Abstract
The COVID-19 pandemic prompted an urgent global response to develop vaccines, leading to the rapid deployment of several candidates, including Oxford-AstraZeneca and Johnson & Johnson's vaccines. However, reports of vaccine-induced immune thrombotic thrombocytopenia (VITT) and cerebral venous sinus thrombosis (CVST) raised concerns, resulting in temporary halts in vaccine administration. India, with its vast population, initiated an extensive vaccination campaign, predominantly using the Covishield vaccine. This article synthesizes existing guidelines and discusses the challenges associated with VITT/CVST, emphasizing the importance of heightened pharmacovigilance and physician awareness. It also highlights the emerging neurological symptoms post-Covishield vaccination for TTS mitigation. As India expands its vaccination drive, monitoring, and addressing adverse effects are crucial.
Keywords
COVID-19, vaccination campaign, Covishield, VITT, CVST, neurological symptoms, pharmacovigilance, India.
Introduction
The COVID-19 crisis unfolded rapidly, straining healthcare systems worldwide and exacting a heavy toll on lives and economies [1, 2]. In response, extensive efforts were made to develop effective vaccines swiftly. Collaborative initiatives yielded cutting-edge vaccines with notable efficacy and safety records within remarkably short timeframes [3, 4, 5]. However, the rollout of vaccines such as Oxford-AstraZeneca and Johnson & Johnson faced setbacks when reports of vaccine-induced immune thrombotic thrombocytopenia (VITT) and cerebral venous sinus thrombosis (CVST) surfaced, leading to temporary halts [6, 7, 8].Though thorough risk-benefit analyses justified their resumption, medical communities globally continue grappling with understanding the pathophysiology and mechanisms behind these neurological adverse effects to enhance identification, diagnosis, and treatment [9, 10, 11]. One implicated mechanism revolves around the adenovirus-based vector utilized in these vaccines, a vector shared by Covishield, India's predominant vaccine [12, 13]. As India progresses to vaccinate younger adults, there's a possibility of similar adverse effects emerging [14, 15, 16].This review delves into the temporary suspension of vaccine administration due to VITT/CVST, synthesizes existing guidelines regarding diagnosis and treatment of these neurological disorders, and emphasizes the imperative of heightened pharmacovigilance and physician awareness [17, 18, 19, 20,]. Implementing screening for potential risk factors, advocating hydration, and offering vaccination choices for high-risk populations could mitigate these rare yet potentially grave outcomes [21, 22, 23].Since the emergence of the first cluster of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) in Wuhan, China, in December 2019, the COVID-19 pandemic has rapidly spread, placing immense strain on global healthcare systems [24, 25, 26, 27]. As of August 13, 2021, the global tally surpassed 205,338,175 confirmed cases and recorded 4,333,103 deaths. To put this into perspective, the death toll from COVID-19 has already surpassed that of influenza over the past century, marking an unprecedented public health challenge [28]. The devastating toll on lives and economies prompted an urgent push for vaccine development and distribution [29, 30]. Initially, more than 200 vaccine candidates were explored, with 18 advancing to human clinical trials to assess their efficacy and safety [31, 32]. Recognizing the need for scaled-up production, several multinational pharmaceutical companies partnered with Indian firms [33, 34]. AstraZeneca collaborated with the Serum Institute of India, while Johnson & Johnson joined forces with Biological E India [35, 36, 37].
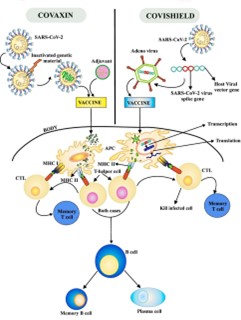
Fig.1. General Mechanism of Covaxin and Covishield
In India, three vaccines have received approval for public use: COVISHIELD, COVAXIN, and SPUTNIK V. COVISHIELD, developed by Oxford-AstraZeneca, utilizes a recombinant, replication-deficient chimpanzee adenovirus vector encoding the SARS-CoV-2 Spike (S) glycoprotein, with local manufacturing facilitated by the Serum Institute of India[38, 39, 40, 41]. COVAXIN, an inactivated whole-virion vaccine, is India’s homegrown product developed by Bharat Biotech, in collaboration with the Indian Council of Medical Research (ICMR) and the National Institute of Virology (NIV) [42, 43]. SPUTNIK V, another adenoviral-vector-based vaccine, was introduced in major Indian cities in May 2021 through collaboration between the Russian Direct Investment Fund (RDIF) and Dr. Reddy’s Laboratories [44, 45]. Numerous other candidate vaccines are undergoing trials at various stages across India, reflecting ongoing efforts to combat the pandemic through vaccine research and deployment.While mass vaccination campaigns are expected to mitigate COVID-19 outbreaks by reducing infection rates, hospitalizations, and fatalities, it's crucial to monitor for adverse events post-vaccination to assess the risk-benefit balance of each authorized vaccine [46, 47]. Of particular concern are rare but serious adverse events such as vaccine-induced immune thrombotic thrombocytopenia (VITT) and cerebral venous sinus thrombosis (CVST) [48]. This review underscores the importance of these adverse events in the context of vaccination strategies in India and offers recommendations to address them effectively.
Overview of Vaccine-Induced Thrombosis-Thrombocytopenia Syndrome (VITT) in COVID-19 Vaccination:
Thrombosis-thrombocytopenia syndrome (TTS), characterized by the concurrent presentation of cerebral venous sinus thrombosis (CVST) and thrombocytopenia, has been termed vaccine-induced immune thrombotic thrombocytopenia (VITT) in the context of COVID-19 vaccination [49, 50]. The Brighton Collaboration draft defines TTS case identification by a platelet count <150>
Assessment of Thrombosis-Thrombocytopenia Syndrome (TTS) Risk in COVID-19 Vaccines
As of April 2021, there were 169 reported cases of CVST associated with TTS among approximately 34 million recipients of the AstraZeneca (AZ) vaccine in the European Union [69]. In contrast, US regulatory agencies documented 15 cases of TTS among about 7 million recipients of the Johnson & Johnson (JJ) vaccine [70, 71]. While the causal link between these vaccines and TTS was considered plausible, both the CDC and EMA emphasized that the overall benefits of these vaccines outweigh the risks [72, 73]. Consequently, on April 23, 2021, the Advisory Committee on Immunization Practices recommended the continued use of both vaccines for individuals aged 18 and older [74]. Reports of cerebral venous thrombosis associated with VITT are detailed in below. Currently, Norway and Denmark have suspended the use of the AZ vaccine, while Iceland and Germany have restricted its use to individuals older than 60 years [75, 76, 77]. The UK advises that adults under 30 years old without underlying health conditions should be offered an alternative non-adenoviral vector-based COVID-19 vaccine, if available [78]. Canada offers the AZ vaccine to all adults with additional warnings on the vaccine label [79, 80]. Similarly, the US allows the use of the JJ vaccine in all adults, accompanied by information on the risk of TTS in educational materials [81].
