Abstract
This review delves into the contemporary use of microwave technology in various cyclization reactions, including heterocyclic ring formation, as well as in significant processes like nucleophilic substitution, hetero-Diels-Alder reactions, and 1,3-dipolar cycloaddition. A comparative analysis with traditional methods highlights the advantages of microwave-assisted approaches in the realm of synthetic heterocyclic chemistry. Microwave (MW) radiation has become a prevalent heat source in organic synthesis, offering notable advantages such as accelerated reactions, higher yields, milder conditions, and reduced environmental impact through solvent-free protocols. This study specifically explores MW-assisted synthesis of N-containing heterocyclic compounds, recognizing their significance in pharmaceuticals and addressing environmental concerns associated with traditional synthesis methods.
Keywords
heterocyclic compound, Green chemistry, synthesis, Microwave Assisted.
Introduction
Heterocyclic compounds are organic compounds that contain at least two different elements. Typically, these elements include carbon along with elements like nitrogen, oxygen, sulfur, or others. These compounds play a crucial role in the field of medicinal chemistry, as many biologically active molecules, including pharmaceuticals, are heterocyclic. The diversity in heterocyclic structures contributes to their wide range of applications, from drugs to agrochemicals. The most common heterocycles include pyridine, furan, thiophene, and pyrrole. The properties and reactivity of heterocyclic compounds vary based on the type of heteroatom present and the overall ring structure. Understanding these compounds is essential for drug discovery, as many pharmaceuticals leverage the unique properties of heterocycles to achieve specific biological effects [1,2,3,4,5,46,47] Green chemistry, also known as sustainable chemistry, is an innovative approach that focuses on designing and implementing processes, products, to minimize environmental impact and promote sustainability. It aims to prevent pollution at the source, reduce the use of hazardous substances, and optimize resource efficiency throughout the entire life cycle of a chemical product. By prioritizing the principles of green chemistry, such as using renewable feedstocks, minimizing waste, and promoting safer chemical synthesis, this discipline seeks safer chemical synthesis, this discipline seeks to foster a more environmentally friendly and economically viable chemical industry.[46,47] Green chemistry focuses on designing products and processes that minimize the use and generation of hazardous substances. It aims to promote sustainability, reduce environmental impact, and enhance efficiency in the production of chemicals. Key principles include using renewable resources, preventing waste, and prioritizing safer and more environmentally friendly alternatives in the development of chemical products and technologies.[47] Microwave-assisted synthesis of heterocyclic compounds involves using microwaves to accelerate chemical reactions, providing a more efficient and rapid method compared to conventional heating. The concept leverages the selective heating of reaction components, enhancing reaction rates and yielding higher product purity. This approach often reduces reaction times, increases yields, and allows for milder reaction conditions, making it a valuable technique in organic synthesis.[1,2,3,46] Microwave assisted chemistry revolutionizes synthetic chemistry by applying microwave radiation to reactions, enabling quicker and more efficient synthesis compared to conventional heating methods. This approach allows chemists to save time, test new theories, and develop processes rapidly. Solvent–related waste issues are addressed by performing reactions without solvents under microwave irradiation. The coupling of microwave irradiation with mineral-supported catalysis in solvent-free conditions enhances reaction rates, yields, selectivity, and ease of manuipulation, making microwave synthesis a potenial tool for green chemistry. Microwave irradiation serves as an alternative heating method, utilizing the transformation of electromagnetic energy into heat by mobile electric charges in liquids or conducting ions in solids. Microwaves with wavelengths of 1mm to 1m and frequencies between 0.3 and 300 GHz, offer a unique range in the electromagnetic spectrum, distinct from infrared radiation and radio waves. This technology, known as microwave dielectric heating, opens up new possibilities for synthetic chemists, enabling reactions not achievable through conventional heating methods.[1,2,3,45]
Microwave assisted synthesis of heterocyclic compounds
Microwave chemistry has transformed organic synthesis, especially in creating heterocyclic compounds. Conventional methods for sulfur and nitrogen-containing molecules often involve complex steps and materials. Microwave-assisted synthesis offers attractive alternatives with its fast, high-yield protocols,making purfication more manageable. The literature survey you mentioned focuses on leveraging microwave technology for efficient synthesis of heterocyclic nuclei, showcasing its benefits over traditional approaches.[47,46]
Pyrrole
Pyrrole is a key organic compound, featuring a five-membered ring with the formula C4H4NH. It holds significance in medicinal chemistry due to various activities. Researchers have devised numerous methods for synthesizing diverse substitutions in pyrroles.
Paal-knorr synthesis
The paal- knorr synthesis is a method employed for the construction of pyrroles, which are five – membered heterocyclic rings containing one nitrogen atom. The process begins with a 1,4- diketone, a molecule featuring two ketone groups separated by three carbon atoms. This diketone reacts with ammonia or a primary amine, leading to the formation of an imine intermediate through condensation. The key step involves intramolecular cyclization, where the imine intermediate through condensation. The key step involves intermolecular cyclization, where the imine undergoes rearrangement and attacks one of the carbonyl carbon atoms, resulting in the creation of a five members ring. This rearrangement establishes a new carbon- nitrogen bond within the ring, yielding the final product an assorted pyrrole derivative. The paal knorr synthesis is widely favored for its straightforward approach in synthesizing pyrroles with good efficiency [18,19]
Scheme 1. Synthesis Of pyrrole by paal-knorr methods

Jayagobi et al.[3] achieved the synthesis of pyrano [4,5-C] pyrroles through a one pot intermolecular knoevenagel-Hetero Diels-Alder reaction. Using alkenyl aldehyde and barbituric acid, they obtained excellent yields (80%) within 2 minutes under microwave heating in toluene. In contrast, the traditional method with refluxing toluene and ethylene diaminediacetate (EDDA) took 6 hours and provided a lower yield of 67%.
