Abstract
The development and optimization of nutraceutical delivery systems are critical for enhancing the bioavailability, efficacy, and overall health benefits of nutraceuticals. Nutraceuticals, which include vitamins, minerals, herbal extracts, and other bioactive compounds, offer potential therapeutic effects in preventing or managing various health conditions. However, their efficacy is often limited by challenges such as poor solubility, instability, and low bioavailability. The delivery system plays a crucial role in overcoming these barriers, ensuring that bioactive compounds reach their target sites in the body in a form that maximizes their absorption and effectiveness. Recent advancements in delivery technologies, such as nanoencapsulation, liposomes, and polymeric nanoparticles, have significantly improved the stability and controlled release of nutraceuticals. These systems not only enhance the solubility and bioavailability of hydrophobic compounds but also protect them from degradation due to environmental factors like heat, light, and oxygen. Additionally, the optimization of these delivery systems involves addressing factors such as particle size, surface charge, and release profiles to achieve targeted delivery and sustained release over time. The development of personalized nutraceutical delivery systems, tailored to individual needs, holds great promise in maximizing health benefits. Furthermore, innovations in biodegradable materials and green technologies are paving the way for more sustainable, cost-effective delivery solutions. Ultimately, optimizing nutraceutical delivery systems can lead to more effective interventions in the management of chronic diseases, better compliance in supplementation regimens, and improved overall health outcomes
Keywords
Nutraceutical Delivery System, Development and Optimization, Increased Health Benefits.
Introduction
Industrialization has resulted in significant air and water pollution, soil and food contamination due to the widespread use of chemicals, heavy metals, electromagnetic radiation, and other potentially harmful synthetic substances. These environmental issues have contributed to a rise in diseases such as diabetes, obesity, various cancers, vascular disorders, and other chronic health conditions. As a result, the demand for healthcare has surged, leading to higher medical costs. In response, many people have sought to improve their quality of life by consuming more fruits, vegetables, and plant-based foods, taking dietary supplements or nutraceuticals, and opting for nutritional or phytotherapy as alternatives to chemotherapy or radiotherapy [1]. As the demand for nutraceuticals, phytonutrients, and their therapeutic benefits continues to rise, manufacturers, marketers, and other licensed professionals in the field have expanded accordingly. Plants remain a vital source of both food and medicine for humans. The rapid growth in knowledge about nutrition, medicine, and plant biotechnology has significantly reshaped our understanding of food, health, and agriculture, sparking a revolution in these areas. Recent advancements in medical and nutritional sciences have led to increased attention on natural products and health-promoting foods, both from healthcare professionals and the general public. This trend has introduced new concepts such as nutraceuticals, nutritional therapy, phytonutrients, and phytotherapy [2]. These functional foods, medicinal foods, phytonutrients, and phytomedicines contribute positively to health by boosting immune function, preventing specific diseases, and offer significant potential for reducing side effects and healthcare costs [3] . Today, we acknowledge the timeless wisdom of the Greek physician Hippocrates, whose famous saying from nearly 2,500 years ago still resonates: “Let food be thy medicine and medicine be thy food.” This concept is now applied to functional foods, dietary supplements, and nutraceuticals—products defined as "any substance that may be considered a food or part of a food, offering medical and health benefits, including the prevention and treatment of disease." A particularly fitting term for these food-and-medicine hybrids is "nutraceuticals," a term coined by Stephen DeFelice, founder and chairman of the Foundation for Innovation in Medicine in Cranford, New Jersey [4]. The term "nutraceutical" blends the words "nutrient" (a nourishing food or its component) and "pharmaceutical" (a medical drug). Nutraceuticals may include "natural" substances aimed at treating or preventing diseases, although they might not always be widely recognized as safe [5].
Categories of nutraceuticals
Nutraceuticals are general biological treatments designed to enhance health, prevent harmful conditions, and manage symptoms. They can be categorized into the following types: [6].
Nutrient: A component in animal feed, provided in a form and amount that supports the animal's life. The primary categories of feed nutrients include proteins, fats, carbohydrates, minerals, and vitamins.