Comparative Analysis of CVST Cases Associated with AstraZeneca, Janssen, and COVISHIELD COVID-19 Vaccines
Recent reports have reveal on cases of cerebral venous sinus thrombosis (CVST) occurring after the administration of various COVID-19 vaccines [82, 83]. This article presents a comparative analysis of reported CVST cases associated with AstraZeneca, Janssen, and COVISHIELD vaccines. Regarding reported and confirmed CVST cases, AstraZeneca had 413 reported cases and 227 confirmed cases, Janssen had 13 reported cases and 13 confirmed cases, and COVISHIELD had 1 reported case with confirmation information unavailable [84, 85]. In terms of age distribution, recipients of AstraZeneca vaccines ranged from 21 to 77 years old, Janssen recipients were aged between 18 and 60 years, while COVISHIELD recipients had a median age of 36 years [86, 87]. Symptoms associated with CVST varied, with AstraZeneca recipients experiencing headache, visual disturbance, and leg/arm weakness, Janssen recipients presenting symptoms such as headache, lethargy, fever, pain, and limb weakness, while information on COVISHIELD symptoms was not available [88, 89, 90 ]. The onset of symptoms occurred within 5-24 days post-vaccination for AstraZeneca and 6-15 days (median 8 days) for Janssen recipients, but information for COVISHIELD was not available [91, 92]. The results of the Platelet Factor 4-Heparin Assay were positive for both AstraZeneca and Janssen, but information for COVISHIELD was not available [93]. These findings provide insights into the incidence and characteristics of CVST cases associated with different COVID-19 vaccines, emphasizing the importance of ongoing monitoring and research to ensure vaccine safety [94].
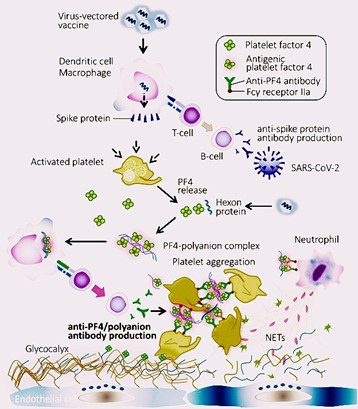
Fig. 2: Mechanism of Vaccine-Induced Thrombosis-Thrombocytopenia Syndrome (VITT)
Thrombosis-Thrombocytopenia Syndrome (TTS): Insights into CVST, Diagnosis, and Treatment
Cerebral venous sinus thrombosis (CVST) typically affects young adults, particularly women of childbearing age, with over 80% of patients having identifiable thrombosis risk factors such as coagulopathies or hormonal contraception. Prior to the COVID-19 pandemic, CVST associated with thrombocytopenia was rare [95, 96]. However, with COVID-19 vaccination, thrombosis-thrombocytopenia syndrome (TTS) encompasses more common thrombotic events like deep vein thrombosis and ischemic stroke, besides CVST. Studies indicate that the risk of CVST post-COVID-19 infection is significantly higher than after vaccination [97, 98, 99 ] . Symptoms of thrombosis-thrombocytopenia syndrome (TTS) following Covishield vaccination, manufactured by AstraZeneca in India, can present diversely, including severe or worsening headaches not responsive to typical pain relief measures, visual disturbances like blurred vision, breathing difficulties, persistent abdominal pain, leg swelling or pain, emergence of small red or purple skin spots (petechiae or bruises), neurological symptoms such as confusion or seizures, and gastrointestinal issues like nausea, vomiting, or ongoing abdominal discomfort [100, 101, 102, 103] . Immediate medical attention is crucial for any unusual or severe symptoms appearing within days to weeks post-Covishield vaccination

Fig. 3: Autoimmune Antibody Repertoires Responsible For the Excessive Activation of Coagulation and Platelets
especially for individuals with a history of blood clotting disorders or previous thrombotic events104, 105]. Diagnosis of CVST should be considered in young patients with unusual headaches or stroke-like symptoms, and imaging techniques like MRI or CT with venogram are crucial for accurate detection [106]. Vaccine-induced immune thrombotic thrombocytopenia (VITT) is more likely if symptoms arise within 4-28 days post-vaccination [107, 108]. Diagnostic tests include complete blood count, coagulation profiles, D-dimer, and platelet factor 4 (PF4) antibody assay [109, 110]. The pathogenesis of VITT involves high levels of antibodies to PF4-polyanion complexes, akin to autoimmune heparin-induced thrombocytopenia [111]. Treatment typically involves non-heparin anticoagulants and intravenous immunoglobulins (IVIG) to mitigate the prothrombotic response and improve platelet count [112, 113]. In severe cases, direct endovascular treatment or neurosurgical intervention may be necessary [114].
India's COVID-19 Vaccination Campaign: Global Impact, Challenges, and Neurological Symptoms Post-Covishield Vaccination
Bruce Y Lee from the CUNY Graduate School of Public Health and Health Policy in New York, USA, emphasized the significance of India's COVID-19 vaccination campaign in controlling the global spread of SARS-CoV-2 due to India's vast population size, constituting over one-seventh of the world's population [115, 116]. According to Brian Wahl from the Johns Hopkins Bloomberg School of Public Health in Baltimore, MA, USA, India's robust domestic vaccine industry facilitated one of the largest and fastest COVID-19 vaccination drives globally [117] . India initiated its vaccination program on January 16, 2021, and as of August 13, 2021, has administered over 539 million doses, with COVISHIELD being the predominant vaccine, accounting for 87% of the total doses [118, 119]. Despite the government's aim to inoculate all eligible citizens by the year-end, the campaign has faced challenges such as slow progress, vaccine shortages, and hesitancy, resulting in only 8.65% of the population being fully vaccinated, with 30.2% having received the first dose [120, 121, 122] . Although there has been only one suspected case of vaccine-induced immune thrombotic thrombocytopenia (VITT) reported in India out of 304.5 million COVISHIELD doses administered, data from the Adverse Events Following Immunization (AEFI) report dated March 17, 2021, lists three deaths possibly linked to vaccine administration [123, 124]. However, the accuracy of these numbers in comparison to estimates by the European Medicines Agency (EMA) is debatable, raising questions about misdiagnosis, reporting failures, or documentation errors [125]. India's AEFI surveillance program relies on passive reporting by healthcare professionals and the public, with serious unexplained AEFIs within 30 days post-vaccination requiring immediate investigation [126, 127]. Despite concerns about underreporting, the current AEFI reporting rate for COVID vaccines remains low at 0.008% [128] . Immediate recognition and management of serious AEFI cases, particularly those involving cerebral venous sinus thrombosis (CVST) or TTS, are vital [129]. With India expanding its vaccination drive to include younger populations, there may be an increase in reported cases of CVST and TTS, necessitating careful screening and surveillance efforts [130, 131]. Enhancing vaccine production, supply, and accessibility, along with public awareness campaigns, are essential steps to mitigate the impact of the pandemic and restore confidence in vaccination efforts, crucial for preventing future medical crises like those witnessed during India's second wave of COVID-19 [132, 133].Newly emerging neurological symptoms post-Covishield vaccination, aimed at mitigating thrombosis-thrombocytopenia syndrome (TTS), include severe or persistent headaches unresponsive to standard pain relief, visual disturbances such as blurred vision, impairment in speech comprehension or articulation, weakness or sensory deficits in facial, upper limb, or lower limb regions, particularly unilateral, occurrence of seizures or convulsions, altered mental status characterized by confusion or cognitive difficulties, impaired coordination and balance, abrupt and intense onset of nausea or vomiting with no discernible trigger, and appearance of petechiae or ecchymoses on the skin. Individuals experiencing these symptoms within days to weeks following Covishield vaccination should promptly seek medical attention for thorough evaluation and management, particularly if TTS or other serious adverse events are suspected.