Scheme 2. Synthesis of ring fused pyrrole under MW irradiation
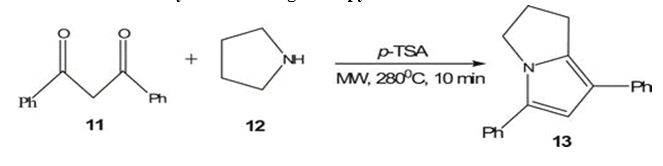
Deb et al. [4] achieved the one step synthesis of ring- fused pyrrole 143 by reacting 1,3- diketone 11 and cyclic aniline 12 under microwave irradiation at 280 C. The reaction, catalyzed by 0.5 equivalent of p- toluenesulfonic acid (p-TSA), resulting in a 53% yield within 10 minutes. Add the ligand free 5-endo- dig cyclization of homopropargyl azide 14, facilitated by 20 mol% ZnCl2 (1.0M in ether) in CH2Cl2 at 105 C, led to the formation of pyrroles 15 (eight examples) with yields ranging from 91 % to 41 % in 40-60 minutes. Comparatively, conventional heating at 160 °C for 16 hours also produced the same product.
Scheme 3. Synthesis of pyrrole by 5- endo- dig cyclization of homopropargyl azide
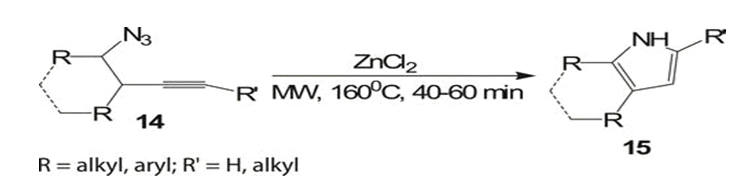
Portela-cubillo et al. [5] explored iminyl radical generation and cyclization using functionalized O-phenyl oxime ethers 17, promoted by microwaves (MWs) to produce dihydropyrrole 21 (for examples) with yields ranging from 68% to 82%. The reaction occurred at 160 °C for 15 minutes, utilizing one equivalent of the ionic liquid 1-ethyl-3-methyl-1H-imidazol-3-ium hexafluorophosphate (emimPF6). Notably, conventional thermolysis of O-phenyl oxime ethers was challenging due to long reaction times, unclear product formation, and disappointingly low yields.
Scheme 4. Synthesis of dihydropyrrole using O-phenyl oxime ethers.
Imidazole:
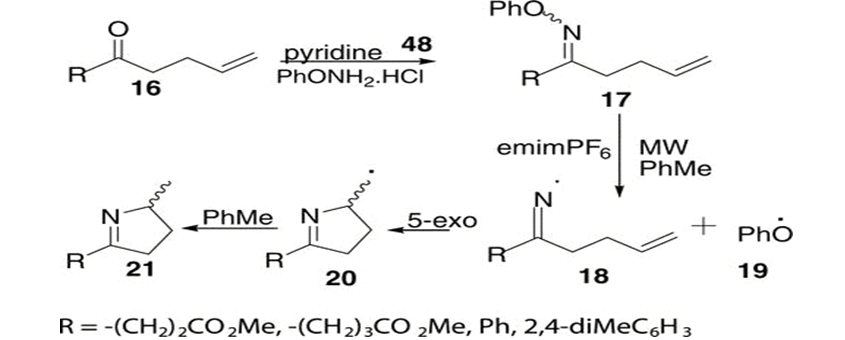
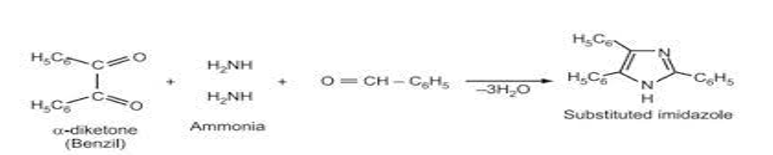
imidazole, a five membered ring compound with nitrogen atoms at positions 1&3, is pivotal molecule in various fields. its aromatic structure, featuring conjugated double bonds, underlies its biological importance as a constituent of histidine, an essential amino acid in proteins. Beyond its role in amino acids, imidazole serves as a biological buffer, stabilizing pH in biochemical studies. Chemically, imidazole’s basic properties enable its participation in diverse reactions, including coordination chemistry with metal ions. Widely utilized in organic synthesis, imidazole plays a crucial role in crafting pharmaceuticals and other organic compounds. Its electron-rich nature allows for versatility in reactions, contributing to its applications in both medicinal and synthetic realms. Imidazole’s multifaceted properties make it a cornerstone in biochemistry, organic synthesis, and coordination chemistry.[38-40] Heinrich debus, a german chemist, first reported imidazole in 1858. The compound was formed through the condensation of glyoxal, formaldehyde, and ammonia, leading to imidazole or glyoxaline, as it was initially named. Despite its low yields, this synthesis method is still employed for producing C-substituted imidazoles. Benzile react with benzaldehyde and two molecules of ammonia reacts to yield 2,4,5-triphenylimidazole.
Scheme 5. synthesis of 2,4,5-triphenylimidazole under MW irradiation

Crouch et al. [45] Described a microwave mediated method for preparing lophine (2,4,5-triphenylimidazole) by irradiating a mixture of benzaldehyde, benzil, glacial acetic acid, and ammonia. This method achieved a 90% yield of compound in shorter reaction time compared to traditional methods, which involve gram quantities of reagents, large solvent amounts, and reaction times exceeding 1 hour.
Scheme 6: reaction under MW irradiation to produce lophine .
In their study, Xia et al.[48] Demonstrated a microwave- assisted three-component synthesis for producing various 2,4,5-trisubstituted imidazole's with good to excellent yields (74-93%). The reactions took place in an ionic liquid ([HeMIM]BF4) without the need for a solvent or additional acid. Notably, the use of microwave irradiation significantly reduced the reaction time from several hours under conventional heating to just a few minutes.