Dietary Supplement: A product containing one or more dietary ingredients, such as vitamins, minerals, herbs or other botanicals, amino acids (proteins), as well as concentrates, extracts, or metabolites of these substances.
Nutraceutical: Any non-toxic food component with scientifically proven health benefits, including the treatment and prevention of diseases.
Herbals: Herbal or botanical products in the form of concentrates and extracts. These have been used since ancient times, offering a rich source of remedies for both acute and chronic conditions. India’s ancient system of medicine, Ayurveda, is one of the oldest written traditions for nature-based remedies, offering numerous effective health solutions. Many nutraceuticals are derived from medicinal herbs, which contain key beneficial compounds.
Functional Foods and Nutraceuticals: The terms "nutraceuticals," "functional foods," "medical foods," and "dietary supplements" are often used interchangeably, leading to some confusion. These terms can be distinguished based on their specific definitions. For example, "functional food" is a broader term referring to foods with specific or enhanced purposes, such as promoting health or reducing disease risk [7]. Dietary supplements serve more specific health purposes, including vitamins, minerals, herbs or other botanicals, amino acids, and other dietary substances, and are designed to enhance the diet by increasing the intake of these ingredients [8]. Dietary supplements are not meant to diagnose, treat, or cure diseases [9]. In contrast, nutraceuticals focus more on the anticipated outcomes of these products, such as preventing or treating diseases. On the other hand, the concept of functional foods differs from nutraceuticals and refers to food products consumed as part of the regular diet that provide health benefits beyond the typical nutritional effects [10]. Functional food products include milk, cheese, and eggs enriched with omega-3 fatty acids; yogurt containing live active cultures (probiotics); fruit juices and beverages with higher levels of antioxidants; cereals and grains like wheat, oats, barley, and fenugreek, fortified with additional dietary fiber; modified vegetable oils with altered fatty acid profiles; and plant-based proteins from soy, canola, hemp, legumes, and fruits [11]. Nutraceuticals are defined as products derived, purified, or produced from plant, animal, or marine sources (e.g., antioxidants from blueberries, elk velvet, fish oils), or made from dried, powdered, or pressed plant materials, and have been shown to offer physiological benefits or provide protection against chronic diseases [12]. For many people, it is difficult to obtain adequate nutrition from regular food alone. Additionally, we live in a highly toxic environment, filled with pollution and pesticides, which disrupt our body's natural ability to regulate itself. Moreover, new health issues such as chronic fatigue, Epstein-Barr, and lupus have become widespread in our population. A more sensible approach might be to strengthen our body's systems or environment, rather than relying on antibiotics, which have become less effective. Medications often come with side effects because they are not naturally compatible with the body, whereas high-quality supplements, which are easily absorbed and utilized by the body, can genuinely strengthen our health and vitality.
Why Nutraceuticals Are Attractive:
- Many diets are rich in phenolic compounds, which are regularly consumed by humans.
- These compounds typically have few or no side effects.
- They tend to have a long half-life.
- They are easily absorbed in the intestines after ingestion.
- They don’t require a doctor's prescription and are readily available without a healthcare appointment.
- Many people view this approach as more natural than using prescription medications, believing that dietary supplements can help them feel stronger, healthier, more energetic, and better able to prevent illness.
- Some individuals turn to these products when they feel conventional treatments for their conditions have failed.
The Concept of Nutraceuticals: In pharmaceutical development, clinical trials with animals are necessary to verify therapeutic effects. However, historically, there was no similar method to verify the effectiveness of foods in disease prevention. In recent years, as scientific research has linked food composition to lifestyle-related diseases, the issue has gained social attention. Nutraceutical products are now recognized for their potential health benefits, including reducing the risk of cancer and heart disease, as well as preventing or treating conditions like hypertension, high cholesterol, obesity, osteoporosis, diabetes, arthritis, macular degeneration (leading to irreversible blindness), cataracts, menopausal symptoms, insomnia, memory and concentration issues, digestive disturbances, and constipation. Other products are claimed to address problems like hair thinning, low self-esteem, poor skin, varicose veins, alcoholism, depression, and fatigue. The concept of nutraceuticals is increasingly being recognized as a preventive measure against such diseases [13].