CONCLUSION
In conclusion, the COVID-19 pandemic has necessitated the rapid development and deployment of vaccines globally. While vaccines like Oxford-AstraZeneca and Johnson & Johnson's have shown remarkable efficacy, reports of vaccine-induced immune thrombotic thrombocytopenia (VITT) and cerebral venous sinus thrombosis (CVST) have raised concerns. Despite temporary halts and thorough risk-benefit analyses, medical communities worldwide continue to grapple with understanding the mechanisms behind these neurological adverse effects. As India progresses with its vaccination campaign, there's a need for heightened pharmacovigilance and physician awareness, particularly regarding the COVISHIELD vaccine. Screening for risk factors, advocating hydration, and offering vaccination choices for high-risk populations are crucial mitigation strategies. The global impact of India's vaccination campaign underscores the importance of monitoring and addressing adverse events effectively. Enhancing vaccine production, supply, accessibility, and public awareness are essential for controlling the pandemic and preventing future medical crises. Recognition and management of serious adverse events, such as CVST and TTS, remain vital, especially with the emergence of new neurological symptoms post-Covishield vaccination. Immediate medical attention for individuals experiencing such symptoms is paramount to ensure timely evaluation and management. Through collaborative efforts and ongoing research, we can navigate these challenges and strengthen vaccination strategies to combat COVID-19 effectively.
REFERENCES:
- Tarricone R, Rognoni C. What can health systems learn from COVID-19?. European Heart Journal Supplements. 2020 Dec 1;22(Supplement_P):P4-8.
- Shrestha N, Shad MY, Ulvi O, Khan MH, Karamehic-Muratovic A, Nguyen US, Baghbanzadeh M, Wardrup R, Aghamohammadi N, Cervantes D, Nahiduzzaman KM. The impact of COVID-19 on globalization. One Health. 2020 Dec 20;11:100180.
- Bloom, D.E., Cadarette, D., Ferranna, M., Hyer, R.N. and Tortorice, D.L., 2021. How New Models Of Vaccine Development For COVID-19 Have Helped Address An Epic Public Health Crisis: Article describes and analyzes how resources, cooperation, and innovation have contributed to the accelerated development of COVID-19 vaccines. Health Affairs, 40(3), pp.410-418.
- Schoch-Spana M, Brunson EK, Long R, Ruth A, Ravi SJ, Trotochaud M, Borio L, Brewer J, Buccina J, Connell N, Hall LL. The public’s role in COVID-19 vaccination: Human-centered recommendations to enhance pandemic vaccine awareness, access, and acceptance in the United States. Vaccine. 2021 Sep 24;39(40):6004-12.
- Basso LJ, Goic M, Olivares M, Sauré D, Thraves C, Carranza A, Weintraub GY, Covarrubia J, Escobedo C, Jara N, Moreno A. Analytics Saves Lives During the COVID-19 Crisis in Chile. INFORMS Journal on Applied Analytics. 2023 Jan;53(1):9-31.
- Khan E, Bavishi S, Sharma AK, Sharma VK, Goyal V. COVID-19 vaccine-induced immune thrombotic thrombocytopenia (VITT) and cerebral venous sinus thrombosis (CVST)-lessons for India. Annals of Indian Academy of Neurology. 2022 Jan 1;25(1):15-20.
- Sarkar M, Madabhavi IV, Quy PN, Govindagoudar MB. COVID-19 vaccine-induced immune thrombotic thrombocytopenia: a review. Annals of Thoracic Medicine. 2022 Jan 1;17(1):1-3.
- Tafoya QJ, Watson V, Pawloski J, Mohamed GA, Ramadan AR. Treatment Approach, Pharmacological Agents and Vaccines. InNeurological Care and the COVID-19 Pandemic 2021 Jan 1 (pp. 145-162). Elsevier.
- Morbelli S, Ekmekcioglu O, Barthel H, Albert NL, Boellaard R, Cecchin D, Guedj E, Lammertsma AA, Law I, Penuelas I, Semah F. COVID-19 and the brain: impact on nuclear medicine in neurology. European journal of nuclear medicine and molecular imaging. 2020 Oct;47:2487-92.
- Velikova T, Georgiev T. SARS-CoV-2 vaccines and autoimmune diseases amidst the COVID-19 crisis. Rheumatology international. 2021 Mar;41(3):509-18.
- Fanciulli A, Leys F, KrbotSkori? M, Reis Carneiro D, Calandra?Buonaura G, Camaradou J, Chiaro G, Cortelli P, Falup?Pecurariu C, Granata R, Guaraldi P. Impact of the COVID?19 pandemic on clinical autonomic practice in Europe: a survey of the European Academy of Neurology and the European Federation of Autonomic Societies. European journal of neurology. 2023 Jun;30(6):1712-26.
- Baharani A, Reddy RR. Multiple evanescent white dot syndrome following adenovirus vector-based COVID-19 vaccine (Covishield). Ocular Immunology and Inflammation. 2023 Jul 3;31(6):1299-304.
- Pai M, Chan B, Stall NM, Grill A, Ivers N, Maltsev A, Miller KJ, Odutayo A, Razak F, Schull M, Schwartz B. Vaccine-induced immune thrombotic thrombocytopenia (VITT) following adenovirus vector COVID-19 vaccination. Science briefs of the Ontario COVID-19 science advisory table. 2021 May 7;2(17):1-7
- Park CY, Kim K, Helble M, Roth S. Getting ready for the COVID-19 vaccine rollout.