Scheme 7: synthesis of 2,4,5-trisubstituted imidazole's under solvent-free condition.
https://www.ijpsjournal.com/uploads/createUrl/createUrl-20240727013820-0.png
R=Ph,ClPh, 4-BrPh,4-FPh,4-FPh,4-CF3Ph,3-O2NPh etc.
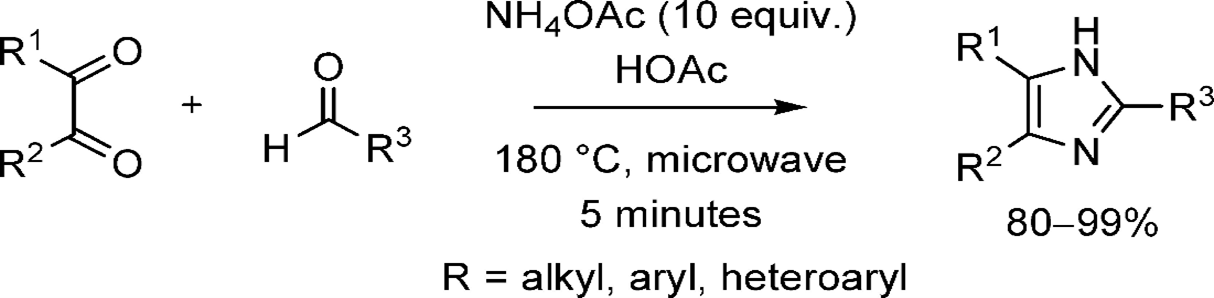
Wolkenberg et al.[49]developed a more efficient synthesis method for 2,4,5-trisubstituted imidazole's using 1,2-diketones and aldehydes. Their approach achieved high yields (80-90%) under mild conditions, specifically with NH40Ac and microwave (MW) irradiation at 180 C for 5 minutes. This contrasts with classical methods, which often required harsher conditions (150-200 C, 4-6 hours) and results in lower yields (40-90%) and mixtures of products. The compounds described are valuable for synthesizing the imidazolium alkaloid lepidine B6 and the platelet aggregation inhibitor trifenagrel.
Scheme 8: synthesis of 2,4,5-trisubsituted imidazole from 1,2-diketone by using MW-assisted reaction.
Sparks RB et al. [50] discovered a rapid microwave assisted method for synthesizing 2,4,5-triarylimidazoles from keto-oximes and aldehydes, achieving moderate to good yields in a one-pot, two-step process. The key N-O reductive bond cleavage step occurs under microwave irradiation at 200 C for 20 minutes, a significant time reduction compared to traditional methods requiring 2 days.
Scheme 9 : synthesis of 2,4,5-triaryl imidazole by using MW -assisted

Shih et al.[51] Synthesized 4,5-diaryl-2-sydnonyl-1-substituted imidazole's using a one-pot condensation method. They employed 3-(4-ethoxyphenyl)-4-formylsydnone benzil derivatives and ammonium acetate under microwave (MW) irradiation, resulting in higher yields (52-85% in 30-90 min) compared to conventional heating at 90-110 C (46-77% yields in 1-3 days). The addition of primary amines led to the formation of (eight examples) in 2-3 hours with 45-60% yields, showcasing the efficieny of MW heating over conventional methods.
Scheme 10 : Synthesis of 4,5-diaryl-2-sydnonyl-1-substituted imidazole by using MW-assisted

Hoz et al.[52] Synthesized 2-imidazolines through microwave-assisted cyclization of nitriles with ethylenediamine . This reaction, conducted in toluene and magtrieve TM (oxidation), resultedin imidazoles (five examples) in 75-105 minutes, whereas conventional heating with MnO2 required longer reaction times (24-48 hours) for comparable reults (76-93%)
Scheme 11: synthesis of 2-imidazole by using MW

Succinic acid and cyclohexane-1,2-diamine, in equimolar ratio, were mixed and exposed to microwave irradiation at 850 W for 4 minutes, resulting in the quantityative yield of octahydro-1H-pyrrolo-[1,2-a]benzimidazol-1-one. This efficient one-step process for synthesizing tricyclic heterocyclic molecules, as reported by sondhi et al.[53] examplifies a rapid and straightfoward approach.
Scheme 12: synthesis of tricyclics heterocyclic molecules by using MW-assisted

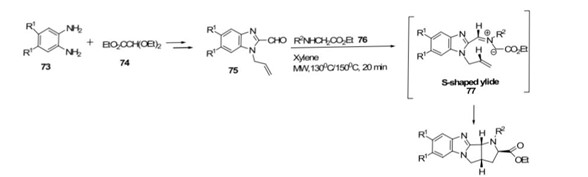
Meng et al.[54] Synthesized parent pyrrolidino[2,3: 3,4] pyrrolidino[1,2-a]benzimidazole-2-carboxylates in examples. They employed a microwave-assisted condensation of carbaldehyde with secondary amino ester followed by a 1,3-dipolar cycloaddition of the S-shaped yilde the reaction, conducted in xylene at 130 or 150 C for 20 minutes, yielded polycyclic pyrrolidine compounds in 52-93%yield. Notably, this approach exhibited higher efficieny compared to azomethine yield cycloadditions under classical reaction conditions, which typically required longer times and resulted in lower yields.
