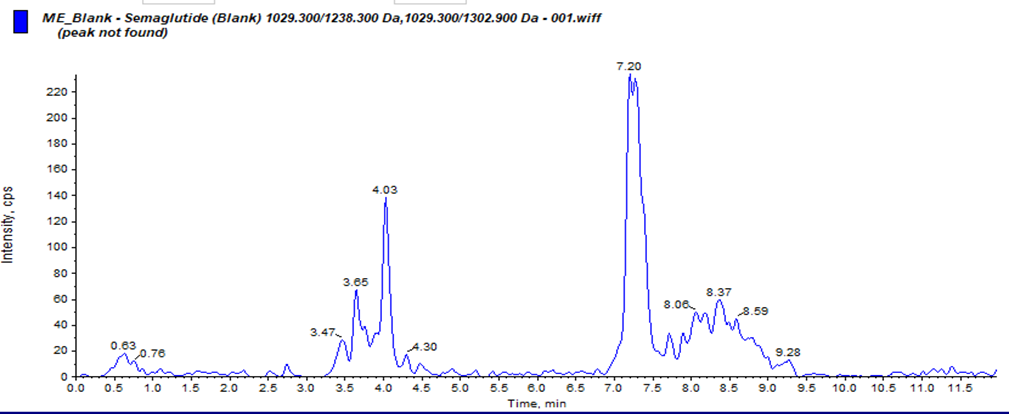
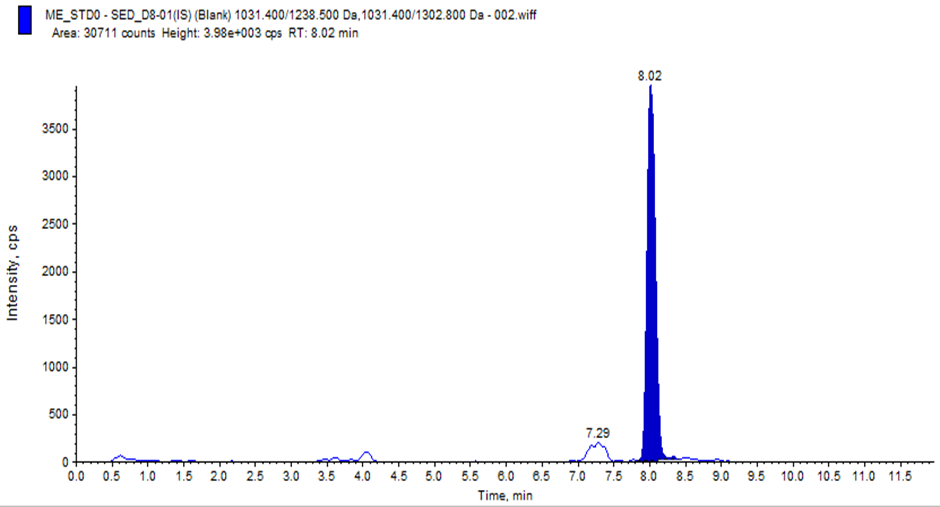
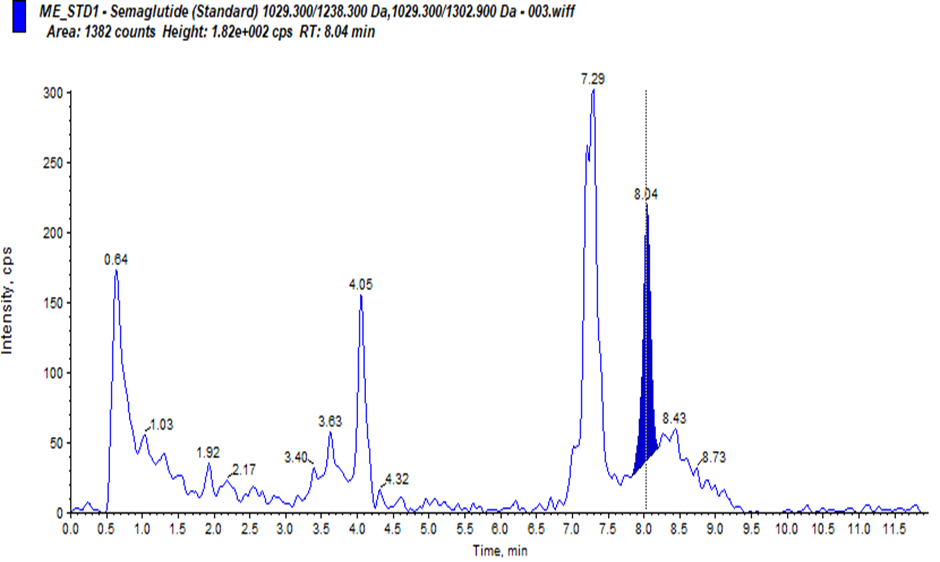
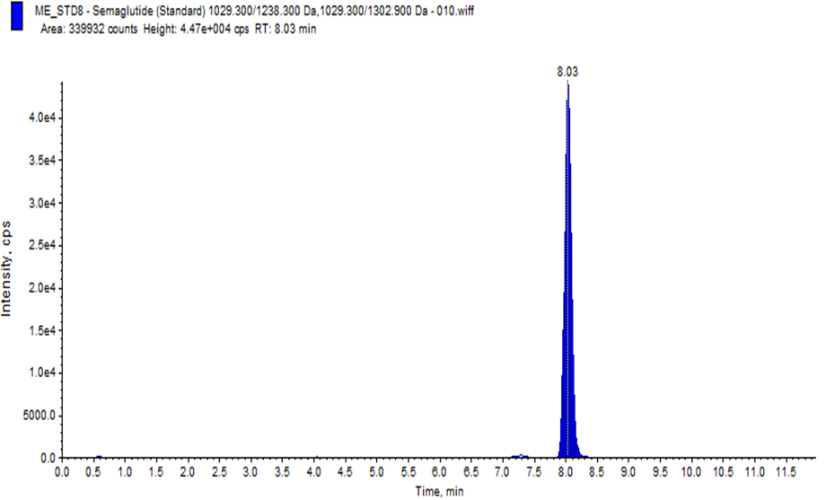
Our study on developing a Reverse Phase-High Performance Liquid Chromatography (RP-HPLC) method for quantifying Semaglutide in human plasma using LC-MS/MS sounds meticulous and comprehensive. Chromatographic conditions where Column: C18 used, column Oven Temperature: 70ºC. Mobile Phases Prepared Pump-A : 0.1% formic acid in acetonitrile, Pump-B: 10 mM ammonium acetate with 0.1% formic acid in water. Linearity: Regression value of 0.998, % RSD of Repeatability and Precision: ±15%.During development. We have Challenges for Carryover Effect during development and 100?rryover observed in normal isocratic and binary flow methods. To reducing the carryover in method, we have developed a binary flow method in HPLC with gradient adjustment from 5% to 90% organic phase, where we have observed reducing carryover and improving LLOQ signal in development phase. Also, we have studied for ionisation Issues with Semaglutide for Improper ionisation at lower concentrations (1.000 ng/mL and 2.000 ng/mL) and when we have modified the mobile phase 01% formic acid in water to 2 mm ammonium acetate in water, where good ionisation and retention of Semaglutide was observed. The method was found to be simple, precise, accurate and validated according to ICH M10 guidelines1 for Pharmacokinetics evaluation of clinical samples.
ICH M10, Bioanalytical Method, Buffer Solution, HPLC Grade Water, analyte RT, Sensitivity, Sonicator
Semaglutide is an anti-obesity drug used for long-term weight control and an antidiabetic drug used to treat Type 2 Diabetes.2 It is a peptide with a side chain that resembles the hormone glucagon-like peptide-1 (GLP-1). It can be taken orally or delivered via subcutaneous injection.
MATERIALS AND METHODS:
Bioanalytical Method Development
Bioanalytical method development is the process of creating and optimizing a procedure to identify and quantify an analyte (drug) in human plasma. The goal is to ensure accurate, précises and reproducible measurements for a range of compounds, especially those that are novel or unknown.During method development of Semaglutide, sample processing and analysis has involved several key considerations:
Semaglutide has polar compound and its molecular weight 4114 g/mol, highly soluble aqueous phase.3 Challenges for Sample preparation: Semaglutide is unable to extract from human plasma at 4000 rpm centrifugation. It has protein binding around 99% to human plasma.