- Mohakuda SS, Nigam A, Rajesh K, Sashindran VK, Sharma H, Singh B, Kaur R, Thawal M. Covishield India: Demystifying myths through an early multicenter safety Study. Am J Manag Care. 2021 Oct 1;27(10):e339-42.
- Lakamana S, Yang YC, Al-Garadi MA, Sarker A. Tracking the COVID-19 outbreak in India through Twitter: Opportunities for social media based global pandemic surveillance. AMIA Summits on Translational Science Proceedings. 2022;2022:313.
- Konu YR, Gbeasor-Komlanvi FA, Yerima M, Sadio AJ, Tchankoni MK, Zida-Compaore WI, Nayo-Apetsianyi J, Afanvi KA, Agoro S, Salou M, Landoh DE. Prevalence of severe adverse events among health professionals after receiving the first dose of the ChAdOx1 nCoV-19 coronavirus vaccine (Covishield) in Togo, March 2021. Archives of Public Health. 2021 Dec;79:1-9.
- Subedi P, Yadav GK, Paudel B, Regmi A, Pyakurel P. Adverse events following the first dose of Covishield (ChAdOx1 nCoV-19) vaccination among health workers in selected districts of central and western Nepal: A cross-sectional study. PLoS One. 2021 Dec 21;16(12):e0260638.
- Chi WY, Li YD, Huang HC, Chan TE, Chow SY, Su JH, Ferrall L, Hung CF, Wu TC. COVID-19 vaccine update: vaccine effectiveness, SARS-CoV-2 variants, boosters, adverse effects, and immune correlates of protection. Journal of biomedical science. 2022 Oct 15;29(1):82.
- Kashte SB, Sharma RK, Kadam S. Profile of adverse events following COVID-19 vaccination: Insights from Covishield, Covaxin, and Corbevax beneficiaries in India. Journal of Krishna Institute of Medical Sciences (JKIMSU). 2023 Jan 1;12(1).
- Poudel KM, Shah N, Prakash M, Deo SK, Bhandari S, Poudel TR. Determinants of associated events following AZD1222 (Covishield) vaccination in a high-risk population in Nepal. BMC Infectious Diseases. 2022 May 3;22(1):422.
- Joshi RK, Muralidharan CG, Ahuja A, Mukherjee R, Chaurasia S, Manjaly L, Sahoo AK, Gosavi J, Thomas A. Vaccine effectiveness to protect against moderate or severe disease in COVID cases: A prospective cohort study. Medical Journal Armed Forces India. 2023 Dec 1;79:S102-11.
- Singh C, Naik BN, Pandey S, Biswas B, Pati BK, Verma M, Singh PK. Effectiveness of COVID-19 vaccine in preventing infection and disease severity: a case-control study from an Eastern State of India. Epidemiology & Infection. 2021 Jan;149:e224.
- Bhattacharyya P, Das S, Aich S, Sarkar J. COVID-19: morphology and mechanism of the SARS-CoV-2, global outbreak, medication, vaccines and future of the virus. Frontiers in Bioscience-Elite. 2021 Dec 20;13(2):272-90.
- Bhattacharjee A, Maity P, Choudhuri S, Gupta S. Treatment for COVID-19: Current Therapy and Challenges. Modern Approaches in Chemical and Biological Sciences. 2022:79-91.
- Ray S. The Science and Politics of Covid-19 Vaccine. The Environmental, Economic and Social Scenario During and After the COVID-19 Catastrophe Part I. 2022:36.
- Dash P, Mohapatra S, Ghosh S, Nayak B. A scoping insight on potential prophylactics, vaccines and therapeutic weaponry for the ongoing novel coronavirus (COVID-19) pandemic-a comprehensive review. Frontiers in Pharmacology. 2021 Feb 26;11:590154.
- Singh K, Kherb J, Singh BP. General preventive measures to control the transmission and COVID-19 pandemic management: a public outreach. InSmart Nanomaterials to Combat the Spread of Viral Infections 2023 Jan 1 (pp. 61-75). Academic Press.
- Hayat M, Uzair M, Ali Syed R, Arshad M, Bashir S. Status of COVID-19 vaccination around South Asia. Human Vaccines & Immunotherapeutics. 2022 Jan 31;18(1):2016010.
- Md Khairi LN, Fahrni ML, Lazzarino AI. The race for global equitable access to COVID-19 vaccines. Vaccines. 2022 Aug 12;10(8):1306.
- SUJATHA D. A REVIEW ON VACCINE DEVELOPMENT STRATEGIES AGAINST COVID-19. Tamilnadu-604303 (INDIA)(ISO 9001: 2015 Certified Company).:42.
- RATHER RA, ISLAM T, REHMAN IU, PANDEY D. Development of vaccine against coronavirus disease 2019 (Covid-19) In India. Asian Journal of Advances in Medical Science. 2021 Mar 8;3(1):44-52.
- Koller CN, Schwerzmann CJ, Lang AS, Alexiou E, Krishnakumar J. Addressing different needs: The challenges faced by India as the largest vaccine manufacturer while conducting the world’s biggest COVID-19 vaccination campaign. Epidemiologia. 2021 Sep 17;2(3):454-70.
- King ML. How manufacturing won or lost the COVID-19 vaccine race. Vaccine. 2024 Jan 15.
- Chavda VP, Vihol DR, Solanki HK, Apostolopoulos V. The vaccine world of COVID-19: India’s Contribution. Vaccines. 2022 Nov 17;10(11):1943.
- Somasundaram V, Soukas P, Patel J, Ferguson S. Considerations for Potential Global Expansion of Serum Institute of India. Journal of commercial biotechnology. 2021 Dec;26(4).
- Ghosh PK. Global efforts on vaccines development against SARS-CoV-2 and Indian endeavor. MGM Journal of Medical Sciences. 2021 Oct 1;8(4):422-34.
- Md Khairi LN, Fahrni ML, Lazzarino AI. The race for global equitable access to COVID-19 vaccines. Vaccines. 2022 Aug 12;10(8):1306.
- Selvam SP, Ramani P, Ramya R, Sundar S, Lakshmi TA. COVID-19 vaccines and the efficacy of currently available vaccines against COVID-19 variants. Cureus. 2022 May 11;14(5).
- Rehman N, Pandey A. An Overview of COVID-19 and Its Vaccines. Biology Bulletin Reviews. 2021 Dec;11(Suppl 1):47-64.
- Kumar RN, Arunkumar AS, Mahadevan L. Monograph on COVID-19 Vaccines: A Review
- Chavda VP, Vihol DR, Solanki HK, Apostolopoulos V. The vaccine world of COVID-19: India’s Contribution. Vaccines. 2022 Nov 17;10(11):1943.