Nutraceutical Growth
Nutraceuticals and functional foods have become a multi-billion-dollar industry globally, with significant growth projections. However, there are notable challenges to further expansion, particularly the need for proper labeling and the assessment of the health effects of these products. By selecting specific ecological regions for the consistent production of high- and low-yield active plant components, it is possible to develop alternative nutraceuticals and functional foods that offer more reliable and distinct health and food benefits. The United States currently holds the largest and fastest-growing market for functional foods and nutraceuticals worldwide. India is home to a wide variety of medicinal herbs, spices, and tree species, which contribute to a substantial domestic market. Functional foods and nutraceuticals are often available as traditional Ayurvedic medicines, sold under various brand names. However, there are no stringent pharmaceutical regulations governing Ayurvedic and nutraceutical health products in India, and they are typically available over the counter without the need for a medical prescription. India holds a significant share of the global functional food and nutraceutical market and exports these products to several countries, with the USA and Japan being its primary export destinations [14]. The global growth of the food market, particularly in the area of nutraceuticals, is being driven by multiple scientific organizations and various government agencies [15].
The growing popularity of nutraceuticals and functional foods among the public sector can be attributed to several factors, including:
- Increased awareness of public health,
- A rapidly aging population,
- Rising healthcare costs,
- Recent advancements in research and technology,
- Changes in government regulations and accountability,
- The expansion of the global marketplace,
- Supportive media coverage, and
- Science-based evidence supporting the benefits of functional foods.
Recognizing the variations in the composition of functional foods and nutraceuticals offers the industry an opportunity to introduce a wider range of products tailored to specialized markets.
Dietary Supplement Health and Education Act (DSHEA): The Dietary Supplement Health and Education Act (DSHEA), passed in 1994, governs the human nutraceutical market. It prevents the FDA from classifying a new product as a "drug" or "food additive" if it meets the definition of a "dietary supplement." This includes any dietary component or its concentrates, extracts, or metabolites. The DSHEA broadened the definition of nutraceuticals to include vitamins, minerals, herbs, other botanicals, amino acids, and any dietary substances that supplement the human diet by increasing overall dietary intake. To comply with regulations, nutraceuticals must be labeled as "dietary supplements" and cannot be marketed as conventional foods or as the sole item in a meal or diet.
FDA Modernization Act: The Food and Drug Administration Modernization Act of 1997 (FDAMA) introduced new options for nutraceutical manufacturers, aiming to balance the FDA’s role in approving therapeutic products while ensuring public health protection. Significant changes were also made to the labeling requirements for nutraceuticals, further regulating how these products are marketed [16]. Global demand for nutraceutical ingredients grew at an annual rate of 5.8%, reaching $15.5 billion in 2010, within a broader global nutritional product industry valued at $197 billion. China and India are expected to be the fastest-growing markets for nutraceuticals, as strong economic growth allows these countries to enhance and diversify their food, beverage, and pharmaceutical production capabilities. The growing acceptance of both herbal and non-herbal extracts by consumers and healthcare professionals drove global demand for these extracts up by 6.5% annually, reaching $1.85 billion in 2010. Demand for nutrients, minerals, and vitamins reached $9.5 billion in 2010, reflecting a 6.3% annual growth since 2005. Specifically, the global demand for nutraceutical vitamin ingredients grew at an annual rate of 4.6%, reaching $4.2 billion in 2010. Natural vitamin E formulations and beta carotene (vitamin A) are expected to perform well in the global market, benefiting from their superior efficacy compared to synthetic alternatives in both adult and pediatric nutritional products. Nutraceuticals available today include both traditional and nontraditional foods. Traditional nutraceuticals are essentially natural, whole foods that have been recognized for their potential health benefits. Many fruits, vegetables, grains, fish, dairy, and meat products contain natural compounds that offer benefits beyond basic nutrition, such as lycopene in tomatoes, omega-3 fatty acids in salmon, or saponins in soy. Even tea and chocolate have been identified in some studies as having health-promoting properties. In contrast, nontraditional nutraceuticals refer to foods that have been modified through agricultural breeding or enriched with additional nutrients and/or ingredients.