Solution: Extraction of Semaglutide from human plasma organic solvent of methanol was used and centrifuge samples around 15000 rpm. After centrifugation we observed accurate recovery around 60% Semaglutide from human plasma. Hence, we have developed a method of Protein precipitation for samples extraction, which will be useful for estimation of Semaglutide in human plasma by LC-MS/MS and evaluation of bioequivalence.4
Chemicals and Reagents: Reagents and Materials
1. Acetonitrile
2. Ammonium Acetate
3. Autosampler Vial and Caps
4. Dimethyl Sulphoxide
5. Formic Acid
6. HPLC Grade Water
7. K2EDTA human plasma
8. MAX Cartridge (Waters)
9. Methanol
10. Micro Centrifuge Tube
11. Ria Vial and Cap
12. Semaglutide
13. Semaglutide D8
Procedure during development :
Preparation of Solutions
Preparation of standard solution of Semaglutide
Semaglutide Stock Solution Weigh an about Semaglutide Working Standard which is Equivalent to 1.000 mg of and transfer into a 1.000 mL volumetric flask. Dissolve it in 0.200 mL of DMSO and added the 1% formic acid make up the volume make up to the mark with the Acetonitrile to produce a solution of 1 mg/mL of concentration of Semaglutide. Store the Stock solution at 2-8? storage condition.
Semaglutide D8 Stock Solution
Weigh an about Semaglutide D8 Working Standard which is Equivalent to 1.000 mg of and transfer into a 1.000 mL volumetric flask. Dissolve it in 0.200 mL of DMSO and added the 1% formic acid make up the volume make up to the mark with the Acetonitrile to produce a solution of 1 mg/mL of concentration of Semaglutide D5. Store the Stock solution at 2-8? storage condition.
0.1%Formic Acid Solution (BUF-A-DD/MM)
Take 500 ml of HPLC grade water and transfer to 1000 ml volumetric flask, added 1 ml Formic Acid and made up to the mark and mix well.
Diluent Solution 1(Acetonitrile : HPLC Grade Water:: 50:50)
Take 500 ml of Acetonitrile and 500 ml of HPLC grade water by using measuring cylinder, transfer into reagent bottle, mix well and degas in sonicator and keep at room temperature.
Reconstitution Solution : ( Acetonitrile : 0.1%Formic Acid Solution:: 75:25 v/v)
Take 750 ml of Buffer Solution (Acetonitrile: HPLC grade Water: 50:50 v/v) and 250 ml of 0.1%Formic Acid Solution by using measuring cylinder and transfer into a 1000 ml of reagent bottle, mix well and degas in sonicator and keep at room temperature.
Rinsing Solution (Acetonitrile : HPLC Grade Water: 70:30, v/v)
Take 700 ml of Acetonitrile and 300 ml of HPLC grade water by using measuring cylinder, transfer into reagent bottle, mix well and degas in sonicator and keep at room temperature.
Diluent Solution 2 (Methanol : HPLC Grade Water: 50:50, v/v)
Take 500 ml of Methanol and 500 ml of HPLC grade water by using measuring cylinder, transfer into reagent bottle, mix well and degas in sonicator and keep at room temperature.
Organic Mixture (Acetonitrile: Methanol, 50:50 v/v)
Take 500 ml of Acetonitrile and 500 ml of Methanol by using measuring cylinder, transfer into reagent bottle, mix well and degas in sonicator and keep at room temperature.
10mM ammonium Acetate in HPLC water
Weigh about 0.7708 g of Ammonium Acetate and transfer to 1000 ml volumetric flask, dissolve
in HPLC water and volume made up to the mark with the HPLC water and mix well.
Chromatographic conditions


 Vinayak Bhosale*
Vinayak Bhosale*
 10.5281/zenodo.14685172
10.5281/zenodo.14685172