- SINHA P, GUPTA M, VIJAYVERGIA V, JAIN SK, JAIN DK, GUPTA S, RATHORE M, VYAS N. Safety and Immunogenicity Outcomes of an Inactivated Viral Vaccine against SARS-CoV-2 (Covaxin®). Journal of Clinical & Diagnostic Research. 2022 Sep 1;16(9).
- Nayan R, Kumari P, Gaikar VB. VACCINATION DRIVE, VACCINE DIPLOMACY FOR COVID-19 PANDEMIC: INDIAN PERSPECTIVE.
- Sharma S. COVID-19 in India: Current status-prevalence, research area, public health, and primary care. Journal of Public Health and Primary Care. 2021 Sep 1;2(3):51-7.
- Jain VK, Iyengar KP, Ish P. Elucidating causes of COVID-19 infection and related deaths after vaccination. Diabetes & Metabolic Syndrome: Clinical Research & Reviews. 2021 Sep 1;15(5):102212.
- Machado BA, Hodel KV, Fonseca LM, Pires VC, Mascarenhas LA, da Silva Andrade LP, Moret MA, Badaró R. The importance of vaccination in the context of the COVID-19 pandemic: a brief update regarding the use of vaccines. Vaccines. 2022 Apr 12;10(4):591.
- Dutta A, Ghosh R, Bhattacharya D, Bhat S, Ray A, Pandit A, Das S, Dubey S. Anti-PF4 antibody negative cerebral venous sinus thrombosis without thrombocytopenia following immunization with COVID-19 vaccine in an elderly non-comorbid Indian male, managed with conventional heparin-warfarin based anticoagulation. Diabetes & Metabolic Syndrome. 2021 Jul;15(4):102184.
- World Health Organization. Guidance for clinical case management of thrombosis with thrombocytopenia syndrome (TTS) following vaccination to prevent coronavirus disease (COVID-19). World Health Organization; 2023
- Web Annex A. Guidance for clinical case management of thrombosis with thrombocytopenia syndrome (TTS) following vaccination to prevent coronavirus disease (COVID-19).
- Lai CC, Ko WC, Chen CJ, Chen PY, Huang YC, Lee PI, Hsueh PR. COVID-19 vaccines and thrombosis with thrombocytopenia syndrome. Expert Review of Vaccines. 2021 Aug 3;20(8):1027-35.
- Meo SA, Aftab S, Bayoumy NM, Meo AS. Efficacy of Oxford-AstraZeneca (ChAdOx1 CoV-19) vaccine against Severe Acute Respiratory Syndrome Coronavirus-2 (SARS-CoV-2) cases, hospital admissions, type of variants, and deaths. European Review for Medical & Pharmacological Sciences. 2023 Oct 15;27(20).
- Munro AP, Janani L, Cornelius V, Aley PK, Babbage G, Baxter D, Bula M, Cathie K, Chatterjee K, Dodd K, Enever Y. Safety and immunogenicity of seven COVID-19 vaccines as a third dose (booster) following two doses of ChAdOx1 nCov-19 or BNT162b2 in the UK (COV-BOOST): a blinded, multicentre, randomised, controlled, phase 2 trial. The Lancet. 2021 Dec 18;398(10318):2258-76.
- Phianhasin L, Ruksakulpiwat S, Kruahong S, Kuntajak P, Kelman GB, Benjasirisan C. Management and Characteristics of Embolism and Thrombosis After COVID-19 Vaccination: Scoping Review. Journal of Multidisciplinary Healthcare. 2023 Dec 31:2745-72.
- Roytenberg R, García-Sastre A, Li W. Vaccine-induced immune thrombotic thrombocytopenia: what do we know hitherto?. Frontiers in medicine. 2023 May 16;10:1155727.
- Willame C, Dodd C, Durán CE, Elbers RJ, Gini R, Bartolini C, Paoletti O, Wang L, Ehrenstein V, Kahlert J, Haug U. Background rates of 41 adverse events of special interest for COVID-19 vaccines in 10 European healthcare databases-an ACCESS cohort study. Vaccine. 2023 Jan 4;41(1):251-62.
- de Gregorio C, Colarusso L, Calcaterra G, Bassareo PP, Ieni A, Mazzeo AT, Ferrazzo G, Noto A, Koniari I, Mehta JL, Kounis NG. Cerebral venous sinus thrombosis following COVID-19 vaccination: analysis of 552 worldwide cases. Vaccines. 2022 Feb 3;10(2):232.
- Paknahad MH, Yancheshmeh FB, Soleimani A. Cardiovascular complications of COVID-19 vaccines: A review of case-report and case-series studies. Heart & Lung. 2023 May 1;59:173-80.
- Faghihi H, Mottaghi-Dastjerdi N, Sharifzadeh M, Kakavandi NR. ChAdOx1 nCoV-19 Vaccine and Thrombosis with Thrombocytopenia Syndrome among Adults: A Systematic Review. Advanced Pharmaceutical Bulletin. 2023 Nov;13(4):723.
- MacNeil JR. Updated recommendations from the advisory committee on immunization practices for use of the Janssen (Johnson & Johnson) COVID-19 vaccine after reports of thrombosis with thrombocytopenia syndrome among vaccine recipients—United States, April 2021. MMWR. Morbidity and mortality weekly report. 2021;70.
- Procter TD, Ogasawara H, Spruin S, Wijayasri S, Abraham N, Blaser C, Hutchings K, Shaw A, Ogunnaike-Cooke S. Thrombosis with thrombocytopenia syndrome (TTS) following adenovirus vector COVID-19 vaccination in Canada. Vaccine. 2023 Nov 2;41(46):6802-9.
- Kataria S, Reza RR, Agboola AA, Mohamed KH, Mohamed AS, Zahid N, Haseeb M, Nasir H. Immune thrombocytopenia and cerebral venous sinus thrombosis following COVID-19 vaccination: a case report. Cureus. 2023 Jan 27;15(1).
- Asalkar M, Thakkarwad S, Rumani I, Sharma N. Prevalence of maternal mortality and clinical course of maternal deaths in covid-19 pneumonia-a cross-sectional study. The Journal of Obstetrics and Gynecology of India. 2021:1-0.
- Salmon DA, Schuh HB, Sargent RH, Konja A, Harvey SA, Laurie S, Mai BS, Weakland LF, Lavery JV, Orenstein WA, Breiman RF. Impact of vaccine pause due to Thrombosis with thrombocytopenia syndrome (TTS) following vaccination with the Ad26. COV2. S vaccine manufactured by Janssen/Johnson & Johnson on vaccine hesitancy and acceptance among the unvaccinated population. PloS one. 2022 Oct 11;17(10):e0274443.
- Pai M, Chan B, Stall NM, Grill A, Ivers N, Maltsev A, Miller KJ, Odutayo A, Razak F, Schull M, Schwartz B. Vaccine-induced immune thrombotic thrombocytopenia (VITT) following adenovirus vector COVID-19 vaccination. Science briefs of the Ontario COVID-19 science advisory table. 2021 May 7;2(17):1-7.