Scheme 13: synthesis of polycyclic pyrrolidine by using MW-assisted
Pyrazoles
Pyrazoles are heterocyclic compounds with a five-membered ring containing three carbon atoms and two adjacent nitrogen atoms. In the realm of research, they areoften investigated for their diverse pharmacological activities, including anti-inflammatory , anti-cancer and anti-microbial properties. Researchers explore synthetic methodologies and structural modification to enhance the biological activities of pyrazole derivatives, contributing to the development of potential drug candidates. Exploring the pharmacological and biological activities of pyrazole and its derivatives, especially pyrazolo[3,4-b]pyridines, can contribute valuable insights into potential treatments for stress related illnesses. Investigating synthesis methods such as pechmann and knorr pyrazole synthesis is crucial for advancing knowledge in medical chemistry and drug development.[56] In their study polshettiwar et al. Utilized microwave assistance for the synthesis of pyrazole derivatives . They employed a nano-organocatalyst in water at 140 C achieving high yields (84-96%) within a short reaction time to 20 minutes.[52]
Scheme 14: synthesis of pyrazole derivative using nano-organocataly by using MW -assisted

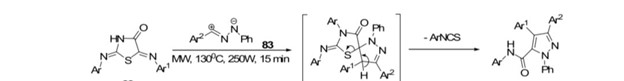
Hatem et al.[57] Successfully synthesized 1,3,4-triaryl-5-N-arylpyrazole-carboxamides through a 1,3-dipolar cycloaddition process. They utilized nitrilimines and 5-arylidene-2-arylimino-4-thiazolidinones employing solvent-free and microwave –assisted conditions at 130C for a duration of 15 minutes.
Scheme 15: synthesis of 1,3,4-triaryl-5-N-arylpyrazole carboxamide by using MW.
R1=H,Me,Cl,-(CH)4 R2=Me,Bn,Ph
Paul and colleagues (58) conducted a reaction involving 4-methylacetophenone and ethyl trifluoroacetate, employing microwave (MW) heating at 160 °C for 10 minutes, resulting in high yield (95%) of enol ketone 88. Non-MW conditions, taking 5 days, yielded 88%.
Scheme 16: synthesis of 1,5-diarylpyrazoles in the presence of silica by using MW irradiation
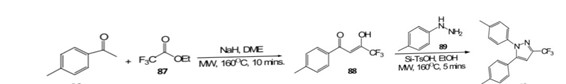
R=Me,Et R1=Me,Et,Pr,i-Pr R2= H
Subsequently, reacted with 4-methylphenylhydrazine, yielding various 1,5-diarylpyrazoles 90 (nine examples) under MW irradiation at 160 °C in 5 minutes, achieving a 95% yield with the assistance of silica-supported toluenesulfonic acid (Si-TsOH) in ethanol. Thermal conditions (100 °C) yielded in 7 hours with an 84% yield. Sauzem and co-authors (59) synthesized 5-trifluoromethyl-4,5-dihydro-1H-pyrazoles 93 (10 examples) through a one-pot cyclocondensation reaction of 4-alkoxy-1,1,1-trifluoromethyl-3-alken-2-ones 91. They utilized microwave-assisted synthesis at 70 °C, completing the reaction in 4 minutes with high yields ranging from 82% to 96%. Notably, some of these compounds exhibited efficacy in addressing neurogenic pain. In comparison, the conventional method resulted in moderate yields and a lengthier process.
Scheme 17: synthesis of 5-trifluoromethyl-4,5-dihydro-1H-pyrazoles by using MW
R=Me,Et R1=Me,Et,Pr,i-Pr R2= H
In their study, Radi et al. [56] employed a multi-component microwave-assisted organocatalytic domino Knoevenagel-hetero Diels-Alder reaction (DKHDA) for synthesizing 2,3-dihydropyran[2,3-c]pyrazoles 98. The reaction involved pyrazolone 94, aldehyde 96, and ethylvinyl ether 95, irradiated at 110 °C for 30 minutes with diaryl-prolinol catalyst 97 and t-BuOH as the solvent. This yielded compounds 98a and 98b in 56% and 12% yield, respectively. The efficiency was notably improved compared to conventional heating at 80 °C for 48 hours in similar compound synthesis.
Scheme 18: synthesis of 2,3-dihydropyran[2,3-c]pyrazoles by using MW -assisted
Ar=Ph,NaPh,3-OMePh
Ju et al.[60] demonstrated a novel approach involving double alkylation of unprotected hydrazines with alkyl dihalides 100 through cyclocondensation under microwave (MW) irradiation. The reactions occurred in aqueous media with a mild base, utilizing MW power between 70–100 W at 120 ?C for 20 minutes. This method yielded a set of pyrazoles with high yields ranging from 60–80%. Notably, the SN2-like sequential heterocyclization protocol was successfully realized under these unconventional reaction conditions.
Scheme 19: synthesis of double alkylation of hydrazine by alkyl dihalides by using MW-assisted
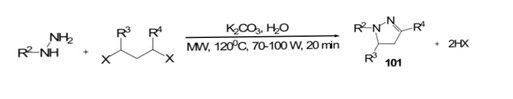
R2=H, alkyl, aryl, R?3;, RH, alkyl, X = Cl, Br, L,TsO
Pyrazolines
Pyrazolines are a class of organic compounds containing a pyrazole ring, which is a five-membered ring with two adjacent nitrogen atoms and three carbon atoms. They exhibit various biological activities and are often used in medicinal chemistry for drug development. Pyrazolines can be synthesized through different methods, such as the reaction of 1,3-diketones with hydrazine derivatives. These compounds have diverse pharmacological properties, including anti-inflammatory, anti-cancer, and anti-microbial activities. Researchers continue to explore their potential in drug discovery due to their structural versatility and biological effects.
In their study, Manna et al. (61) employed a microwave-assisted route to synthesize novel derivatives labeled as 2-[1-(5,8-dihydroquinoxalino[2,3-b]indoloacetyl)-3-(1-benzofuran-2-yl)-4,5-dihydro-1H-pyrazol-5-yl] phenyl (104 examples). This method yielded excellent results, achieving yields ranging from 84% to 98% within a short timeframe of 20 to 30 minutes. In comparison, the conventional reflux synthetic route yielded lower results, ranging from 28% to 72% yield, but required a longer reaction time of 8 to 9.5 hours.
