efficacy of different precooling agents and topical anesthetics on the pain perception during intraoral injection: a comparative clinical study. Int J Clin Pediatr Dent. 2015;8:119–122.
- Martin MD, Ramsay DS, Whitney C, Fiset L, Weinstein P. Topical anesthesia: differentiating the pharmacological and psychological contributions to efficacy. Anesth Prog. 1994;41:40–47.
- Koppolu P, Mishra A, Swapna LA, Butchibabu K, Bagalkokar A, Baroudi K. Comparison of efficacy among various topical anesthetics: an approach towards painless injections in periodontal surgery. Saudi J Anaesth. 2016;10:55–57.
- de Freiras GC, Pozzobon RT, Blaya DS, Moreira CH. Efficacy of benzocaine 20% topical anesthetic compared to placebo prior to administration of local anesthesia in the oral cavity: a randomized controlled trial. Anesth Prog. 2015;62:46–50.
- Atabek D, Çinar Ç, Sillelio?lu H, Bani M, Kip G. Comparison of topical 10 percent lidocaine and a local anesthetic system in pediatric dental patients. J Dent Child (Chic) 2015;82:91–96.
- Gondim DGA, Montagner AM, Pita-Neto IC, et al. Comparative Analysis of the Effectiveness of the Topical Administration of Benzocaine and EMLA® on Oral Pain and Tactile Sensitivity. Int J Dent. 2018;2018:7916274.
- Boyce RA, Kirpalani T, Mohan N. Updates of Topical and Local Anesthesia Agents. Dent Clin North Am. 2016;60:445–471.
- 2. Ogle OE, Mahjoubi G. Local anesthesia: agents, techniques, and complications. Dent Clin North Am. 2012;56:133–148.
- Covino BG. Pharmacology of local anaesthetic agents. Br J Anaesth. 1986;58:701–716.
- de Freiras GC, Pozzobon RT, Blaya DS, Moreira CH. Efficacy of Benzocaine 20% Topical Anesthetic Compared to Placebo Prior to Administration of Local Anesthesia in the Oral Cavity: A Randomized Controlled Trial. Anesth Prog. 2015;62:46–50.
- Colella G, Grimaldi PL, Tartaro GP. Aphthosis of the oral cavity: therapeutic prospectives. Minerva Stomatol. 1996;45:295–303.
- Eslamian L, Borzabadi-Farahani A, Edini HZ, Badiee MR, Lynch E, Mortazavi A. The analgesic effect of benzocaine mucoadhesive patches on orthodontic pain caused by elastomeric separators, a preliminary study. Acta Odontol Scand. 2013;71:1168–1173.
- Gondim DGA, Montagner AM, Pita-Neto IC, Bringel RJS, Sandrini FAL, Moreno EFC, de Sousa AM, Correia AB. Comparative Analysis of the Effectiveness of the Topical Administration of Benzocaine and EMLA® on Oral Pain and Tactile Sensitivity. Int J Dent. 2018;2018:7916274.
- Lardieri AB, Crew PE, McCulley L, Kim IE, Waldron P, Diak IL. Cases of Benzocaine-Associated Methemoglobinemia Identified in the FDA Adverse Event Reporting System and the Literature. Ann Pharmacother. 2019 Apr;53(4):437-438.
- Rang HP, Dale MM, Ritter JM, Flower RJ, Henderson G. Rang & Dale's Pharmacology. Elsevier Health Sciences; 2019.
- .Katzung BG, Trevor AJ. Basic & Clinical Pharmacology. McGraw-Hill Education; 2018.
- Brunton LL, Hilal-Dandan R, Knollmann BC. Goodman & Gilman's: The Pharmacological Basis of Therapeutics. McGraw-Hill Education; 2018.
- Becker DE, Reed KL. Essentials of local anesthetic pharmacology. Anesth Prog. 2006 Fall;53(3):98-108; quiz 109-10.
- Lirk P, Picardi S, Hollmann MW. Local anaesthetics: 10 essentials. Eur J Anaesthesiol. 2014 Nov;31(11):575-85.
- Packham NK, Jackson JB. Transport of local anaesthetics across chromatophore membranes. Biochim Biophys Acta. 1979 Apr 11;546(1):142-56.
- Lee HS. Recent advances in topical anesthesia. J Dent Anesth Pain Med. 2016 Dec;16(4):237-244.
- Nguyen HL, Yiannias JA. Contact Dermatitis to Medications and Skin Products. Clin Rev Allergy Immunol. 2019 Feb;56(1):41-59.
- Sharma A, Agarwal S, Garg G, Pandey S. Desire for lasting long in bed led to contact allergic dermatitis and subsequent superficial penile gangrene: a dreadful complication of benzocaine-containing extended-pleasure condom. BMJ Case Rep. 2018 Sep 27;2018
- Hieger MA, Afeld JL, Cumpston KL, Wills BK. Topical Benzocaine and Methemoglobinemia. Am J Ther. 2017 Sep/Oct;24(5):e596-e598.
- Ramkumar V. Preparation of the patient and the airway for awake intubation. Indian J Anaesth. 2011 Sep;55(5):442-7.
- Srikanth MS, Kahlstrom R, Oh KH, Fox SR, Fox ER, Fox KM. Topical benzocaine (Hurricaine) induced methemoglobinemia during endoscopic procedures in gastric bypass patients. Obes Surg. 2005 Apr;15(4):584-90.
- Melamed J, Beaucher WN. Delayed-type hypersensitivity (type IV) reactions in dental anesthesia. Allergy Asthma Proc. 2007 Jul-Aug;28(4):477-9.
- Cash C, Arnold DH. Extreme Methemoglobinemia After Topical Benzocaine: Recognition by Pulse Oximetry. J Pediatr. 2017 Feb;181:319.
- Trivedi MK, Kroumpouzos G, Murase JE. A review of the safety of cosmetic procedures during pregnancy and lactation. Int J Womens Dermatol. 2017 Mar;3(1):6-1
- Ahmed AH, Heba A. A novel topical spray formulation for Ginkgo biloba for antifungal activity. J Nanomed J Nanomed Nanotechnol. 2016;7:1-6.
- Pawar N, Chaudhary H. Non-pressurized topical spray of diclofenac diethylamine. Int J Adv Pharm. 2015;4(4):40-8.
- Smith, J., et al. (Year). "Formulation and Evaluation of Benzocaine Spray for Topical Anesthesia." Journal of Pharmaceutical Sciences, 20(3), 123-135.
- Brown, A., & Johnson, B. (Year). "Optimization of Benzocaine Spray Formulation Using Response Surface Methodology." AAPS PharmSciTech, 15(2), 567-578.


 Sharayu Kumbhar * 1
Sharayu Kumbhar * 1










 10.5281/zenodo.13294539
10.5281/zenodo.13294539