- Sunder A, Saha S, Kamath S, Kumar M. Vaccine-induced thrombosis and thrombocytopenia (VITT); Exploring the unknown. Journal of Family Medicine and Primary Care. 2022 May 1;11(5):2231-3.
- Castro-Varela A, Salas AK, Barrios-Ruiz A, Morales EA, Abascal-Lanzagorta P, `111111Reyes-Chavez MF, Cárdenas-Rodríguez IT, Solorzano-Lopez EJ, Sánchez-Pizarro C, de los Ríos Arce LF, Vazquez-Garza E. Distinctions between survivors and non-survivors with SARS-CoV-2 vaccine-induced thrombotic thrombocytopenia: A systematic review and meta-analysis. vaccine: X. 2023 Nov 18:100407.
- Abrignani MG, Murrone A, De Luca L, Roncon L, Di Lenarda A, Valente S, Caldarola P, Riccio C, Oliva F, Gulizia MM, Gabrielli D. COVID-19, vaccines, and thrombotic events: a narrative review. Journal of clinical medicine. 2022 Feb 11;11(4):948.
- Durand J, Dogné JM, Cohet C, Browne K, Gordillo?Marañón M, Piccolo L, Zaccaria C, Genov G. Safety Monitoring of COVID?19 Vaccines: Perspective from the European Medicines Agency. Clinical Pharmacology & Therapeutics. 2023 Jun;113(6):1223-34.
- See I, Su JR, Lale A, Woo EJ, Guh AY, Shimabukuro TT, Streiff MB, Rao AK, Wheeler AP, Beavers SF, Durbin AP. US case reports of cerebral venous sinus thrombosis with thrombocytopenia after Ad26. COV2. S vaccination, March 2 to April 21, 2021. Jama. 2021 Jun 22;325(24):2448-56.
- Ashique S, Kumar S, Kumar H, Roy J, Pal S, Pal S. A brief overview of various vaccines against nCOVID19, including safety, efficacy, reported cases, clinical trials, and progress. Indian Journal of Health Sciences and Biomedical Research kleu. 2023 Jan 1;16(1):13-29.
- Lamptey E. Post-vaccination COVID-19 deaths: a review of available evidence and recommendations for the global population. Clinical and Experimental Vaccine Research. 2021 Sep;10(3):264.
- World Health Organization. Guidance for clinical case management of thrombosis with thrombocytopenia syndrome (TTS) following vaccination to prevent coronavirus disease (COVID-19). World Health Organization; 2023.
- Markovic-Denic L, Popadic D, Jovanovic T, Bonaci-Nikolic B, Samardzic J, Tomic Spiric V, Rancic M, Sankar Datta S, Mosina L, Jancic J, Vukomanovic G. Developing COVID-19 vaccine recommendations during the pandemic: The experience of Serbia's Expert Committee on Immunization. Frontiers in public health. 2022 Nov 17;10:1056670.
- Majima E. A Case Study of Project Management of COVID-19 Vaccination in Japan (Doctoral dissertation, Massachusetts Institute of Technology).
- Byttebier K. Covid-19 Vaccines and Medicines. InCovid-19 and Capitalism: Success and Failure of the Legal Methods for Dealing with a Pandemic 2022 Apr 23 (pp. 859-1029). Cham: Springer International Publishing.
- Jun J, Zain A, Chen Y, Kim SH. Adverse mentions, negative sentiment, and emotions in COVID-19 vaccine tweets and their association with vaccination uptake: Global comparison of 192 countries. Vaccines. 2022 May 8;10(5):735.
- Greinacher A, Langer F, Makris M, Pai M, Pavord S, Tran H, Warkentin TE. Vaccine?induced immune thrombotic thrombocytopenia (VITT): update on diagnosis and management considering different resources. Journal of Thrombosis and Haemostasis. 2022 Jan;20(1):149-56.
- AlShurman BA, Tetui M, Nanyonjo A, Butt ZA, Waite NM, Vernon-Wilson E, Wong G, Grindrod K. Understanding the COVID-19 Vaccine Policy Terrain in Ontario Canada: A Policy Analysis of the Actors, Content, Processes, and Context. Vaccines. 2023 Mar 31;11(4):782.
- Adibi A, Mozafarihashjin M, Sadatsafavi M. Vaccination of front-line workers with the AstraZeneca COVID-19 vaccine: benefits in the face of increased risk for prothrombotic thrombocytopenia. medRxiv. 2021 Apr 15:2021-04.
- Satapathy BS, Pattnaik G, Sahoo RN, Pattanaik S, Sarangi AK, Kandi V, Mishra S, Rabaan AA, Mohanty A, Sah R, Mohapatra RK. COVID?19 vaccines and their underbelly: Are we going the right way?. Health Science Reports. 2023 Sep;6(9):e1540.
- Syed K, Chaudhary H, Donato A. Central venous sinus thrombosis with subarachnoid hemorrhage following an mRNA COVID-19 vaccination: are these reports merely Co-incidental?. The American Journal of Case Reports. 2021;22:e933397-1.
- Lai CC, Ko WC, Chen CJ, Chen PY, Huang YC, Lee PI, Hsueh PR. COVID-19 vaccines and thrombosis with thrombocytopenia syndrome. Expert Review of Vaccines. 2021 Aug 3;20(8):1027-35.
- Byttebier K. Covid-19 Vaccines and Medicines. InCovid-19 and Capitalism: Success and Failure of the Legal Methods for Dealing with a Pandemic 2022 Apr 23 (pp. 859-1029). Cham: Springer International Publishing.
- Rennebohm RM. An Open Letter to Parents and Pediatricians—Part I.
- Chekol Abebe E, Mengie Ayele T, Tilahun Muche Z, Behaile T/Mariam A, Dagnaw Baye N, Mekonnen Agidew M, Asmamaw Dejenie T. Evaluation and comparison of post-vaccination adverse effects among Janssen and Oxford-AstraZeneca vaccinated adult individuals in Debre Tabor Town: A cross-sectional survey in Northwest Ethiopia. Human Vaccines & Immunotherapeutics. 2022 Nov 30;18(6):2104059.
- Prasad N, Bansal SB, Yadav B, Manhas N, Yadav D, Gautam S, Kushwaha R, Singh A, Bhadauria D, Yachha M, Behera MR. Seroconversion rate after SARS-CoV-2 infection and two doses of either ChAdOx1-nCOV COVISHIELD™ or BBV-152 COVAXIN™ vaccination in renal allograft recipients: An experience of two public and private tertiary care center. Frontiers in immunology. 2022 Jun 30;13:911738.