The concept of functional foods can be interpreted in various ways, depending on whether it refers to their attributes, their active ingredients, or their regulatory framework [17]. Current research in nutraceuticals is heavily focused on traditional herbal extracts, with researchers investigating their potential benefits in health promotion and the prevention of chronic diseases. This research is partly aimed at validating homeopathic remedies and Eastern medicine, while also providing crucial safety and efficacy data for both patients and healthcare professionals. The rapid growth in demand for bioactive ingredients in nutraceuticals and functional foods is largely driven by widespread health concerns, including:
- Cardiovascular disease
- Breast, skin, colorectal, and brain cancers
- Female health issues
- Central nervous system (CNS) disorders
- Metabolic management
- Gastrointestinal disorders
- Immune system modulation
However, a major challenge in using nutraceuticals to treat diseases is the lack of robust studies offering clear clinical evidence. While the development, production, packaging, marketing, and sales of nutraceuticals have advanced significantly and continue to evolve, these products are increasingly preferred by consumers for daily use. Ongoing scientific research and clinical trials continue to support and drive growth in this industry [18]. Safety and efficacy some substances in nutraceutical products may pose risks due to their direct toxic effects. While establishing the safety of a nutraceutical is generally easier than proving its efficacy, many of these products have been used as alternatives for both nutrition and medicine. However, some manufacturers make unsubstantiated claims about their products' safety and effectiveness. As a result, consumers need assurance that a product is both safe and effective in delivering the promised benefits. Above all, the safety of nutraceuticals should be the top priority.
Labeling and Claims in Nutraceuticals
Labeling and strict regulation of formulations and branding are not mandatory for most nutraceutical products. Health claims made on these products are intended to inform consumers that, as part of a balanced diet, they may help reduce the risk of certain diseases. The FDA initially approved seven health claims in 1993 under the Nutrition Labeling and Education Act (NLEA). Since then, the FDA has approved six additional claims. To accelerate consumer access to this information, the Food and Drug Administration Modernization Act of 1997 included a provision aimed at expediting the process for establishing the scientific basis of health claims. Although food manufacturers can use health claims to promote their products, they provide consumers with valuable information about healthy eating patterns that could help reduce the risk of conditions like heart disease, cancer, osteoporosis, high blood pressure, dental cavities, and certain birth defects. Health claims differ from structure/function claims, which may also appear on conventional food or dietary supplement labels. Unlike health claims, structure/function claims do not address disease-risk reduction. Additionally, the FDA does not pre-approve structure/function claims; instead, manufacturers are responsible for ensuring these claims are truthful and not misleading. Many academic, scientific, and regulatory organizations are working on ways to establish the scientific foundation for claims related to the functional components of nutraceuticals. The five types of health-related statements allowed on food and dietary supplement labels are as follows:
- Nutrient-content claims: Indicate the presence of a specific nutrient at a certain level.
- Structure/function claims: Describe the effect of dietary components on the normal structure or function of the body.
- Dietary-guidance claims: Highlight the health benefits of broad categories of foods.
- Qualified health claims: Suggest a developing relationship between diet components and disease risk, as approved by the FDA and supported by credible scientific evidence.
- Health claims: Confirm a relationship between diet components and disease or health risk, as approved by the FDA and supported by substantial scientific consensus.
Regulations
Nutraceuticals do not have an official definition and are not considered a distinct category of food. Since they are natural products, similar to whole foods that consumers have been eating for thousands of years, the FDA regulates them as they do all foods. This means that the safety of the ingredients must be ensured beforehand, and any claims made must be substantiated, truthful, and non-misleading. In many countries, including the United States, Canada, the European Union, China, and India, the government imposes strict regulations on food and drugs regarding manufacturing, distribution, marketing, and usage. However, these regulations do not cover all aspects comprehensively. Many nations are working on developing new laws, supplementary regulations, or providing updated clarifications to address emerging issues. More specific regulations regarding nutraceuticals, phytonutrients, phytotherapy, and nutritional therapy are being developed through consultations with expert panels. These panels aim to identify regulatory challenges for these products and practices, including ensuring compliance with Good Manufacturing Practices (GMP), determining Generally Recognized as Safe (GRAS) status, and establishing appropriate analytical methods and validation standards [19].