Scheme 20: synthesis of 2-[1-(5,8-dihydro quinoxalino[2,3-b]indolacetyl-3-(1-benzofuran-2-yl)-4,5-dihydro-1H-pyrazol-5-yl]phenyl derivative by using MW
![synthesis of 2-[1-(5,8-dihydro quinoxalino[2,3-b]indolacetyl-3-(1-benzofuran-2-yl)-4,5-dihydro-1H-pyrazol-5-yl]phenyl derivative by using MW - Copy.jpg](https://www.ijpsjournal.com/uploads/createUrl/createUrl-20240726183839-0.jpg)
R= Oh, OMe, N(Me), COOH, NO?, Cl, furan ring, -CH=CH-Ar
Martins et al. (62) detailed the synthesis of new 4,5-dihydro-1H-pyrazole derivatives. This was achieved through a cyclocondensation reaction involving enones 105 and hydrazine methyl carboxylate 106 under solvent-free conditions at 50–55 °C, completing in a swift 6 minutes with yields ranging from 70% to 98% . In contrast, the traditional heating method resulted in only moderate yields (70–79%) but required an extended time frame of 24 hours.
Scheme 21: synthesis of 4,5-dihydro-1H-pyrazole under solvent-free conditions by using MW
R-Me, Et; R = CO?Me; R = H, Me, R?3; = H, Me, Pr, i-Pr, Bu, i-Bu, t-Bu, Ph, 4-O?N-Ph
Mutairi et al. [63] employed microwave irradiation (300 W) for 1–15 minutes, under solvent-free conditions, to irradiate a mixture of hydrazine derivatives and b-keto esters. This process yielded pyrazolones in moderate to good yields (40–91%). Additionally, these pyrazolones were also obtained under conventional conditions by stirring in ethanol at room temperature for 1–5 hours.
Scheme 22: synthesis of pyrazolones Under Microwave irradiation
R= H, Ph, 2-pyridyl, R?1;= Me, Et, Ph, CF3
Deshmukh et al. (64) demonstrated a microwave-assisted method for synthesizing 5-aminopyrazolone 116 with 88% yields under solvent-free conditions at 130°C in just 2 minutes. This outperformed the conventional thermal heating method, which took 4 hours and yielded the product at 80%.
Scheme 23: synthesis of 5-aminopyrazolones under solvent- free condition by using MW R = Benzo[d]thiazol-2-yl
![synthesis of 5-aminopyrazolones under solvent- free condition by using MW R = Benzo[d]thiazol-2-yl.jpg](https://www.ijpsjournal.com/uploads/createUrl/createUrl-20240727012912-0.jpg)
Pyrimidinones
Pyrimidinones are a class of organic compounds containing a pyrimidine ring fused with a ketone group. Pyrimidine is a six-membered heterocyclic ring consisting of four carbon atoms and two nitrogen atoms. The ketone group adds a carbonyl functionality to the pyrimidine structure. These compounds have diverse applications in medicinal chemistry, as they are often found in pharmaceuticals and bioactive molecules. Their structural versatility makes them valuable for drug design and synthesis. Researchers explore pyrimidinones for their potential in developing agents with various biological activities, including antiviral, anticancer, and anti-inflammatory properties. An efficient and simple microwave-assisted synthesis of 3,4-dihydropyrimidinones (example 230) with 14 instances yielded excellent results in the presence of water, without the need for additional solvent or acid catalyst. The reaction took only 2 minutes, achieving yields between 88% and 98%. This method, reported by Singhal et al.[65], outperformed conventional heating, where completion of the reaction took significantly longer, ranging from 45 to 75 minutes.
Scheme 24: synthesis of pyrimidinones under solvent-free by using MW irradiation
R’ Me, OMe, OEt
Ar = Ph, 4-Me-Ph, 4-OMe-Ph, 4-NO?-Ph, 4-CI-Ph, 2-CI-Ph, 2-pyridyl, 2-Furyl, Ph-CH=CH
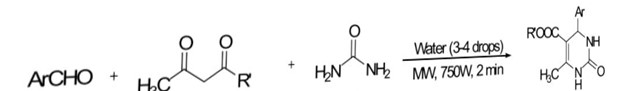
Shingare et al. [66] efficiently synthesized diverse pyrimido[4,5-d]pyrimidine-2,4,7-triones through a multicomponent reaction involving aldehyde, urea 30/thiourea 209, barbituric acid, and alumina (Al2O3) under microwave irradiation (600 W) in just 25–40 seconds. This approach outperformed traditional methods in terms of both time and yield.
Scheme 25: synthesis of pyrimido[4,5-d] pyrimidinones by using MW irradiation
X= O,S R = aryl groups
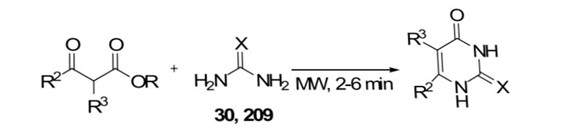
Mojtahedi et al.[66] described synthesizing pyrimidinones and thiopyrimidinones using solvent-free and microwave-assisted conditions, achieving good yields (53–81%) in a short time (2–6 min). In comparison, the conventional refluxing ethanol method, which includes metallic sodium, required 6–7 hours with yields ranging from 4% to 78%.
Scheme 26: synthesis of pyrimidinones by using MW -assisted
X = O (30, 232); X = S (209, 233)
R = Me, Et; R?2; = Et, H; R = Me, Ph, CH,Cl
Khunt et al. [67] developed a solvent-free microwave-assisted process to prepare tetrahydropyrimidinones 236 and tetrahydrothiopyrimidinones 237 using equimolar amounts of 1,3-dicarbonyl compounds 234, various aromatic aldehydes 235, and urea 30 (or thiourea 209) in 1.5–6.5 minutes with 78–85% yield (15 examples). In contrast, the conventional thermal heating method with THF as a solvent and InCl3 as a catalyst required hours to produce the products.