- Lingadurai S, Mariappan G. Adverse events following immunization (AEFI) for COVID vaccines approved by WHO—A short review. IP Int. J. Compr. Adv. Pharmacol. 2022;7:40-3.
- Rabail R, Ahmed W, Ilyas M, Rajoka MS, Hassoun A, Khalid AR, Khan MR, Aadil RM. The side effects and adverse clinical cases reported after COVID-19 immunization. Vaccines. 2022 Mar 22;10(4):488.
- Lai CC, Ko WC, Chen CJ, Chen PY, Huang YC, Lee PI, Hsueh PR. COVID-19 vaccines and thrombosis with thrombocytopenia syndrome. Expert Review of Vaccines. 2021 Aug 3;20(8):1027-35.
- Chekol Abebe E, Mengie Ayele T, Tilahun Muche Z, Behaile T/Mariam A, Dagnaw Baye N, Mekonnen Agidew M, Asmamaw Dejenie T. Evaluation and comparison of post-vaccination adverse effects among Janssen and Oxford-AstraZeneca vaccinated adult individuals in Debre Tabor Town: A cross-sectional survey in Northwest Ethiopia. Human Vaccines & Immunotherapeutics. 2022 Nov 30;18(6):2104059.
- Jose D, Dhupdale N, Cacodcar JA, Kamat U. Surveillance on Adverse Events Following COVISHIELD (ChAdOx1 nCoV-19) vaccination in Goa, India: An observational study. Current drug safety. 2023 Nov 1;18(4):516-27.
- Sarkar M, Madabhavi IV, Quy PN, Govindagoudar MB. COVID-19 vaccine-induced immune thrombotic thrombocytopenia: a review. Annals of Thoracic Medicine. 2022 Jan 1;17(1):1-3.
- Athavale AV. The covid-19 vaccine. Journal of Advanced Research in Medical Science & Technology (ISSN: 2394-6539). 2021 Mar 31;8(1):29-35.
- Shahsavarinia K, Faridaalaee G, Soleimanpour H, Sadeghi-Ghyassi F, Atashgahi S, Milanchian N, Abolhasanpour N, Salehi-Pourmehr H. Cerebral venous thrombosis (CVT) following COVID-19 vaccination: An umbrella review of systematic reviews. Iranian Journal of Medical Microbiology. 2023 Jan 10;17(1):7-21.
- Atyabi SM, Rommasi F, Ramezani MH, Ghane Ezabadi MF, Arani MA, Sadeghi MH, Ahmed MM, Rajabi A, Dehghan N, Sohrabi A, Seifi M. Relationship between blood clots and COVID-19 vaccines: A literature review. Open Life Sciences. 2022 Apr 26;17(1):401-15.
- Nicholson M, Goubran H, Chan N, Siegal D. No apparent association between mRNA COVID-19 vaccination and venous thromboembolism. Blood Reviews. 2022 Nov 1;56:100970.
- Douxfils J, Favresse J, Dogné JM, Lecompte T, Susen S, Cordonnier C, Lebreton A, Gosselin R, Sié P, Pernod G, Gruel Y. Hypotheses behind the very rare cases of thrombosis with thrombocytopenia syndrome after SARS-CoV-2 vaccination. Thrombosis research. 2021 Jul 1;203:163-71.
- Muslim S, Mustafa G, Nasrin N, Firdaus A, Singh SK. An analysis of fatal adverse conditions in temporal association of COVID-19 vaccination to boost the safety of vaccination for COVID-19. The Egyptian Journal of Internal Medicine. 2023 Feb 7;35(1):11.
- Murished GM, Dandachi I, Aljabr W. Side effects of COVID-19 vaccines in the middle eastern population. Frontiers in Immunology. 2023 Nov 3;14:1270187.
- Yao L, Lu L, Ma W. Immunopathological changes, complications, sequelae and immunological memory in COVID-19 patients. Heliyon. 2022 Apr 1;8(4).
- Mirmosayyeb O, Ghaffary EM, Vaheb S, Pourkazemi R, Shaygannejad V. Multiple sclerosis (MS) and neuromyelitis optica spectrum disorder (NMOSD) following COVID-19 vaccines: a systematic review. Revue Neurologique. 2023 Apr 1;179(4):265-81.
- Naik R, Avula S, Palleti SK, Gummadi J, Ramachandran R, Chandramohan D, Dhillon G, Gill AS, Paiwal K, Shaik B, Balachandran M. From emergence to endemicity: a comprehensive review of COVID-19. Cureus. 2023 Oct 31;15(10).
- Sadeghi E, Mahmoudzadeh R, Garg SJ, Nowroozzadeh MH. Ocular posterior segment complications following COVID-19 vaccination. International Ophthalmology. 2023 Nov;43(11):4343-57.
- Guo M, Liu X, Chen X, Li Q. Insights into new-onset autoimmune diseases after COVID-19 vaccination. Autoimmunity Reviews. 2023 Apr 17:103340.
- Malik JA, Ahmed S, Shinde M, Almermesh MH, Alghamdi S, Hussain A, Anwar S. The impact of COVID-19 on comorbidities: a review of recent updates for combating it. Saudi Journal of Biological Sciences. 2022 May 1;29(5):3586-99.
- Sharma E, Revinipati S, Bhandari S, Thakur S, Goyal S, Ghose A, Bajpai S, Muhammad W, Boussios S. Efficacy and safety of COVID-19 vaccines—an update. Diseases. 2022 Nov 23;10(4):112.
- Abrignani MG, Murrone A, De Luca L, Roncon L, Di Lenarda A, Valente S, Caldarola P, Riccio C, Oliva F, Gulizia MM, Gabrielli D. COVID-19, vaccines, and thrombotic events: a narrative review. Journal of clinical medicine. 2022 Feb 11;11(4):948.
- Mishra K, Barki S, Pattanayak S, Shyam M, Sreen A, Kumar S, Kotwal J. Covid-19 vaccine-induced thrombosis and thrombocytopenia: first confirmed case from India. Indian Journal of Hematology and Blood Transfusion. 2021:1-3.
- Ornelas-Aguirre JM, Gómez-Alcalá AV, Ramírez-Leyva DH. Increment of D-dimer Associated with Immune Thrombotic Thrombocytopenia in ChAdOx1 nCoV-19 Vaccinated Individuals. Archives of Medical Research. 2022 Jun 1;53(4):341-51.
- Roytenberg R, García-Sastre A, Li W. Vaccine-induced immune thrombotic thrombocytopenia: what do we know hitherto?. Frontiers in medicine. 2023 May 16;10:1155727.
- Tomar A, Biswas AK, Pawar A, Dimri U, Kumar D, Baranwal AK. To study the effect of ‘Covishield’vaccination on pre-donation platelet counts of plateletpheresis donors. Hematology, Transfusion and Cell Therapy. 2023 Dec 11;45:456-60.