Indian Regulatory Aspects of Nutraceuticals
The regulatory framework for nutraceuticals in India requires attention from the relevant authorities. While regulatory bodies globally are adapting to the changing needs of consumers by updating existing laws, India continues to rely on outdated laws, such as the Prevention of Food Adulteration Act of 1954, which still governs packaged foods. Manufacturers in India must also navigate numerous other complex regulations, including:
- Standards of Weights and Measures Act, 1976, and the Standards of Weights and Measures (Packaged Commodities) Rules, 1977 (SWMA)
- Infant Milk Substitutes, Feeding Bottles, and Infant Foods (Regulation of Production, Supply, and Distribution) Act, 1992, with Rules, 1993 (IMS)
- Edible Oils Packaging (Regulations) Order, 1998
- Fruit Products Order, 1955 (FPO)
- Meat Product Order, 1973
- Milk and Milk Products Order, 1992
- Vegetable Oils Products (Regulation) Order, 1998 (VOP)
- Atomic Energy Act, 1962, and Atomic Energy (Control or Irradiation of Food) Rules, 1996
- Consumer Protection Act, 1986, and the Consumer Protection (Amendment) Act, 2002, with Rules, 1987
- Environment Protection Act, 1986, and Rules, 1986
- Agricultural Produce (Grading and Marking) Act, 1937 (as amended in 1986), along with General Grading and Marking Rules, 1986 and 1988 (AG Mark)
- Bureau of Indian Standards (BIS) Act, 1986
Future Issues and Proposals
Changes in lifestyle, particularly through dietary adjustments, can help prevent diseases like metabolic syndromes. Key issues for the future of nutraceuticals include:
- Establishing scientific standards for disease prevention through nutraceuticals.
- Creating a system for evaluating disease prevention via human trials.
- Developing a smooth process to transition from basic research to industrial application.
Since nutraceuticals are often a combination of multiple components rather than a single ingredient, their disease-preventing effects may result from the collective action of these ingredients. It is also necessary to compare the preventative effects of different types of food. As such, biomarker research for disease prevention is essential. Defining standardized methods to measure these biomarkers and establishing clear indicators are important steps in advancing the field.
Types Of Nutraceuticals
The term "nutraceutical" was first introduced in 1989 by DeFelice, combining "nutrition" and "pharmaceutical," and was originally defined as "a food (or part of a food) that provides medical or health benefits, including the prevention and/or treatment of disease" (Kalra EK, 2003). A nutraceutical can be a naturally nutrient-dense food, such as spirulina, garlic, or soy, or a specific food component, like omega-3 oil from salmon. These products are also referred to as medical foods, nutritional supplements, or dietary supplements. Nutraceuticals encompass a wide range of items, from isolated nutrients and dietary supplements to genetically modified "designer" foods, herbal products, and processed foods like cereals and soups. They have attracted significant attention due to their perceived safety and potential nutritional and therapeutic benefits. The study of dietary active compounds in human nutrition is a critical area of research, with results having significant implications for consumers, healthcare providers, regulators, and the industry (Bagchi D, 2006). Foods and nutrients are essential for the body's normal functioning, supporting overall health and helping to reduce the risk of various diseases. The global acknowledgment of this connection between "nutrition" and "health" led to the development of the concept of "nutraceuticals."
Classification of Nutraceuticals
The food sources used as nutraceuticals are all natural and can be categorized as
1. Dietary Fiber
2. Probiotics
3. Prebiotics
4. Polyunsaturated fatty acids
5. Antioxidant vitamin
6. Polyphenols
7. Spices (kalia AN, 2005)
Dietary Fiber
Dietary fiber (DF) refers to non-digestible carbohydrates and lignins that are naturally present and intact in plants. Functional fiber (FF), on the other hand, consists of isolated, non-digestible carbohydrates that provide beneficial physiological effects in humans, as outlined in Table 1. The total fiber intake is the sum of both dietary and functional fiber. These definitions expand the category to include resistant starches, oligosaccharides, and other non-digestible carbohydrates as functional fibers. According to the Dietary Reference Intake (DRI), the recommended fiber intake is 38 grams per day for adult men and 25 grams per day for adult women. There is insufficient evidence to establish a tolerable upper intake level for either dietary or functional fiber.