Scheme 27: synthesis of tetrahydropyrimidinone and tetrahydropyrimidinone by using MW heating.
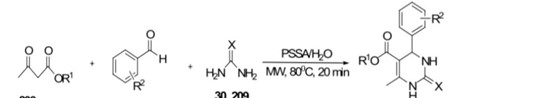
Dihydropyrimidinones were synthesized efficiently using the aqueous Biginelli protocol with polystyrenesulfonic acid (PSSA) as a catalyst under microwave irradiation at 80 ?C for 20 minutes, yielding 86–92%. Notably, this process eliminates the need for a phase-transfer catalyst, and the conventional oil bath heating method takes 5–6 hours for completion.
Scheme 28 : synthesis of biginelli in an aqueous medium
R1=H. Me, Et, Pr, Bu
CONCLUSION
The microwave-assisted synthesis of our heterocyclic compound proved to be a promising and efficient method. The use of microwave irradiation facilitated faster reaction rates and higher yields compared to traditional methods. This research contributes valuable insights to the field, highlighting the potential of microwave-assisted synthesis for the development of novel heterocyclic compounds with improved efficiency and reproducibility. Further studies can explore the broader applicability of this technique in diverse chemical syntheses.
REFERENCE
- Bacolini G. Topics Heterocycl. Syst. Synth. React.Prop. 1996;vol. 1 page no..:103.
- Alca´zar J, Oehlrich D. Recent applications of micro-Wave irradiation to medicinal chemistry. Future Med.Chem. 2010;vol. 2 page no.:169–176.
- Katritzky AR, Rees CW. Comprehensive heterocyclic Chemistry. New York: Pergamon Press; 1984 (Vols.1–8).
- Balaban AT, Oniciu DC, Katritzky AR. Aromaticity As a Cornerstone of Heterocyclic Chemistry. Chem.Rev. 2004;104 page no.:2777–2812.
- Brichacek M, Njardarson JT. Creative approaches Towards the synthesis of 2,5-dihydro- furans, thio-Phenes, and pyrroles. One method does not fit all.Org. Biomol. Chem. 2009;vol.7 page no.:1761–1770.
- Sheldon RA. Catalysis: the key to waste minimization. J. Chem. Technol. Biotechnol. 1997;68 page no.:381–388.
- Dabholkar VV, Ansari FY. Novel pyrimidine derivatives by sonication and traditional thermal meth-Ods. Green Chem. Lett. Rev. 2010;vol. 3 page no.:245–248.
- Ballini R, editor. Eco-friendly synthesis of fine Chemicals. UK: Royal Society of Chemistry; 2009.Page:275–292.
- Santagada V, Perissutti E, Caliendo G. The application of microwave irradiation as new convenient Synthetic procedure in drug discovery. Curr. Med.Chem. 2002;vol.9 page no.:1251–1283.
- Santagada V, Frecentesep F, Perissutti E, Fiorino F, Severino B, Caliendo G. The application of micro-Wave irradiation as new convenient synthetic Procedure in drug discovery. Mini Rev. Med. Chem.2009;vol. 9 page:340–358.
- Phukan M, Borah KJ, Borah R. Henry reaction in Environmentally benign methods using imidazole as Catalyst. Green Chem. Lett. Rev. 2009;vol. 2 page :249–253.
- Anastasa PT, Beacha ES. Green chemistry: the Emergence of a transformative framework. GreenChem. Lett. Rev. 2007;vol.1 page no.9–24.
- Villar H, Frings M, Bolm C. Ring closing enyne Metathesis: a powerful tool for the synthesis of Heterocycles. Chem. Soc. Rev. 2007;36 page no.:55–66.
- Polshettiwar V, Varma RS. Microwave-assisted Organic synthesis and transformations using Benign Reaction Media. Acc. Chem. Res. 2008;41 page:629–639.
- Tucker JL. Green chemistry, a pharmaceutical Perspective. Org. Process Res. Dev. 2006;Vol. 10 page no.:315–319.
- Pasunooti KK, Chai H, Jensen CN, Gorityala BK, Wang S, Liu X-W. A microwave-assisted, copper- Catalyzed three-component synthesis of dihydropyr Imidinones under mild conditions. Tetrahedron Lett. 2011;52 page:80–84.
- Kidwai M, Saxena S, Mohan R, Venkataramanan R. A novel one pot synthesis of nitrogen containin Heterocycles: an alternate methodology to the Bigi Nelli and Hantzsch reactions. J. Chem. Soc. Perki Trans. 2002;Vol.1 ,Page no.1845–1846.
- Knorr L. Synthese von pyrrolderivaten. Ber. Dtsch. Chem. Ges. 1884;17:1635–1642.
- Paal C. Synthese von thiophen-und pyrrolderivaten (synthesis of thiophene and pyrrole). Ber. Dtsch. Chem. Ges. 1885;18 Page no.:367–371.
- Hantzsch A. Neue Bildungsweise von Pyrrolderiva- Ten. Ber. Dtsch. Chem. Ges. 1890;23 page no.:1474–1476.
- Jayagobi M, Raghunathan R. An efficient synthesis Of pyrano [4,5-c] pyrrole derivatives through micro- Wave–accelerated intramolecular Knoevenagal hetero
- Diels–Alder reaction. Tetrahedron Lett. 2009;50: page no. 6886–6890.
- Deb I, Seidel D. Retro-Claisen condensation versus Pyrrole formation in reactions of amines and 1,3-Diketones. Tetrahedron Lett. 2010;51:2945–2947.
- Wyrbek P, Sniady A, Bewick N, Li Y, Mikus A, Wheeler KA, Dembinski R. Microwave-assisted zinc Chloride-catalyzed synthesis of substituted pyrroles From homopropargyl azides. Tetrahedron. 2009;65: page no. 1268–1275.