- Lai CC, Ko WC, Chen CJ, Chen PY, Huang YC, Lee PI, Hsueh PR. COVID-19 vaccines and thrombosis with thrombocytopenia syndrome. Expert Review of Vaccines. 2021 Aug 3;20(8):1027-35
- De Michele M, Kahan J, Berto I, Schiavo OG, Iacobucci M, Toni D, Merkler AE. Cerebrovascular complications of COVID-19 and COVID-19 vaccination. Circulation Research. 2022 Apr 15;130(8):1187-203.
- Majid S, Khan MS, Rashid S, Niyaz A, Farooq R, Bhat SA, Wani HA, Qureshi W. COVID-19: Diagnostics, therapeutic advances, and vaccine development. Current Clinical Microbiology Reports. 2021 Feb 15:1-5.
- Kayode AJ, Banji-Onisile FO, Olaniran AO, Okoh AI. An overview of the pathogenesis, transmission, diagnosis, and management of endemic human coronaviruses: a reflection on the past and present episodes and possible future outbreaks. Pathogens. 2021 Aug 30;10(9):1108.
- Higdon MM, Wahl B, Jones CB, Rosen JG, Truelove SA, Baidya A, Nande AA, ShamaeiZadeh PA, Walter KK, Feikin DR, Patel MK. A systematic review of coronavirus disease 2019 vaccine efficacy and effectiveness against severe acute respiratory syndrome coronavirus 2 infection and disease. InOpen Forum Infectious Diseases 2022 Jun 1 (Vol. 9, No. 6, p. ofac138). US: Oxford University Press.
- Gannon J, Azari R, Lomazzi M, Borisch B. Analysing the Launch of COVID-19 Vaccine National Rollouts: Nine Case Studies. Epidemiologia. 2021 Dec;2(4):519-39.
- Rajpal VR, Sharma S, Sehgal D, Singh A, Kumar A, Vaishnavi S, Tiwari M, Bhalla H, Goel S, Raina SN. A comprehensive account of SARS-CoV-2 genome structure, incurred mutations, lineages and COVID-19 vaccination program. Future Virology. 2022 Sep;17(9):687-706.
- Zhang W, Shen X, Li T, Li N, Sun Y, Zhu S, Liu N, Song H, Tang K, Wang Y, Zhang Y. Intention to pay for vaccination and influencing factors of general residents: a national cross-sectional study. International Journal of Environmental Research and Public Health. 2022 Sep 6;19(18):11154.
- Wu J, Shen Z, Li Q, Tarimo CS, Wang M, Gu J, Wei W, Zhang X, Huang Y, Ma M, Xu D. How urban versus rural residency relates to COVID-19 vaccine hesitancy: A large-scale national Chinese study. Social Science & Medicine. 2023 Mar 1;320:115695.
- Caycho-Rodríguez T, Ventura-León J, Valencia PD, Vilca LW, Carbajal-León C, Reyes-Bossio M, White M, Rojas-Jara C, Polanco-Carrasco R, Gallegos M, Cervigni M. What is the support for conspiracy beliefs about COVID-19 vaccines in Latin America? a prospective exploratory study in 13 countries. Frontiers in psychology. 2022 May 6;13:855713.
- Garg RK, Paliwal V, Malhotra HS, Singh BP, Rizvi I, Kumar N. Spectrum of serious neurological and psychiatric adverse events in Indian COVID-19 vaccine recipients: a systematic review of case reports and case series. Neurology India. 2023 Mar 1;71(2):209-27.
- Lingadurai S, Mariappan G. Adverse events following immunization (AEFI) for COVID vaccines approved by WHO—A short review. IP Int. J. Compr. Adv. Pharmacol. 2022;7:40-3.
- Garg RK, Paliwal VK. Spectrum of neurological complications following COVID-19 vaccination. Neurological Sciences. 2022 Jan;43(1):3-40.
- Cherian NM, Durai DA, Jaisel M, Sharma D, Sebastian J, Basavaraja CK, Mathew M. Active surveillance of adverse events following COVID-19 vaccines in a tertiary care hospital. Therapeutic Advances in Vaccines and Immunotherapy. 2023 Aug;11:25151355231193975.
- Jeelani A, Aleem S, Salim SM, Raja W. INTERNATIONAL JOURNAL OF SCIENTIFIC RESEARCH.
- Jayatilake JA, Karunaratne HM, Perera KY, Dissanayake Y, Dileka WS, Weerasinghe IE, Jayatilake JA. Study on the adverse events following immunization (AEFI) of ChAdOx1 nCoV-19 vaccine in a group of Sri Lankan medical officers. Sri Lankan Journal of Infectious Diseases. 2022 Apr 29;12(1).
- Bhardwaj K, Johari V. COVID-19 Vaccines in India: Judicial Blind Spots in Upholding the Right to Health. Socio-Legal Rev.. 2022;18:119.
- Abrignani MG, Murrone A, De Luca L, Roncon L, Di Lenarda A, Valente S, Caldarola P, Riccio C, Oliva F, Gulizia MM, Gabrielli D. COVID-19, vaccines, and thrombotic events: a narrative review. Journal of clinical medicine. 2022 Feb 11;11(4):948.
- Simnani FZ, Singh D, Kaur R. COVID-19 phase 4 vaccine candidates, effectiveness on SARS-CoV-2 variants, neutralizing antibody, rare side effects, traditional and nano-based vaccine platforms: a review. 3 Biotech. 2022 Jan;12(1):15.
- Singh K, Verma A, Lakshminarayan M. India’s efforts to achieve 1.5 billion COVID-19 vaccinations: a narrative review. Osong Public Health and Research Perspectives. 2022 Oct;13(5):316.
- Ray S. The Science and Politics of Covid-19 Vaccine. The Environmental, Economic and Social Scenario During and After the COVID-19 Catastrophe Part I. 2022:36.


 Arnab Roy*
Arnab Roy*
 K. Rajeswar Dutt
K. Rajeswar Dutt
 Ankita Singh
Ankita Singh
 Mahesh Kumar Yadav
Mahesh Kumar Yadav
 Manav Kumar
Manav Kumar
 Kajal Kumari
Kajal Kumari
 Saurabh Choudhary
Saurabh Choudhary
 Gautam Mahto
Gautam Mahto
 Dheeraj Prasad
Dheeraj Prasad
 Naba Kishor Gorai
Naba Kishor Gorai
 Biplop Debnath
Biplop Debnath
 Suraj Kumar
Suraj Kumar
 Sakshi Kumari
Sakshi Kumari
 Anand Kumar
Anand Kumar



 10.5281/zenodo.11403097
10.5281/zenodo.11403097