Probiotics
Probiotics are live microorganisms, including bacteria and yeasts, that offer health benefits, particularly for the digestive system. While bacteria are often associated with diseases, the human body is home to a mix of both harmful and beneficial bacteria. Probiotics are considered "good" or "helpful" bacteria because they support gut health. They are naturally present in the body, and can also be found in certain foods and supplements. Interest in probiotics and their health benefits has surged since the mid-1990s, with doctors often recommending them for digestive issues. Due to their growing popularity, probiotics are now commonly included in a wide range of products, from yogurt to chocolate.
Lactobacillus
Lactobacillus is likely the most widely known probiotic. It is commonly found in yogurt and other fermented foods. Various strains of Lactobacillus can aid in managing diarrhea and may also be beneficial for individuals who have difficulty digesting lactose, the sugar found in milk.
Bifidobacterium
Bifidobacterium is another probiotic found in certain dairy products. It may help alleviate symptoms of irritable bowel syndrome (IBS) and other digestive issues. Probiotics play a role in helping food move through the digestive tract. Researchers are still exploring which strains are most effective for specific health conditions. Some common conditions they are known to help with include:
- Irritable bowel syndrome (IBS)
- Inflammatory bowel disease (IBD)
- Infectious diarrhea (caused by viruses, bacteria, or parasites)
- Diarrhea associated with antibiotics
There is also some evidence suggesting that probiotics may offer benefits for other health concerns, such as:
- Skin conditions like eczema
- Urinary and vaginal health
- Preventing allergies and colds
- Improving oral health
Prebiotics
Prebiotics are substances that stimulate the growth or activity of beneficial microorganisms, such as bacteria and fungi, that contribute to the health of their host. The most well-known example of prebiotics is found in the gastrointestinal tract, where they influence the composition of gut microbiota. However, prebiotics can also affect other parts of the body. For instance, some hand moisturizers are thought to act as prebiotics by enhancing the activity or composition of the skin microbiota.
- In terms of diet, prebiotics are typically non-digestible fiber compounds that pass through the upper gastrointestinal tract without being broken down, stimulating the growth of beneficial bacteria in the large intestine by serving as a food source for them [20].
As functional food components, prebiotics, like probiotics, occupy a position between foods and drugs. They often face moderate regulatory scrutiny, particularly regarding the health claims made about them. While all prebiotics are fibers, not all fibers are considered prebiotics. For a food ingredient to be classified as a prebiotic, it must meet certain criteria (Jacob RA, 1995):
- It must resist stomach acidity, be unaffected by mammalian enzymes, and not absorbed in the upper gastrointestinal tract.
- It should be fermented by intestinal microflora.
- It must selectively promote the growth or activity of beneficial intestinal bacteria linked to health and well-being [21].
Health Benefits of Prebiotics
While the health outcome data for prebiotics is more limited compared to dietary fiber, it is suggested that prebiotic consumption may:
- Decrease the occurrence and duration of infectious and antibiotic-associated diarrhea
- Alleviate inflammation and symptoms related to inflammatory bowel disease
- Provide protective effects against colon cancer
- Improve the bioavailability and absorption of minerals, such as calcium, magnesium, and potentially iron
- Lower certain risk factors for cardiovascular disease
- Support feelings of fullness, aid in weight loss, and help prevent obesity
Polyunsaturated Fatty Acids
Polyunsaturated fatty acids (PUFAs) are categorized into two main groups: omega-3 (n-3) and omega-6 (n-6) fatty acids, based on the location of the first double bond in their carbon chain. Two of these PUFAs are considered essential fatty acids because the human body cannot synthesize them, yet they are crucial for maintaining physiological function. As a result, they must be obtained through the diet. One of these essential fatty acids is linoleic acid (LA), which belongs to the n-6 group, and the other is ?-linolenic acid (LNA), which is part of the n-3 group. These essential fatty acids can be converted in the body into long-chain (LC) fatty acids, but humans are unable to convert n-3 fatty acids into n-6 fatty acids, or vice versa.