- Portela-Cubillo F, Scottb JS, Walton JC. Microwave-Assisted preparations of dihydropyrroles from alkenone O-phenyl oximes. Chem. Commun. 2007;4041–4043.
- Oskooie HA, Heravi MM, Behbahani FK. A facile, Mild and efficient one-pot synthesis of 2-substituted indole derivatives catalyzed by Pd(PPh3)2Cl2 Molecules. 2007;12:1438–1446.
- Karthikeyan SV, Perumal S, Shetty KA, YogeeswariP, Sriram D. A microwave-assisted facile regioselective Fischer indole synthesis and antitubercular
- Evaluation of novel 2-aryl-3,4-dihydro-2H-thieno [3,2-b]indoles. Bioorg. Med. Chem. Lett. 2009;19 page no.3006–3009.
- Lehmann F, Holm M, Laufer S. Rapid and Easy access to indoles via microwave-assisted
- Hemetsberger–Knittel synthesis. Tetrahedron Lett.m 2009;50 page no.:1708–1709.
- Borthakur M, Gogoi Y, Gogoi J, Boruah RC. Lewis Acid catalyzed rapid synthesis of 5-hydroxy-benzo[g] Indole scaffolds by a modified Nenitzescu reaction. Tetrahedron Lett. 2010;51:5160–5163.
- Bhella SS, Pannu APS, Elango M, Kapoor A, Hundal MS, Ishar MPS. Investigations on synthesis Of indole based constrained mimetic scaffold Through 1,3-dipolar cycloadditions of the C-(3-indo-Lyl)-N-phenylnitrone with a variety of olefinic and
- Allenic dipolarophiles under microwave irradiation. Tetrahedron. 2009;65 page no.:5928–5935.
- Carpita A, Ribecai A. Microwave-assisted synthesis Of indole-derivatives via cycloisomerization of 2-Alkynylanilines in water without added catalysts,
- Acids, or bases. Tetrahedron Lett. 2009;50 page no.:6877–6881.
- Carpita A, Ribecai A, Stabile P. Microwave-assisted
- Synthesis of indole- and azaindole -derivatives in Water via cycloisomerization of 2-alkynylanilines And alkynylpyridinamines promoted by amines or Catalytic amounts of neutral or basic salts. Tetrahedron. 2010;66 page no.:7169–7178.
- Chen Y, Markina NA, Larock RC. An efficient, Microwave-assisted, one-pot synthesis of indoles Under Sonogashira conditions. Tetrahedron. 2009; 65 page no :8908–8915.
- Waldmann H, Eberhardt L, Wittsteinab K, Kumar K. Silver catalyzed cascade synthesis of alkaloid ring Systems: concise total synthesis of fascaplysin, homo- Fascaplysin C and analogues. Chem. Commun. 2010;46 page no.:4622–4624.
- Zang H, Su Q, Mo Y, Cheng BW, Jun S. Ionic liquid [EMIM]OAc under ultrasonic irradiation towards the First synthesis of trisubstituted imidazoles. Ultrason. Sonochem. 2010;17 page no.:749–751.
- Debus H. Umber die einwirkung des ammoniaks auf Glyoxal. Liebigs Ann. Chem. 1858;107 page no.199–208.
- Wallach O, Schulze E. Ueber Basen der Oxalsa¨urer- Eihe. Ber. Dtsch. Chim. Ges. 1881;14:420.
- Lantos I, Zhang W-Y, Shui X, Eggleston DS. Synthesis of imidazoles via Hetero-Cope Rearrange-
Ments. J. Org. Chem. 1993;58 page no.:7092–7095.
- Tymoshenko DO. On the development of organic Chemistry in Ukraine. Arkivoc. 2005;viii: page no.1–3.
- Crouch RD, Howard JL, Zile JL, Barker KH. Microwave-mediated synthesis of lophine: developing A mechanism to explain a product. J. Chem. Edu. 2006;83 page no.:1658–1660.
- Arpi Majumder, Ragini Gupta & Anshu Jain Microwave-assisted synthesis of nitrogen-Containing heterocycles Green Chemistry Letters and Reviews vol. 6 page no.151–182,
- Xia M, Lu Y-D. A novel neutral ionic liquid- Catalyzed solvent-free synthesis of 2,4,5-trisubstituted Imidazoles under microwave irradiation. J. Mol. Catal. A: Chem. 2007;265: page no. 205–208.
- Wolkenberg SE, Wisnoski DD, Leister WH, Wang Y, Zhao Z, Lindsley CW. Efficient synthesis of imida-zoles from aldehydes and 1,2-diketones using micro-Wave irradiation. Org. Lett. 2004;6: page no. 1453–1456.
- Sparks RB, Combs PA. Microwave-assisted synthesis Of 2,4,5-triaryl-imidazole; A novel thermally induced N-hydroxyimidazole N–O bond cleavage. Org. Lett. 2004;6:2473–2475.
- Shih M-H, Tsai C-H, Wang Y-C, Shieh MY, Lin, G-L, Wei C-Y. Microwave-assisted synthesis of sydnonyl -substituted imidazoles. Tetrahedron. 2007;63: 2990–2999.
- Hoz A de la,D?´az-Ortiz A´ , Mateo Mdel C, Moral M, Moreno A, Elguero J, Foces-Foces C, Rodr?´guez ML, Sa´nchez-Migallo´n A. Microwave assisted synth-Esis and crystal structures of 2-imidazolines and Imidazoles. Tetrahedron. 2006;62 page no. :5868–5874.
- Sondhi SM, Rani R. Microwave-mediated one step Synthesis of tri- and tetracyclic heterocyclic molecules. Green. Chem. Lett. Rev. 2010;3:115–120.
- Meng L, Fettinger JC, Kurth MJ. Intramolecular Cycloaddition of azomethine ylides in the preparationOf pyrrolidino[2¢,3¢:3,4]pyrrolidino[1,2-a]benzimida- Zoles. Org. Lett. 2007;vol.9 page no.:5055–5058.
- Li J-T, Yin Y, Li Li, Sun M-X. A convenient and Efficient protocol for the synthesis of 5-aryl-1,3-Diphenylpyrazole catalyzed by hydrochloric acid Under ultrasound irradiation. Ultrason. Sonochem. 2010;17 page no.11–13.
- Sahu SK, Banerjee M, Samantray A, Behera C, AzamMA. Synthesis, analgesic, anti-inflammatory and Antimicrobial activities of some novel pyrazoline Derivatives. Trop. J. Pharm. Res. 2008; vol. 7 page no.961–968
- Polshettiwar V, Varma RS. Nano-organocatalyst: Magnetically retrievable ferrite-anchored glutathione For microwave-assisted Paal–Knorr reaction, aza- Michael addition, and pyrazole synthesis. Tetrahedron. 2010;66 page no.:1091–1097.
- Hatem AA-A, Heba SAE-Z, Kamal MD. Micro-Wave-assisted synthesis and in-vitro anti-tumor activiIty of 1,3,4-triaryl-5-N-arylpyrazole-carboxamides. Eur. J. Med. Chem. 2010;45 page no.2427–2432.
- Paul SH, Jennifer MF. Microwave-assisted synthesis Utilizing supported reagents: a rapid and versatile Synthesis of 1,5-diarylpyrazoles. Tetrahedron Lett. 2006;47:2443–2446.
- Sauzem PD, Machado P, Rubin MA, Sant’Anna G Da S, Faber HB, De Souza AH, Mello CF, Beck P, Burrow RA, Bonacorso HG, Zanatta N, Martins MAP. Design and microwave-assisted synthesis of 5-Trifluoromethyl-4,5-dihydro-1H-pyrazoles: Novel Agents with analgesic and anti-inflammatory properties’. Eur. J. Med. Chem. 2008;vol 43 page no.:1237–1247.
- Radi M, Bernardo V, Bechi B, Castagnolo D, Pagano M, Botta M. Microwave-assisted organocatalytic Multicomponent Knoevenagel/hetero Diels–Alder Reaction for the synthesis of 2,3-dihydropyran[2,3-c]Pyrazoles. Tetrahedron Lett. 2009;vol. 50 page no.:6572–6575.
- Ju Y, Varma RS. Aqueous N-heterocyclization Primary amines and hydrazines with dihalides: Microwave-assisted syntheses of N-Azacycloalkanes, Isoindole, pyrazole, pyrazolidine, and phthalazine Derivatives. J. Org. Chem. 2006;71: page no.135.
- Candeias NR, Branco LC, Gois PMP, Afonso CAM, Trindade AF. More sustainable approaches for the Synthesis of N-based heterocycles. Chem. Rev. 2009;109 page no.:2703–2802.
- Manna K, Agrawal YK. Microwave assisted synthesis of new indophenazine 1,3,5-trisubstruted pyrazoline derivatives of benzofuran and their antimicrobial Activity. Bioorg. Med. Chem. Lett. 2009;vol. 19 page no.:2688– 2692.
- Martins MAP, Beck P, Machado P, Brondani S, Moura S, Zanatta N, Bonacorso HG, Flores AFC.Microwave-assisted synthesis of novel 5-trichloro-Methyl-4,5-dihydro-1H-1-pyrazole methyl esters under solvent free conditions. J. Braz. Chem. Soc.2006;vol. 17 page no. 408–411.
- Patel VM, Desai KR. Eco-friendly synthesis of Pyrazoline derivatives over potassium carbonate Arkivoc. 2004;vol. 1 page no.123–129.
- Al-Mutairi AA, El-Baih FEM, Al-Hazimi HM. Microwave versus ultrasound assisted synthesis of Some new heterocycles based on pyrazolone moiety. J. Saudi Chem. Soc. 2010; vol. 14 page no. :287–299.
- Pal S, Mareddy J, Devi NS. High speed synthesis of Pyrazolones using microwave-assisted neat reaction Technology. J. Braz. Chem. Soc. 2008;19:1207–1214.
- Deshmukh MB, Jagtap SS, Desmukh SA. Solvent Free accelerated synthesis of 2-hydrazinobenzothia-zoles derivatives using microwave. J. Indian Chem. Soc. 2006;83 page no.1055–1057.


 Teena Satish Dubey *
Teena Satish Dubey *
 Prasad Vijay Kate
Prasad Vijay Kate
 Runita Sharad karale
Runita Sharad karale
 Vilas sawale
Vilas sawale
















![synthesis of 2,3-dihydropyran[2,3-c]pyrazoles by using MW -assisted - Copy.jpg](https://www.ijpsjournal.com/uploads/createUrl/createUrl-20240726190237-6.jpg)

![synthesis of 2-[1-(5,8-dihydro quinoxalino[2,3-b]indolacetyl-3-(1-benzofuran-2-yl)-4,5-dihydro-1H-pyrazol-5-yl]phenyl derivative by using MW - Copy.jpg](https://www.ijpsjournal.com/uploads/createUrl/createUrl-20240726183839-0.jpg)


![synthesis of 5-aminopyrazolones under solvent- free condition by using MW R = Benzo[d]thiazol-2-yl.jpg](https://www.ijpsjournal.com/uploads/createUrl/createUrl-20240727012912-0.jpg)
![synthesis of pyrimido[4,5-d] pyrimidinones by using MW irradiation.jpg](https://www.ijpsjournal.com/uploads/createUrl/createUrl-20240726183839-14.jpg)

 10.5281/zenodo.12937857
10.5281/zenodo.12937857