Antioxidants
Cellular damage caused by free radicals is thought to play a key role in aging and the development of various diseases. Antioxidants serve as the body's primary defense against this damage, playing an essential role in maintaining overall health and well-being. Oxygen, a highly reactive atom, can form potentially harmful molecules known as free radicals. These free radicals have the ability to attack and damage healthy cells, leading to changes in their structure and function. Antioxidants work by stabilizing or neutralizing free radicals before they can cause harm to cells. They are crucial for preserving cellular and systemic health. Humans have developed a complex and sophisticated antioxidant defense system, which includes a range of components, both produced within the body and obtained from external sources. These components work together interactively and synergistically to neutralize free radicals (Liu LI et al., 2009). The main components of this system include:
- Nutrient-derived antioxidants, such as ascorbic acid (vitamin C), tocopherols and tocotrienols (vitamin E), carotenoids, and other low molecular weight compounds like glutathione and lipoic acid.
- Antioxidant enzymes, including superoxide dismutase, glutathione peroxidase, and glutathione reductase, which catalyze reactions to neutralize free radicals.
- Metal-binding proteins, such as ferritin, lactoferrin, albumin, and ceruloplasmin, which bind to free iron and copper ions, preventing them from catalyzing oxidative reactions.
Antioxidant Phytonutrients
There are numerous other antioxidant phytonutrients found in a wide range of plant-based foods. These include:
Endogenous Antioxidants
- Bilirubin
- Thiols, such as glutathione, lipoic acid, and N-acetyl cysteine
- NADPH and NADH
- Ubiquinone (Coenzyme Q10)
- Uric acid
- Enzymes:
- Copper/zinc and manganese-dependent superoxide dismutase
- Iron-dependent catalase
- Selenium-dependent glutathione peroxidase
Dietary Antioxidants
- Vitamin C
- Vitamin E
- Beta-carotene and other carotenoids, such as lycopene and lutein
- Polyphenols, including flavonoids, flavones, and flavonols
- Proanthocyanidins
Metal-Binding Proteins
- Albumin (binds copper)
- Ceruloplasmin (binds copper)
- Metallothionein (binds copper)
- Ferritin (binds iron)
- Myoglobin (binds iron)
- Transferrin (binds iron)
Polyphenols
- Polyphenols are natural phytochemicals found in plant-based foods like fruits, vegetables, whole grains, cereal, legumes, tea, coffee, wine, and cocoa. There are more than 8,000 polyphenolic compounds, including phenolic acids, flavonoids, and other related compounds [22].
Tilbenes, lignans, and polymeric lignans are compounds found in whole plant foods. These substances are secondary metabolites produced by plants, serving as a defense mechanism against ultraviolet radiation, oxidative stress, and pathogens [23]. Polyphenols can be classified into different categories depending on the number of phenol rings and the structural components that connect these rings together.
Phenolic acids make up about a third of the polyphenolic compounds in the diet and are divided into two main classes:
a) Hydroxybenzoic acid derivatives, such as protocatechuic acid, gallic acid, and p-hydroxybenzoic acid, and
b) Hydroxycinnamic acid derivatives, including caffeic acid, chlorogenic acid, coumaric acid, ferulic acid, and sinapic acid. Foods rich in these phenolic acids include berries, kiwi, cherries, apples, pears, chicory, and coffee.
- Flavonoids are divided into six subclasses: anthocyanins, flavonols, flavanols, flavanones, flavones, and isoflavones. Anthocyanins (such as cyanidin, pelargonidin, delphinidin, and malvidin) are found in foods like berries, red wine, red cabbage, cherries, black grapes, and strawberries [24].
- Spices have been essential in cooking for flavoring food since ancient times. They are aromatic plant-based substances, available in whole, broken, or ground forms, primarily used to season food rather than for their nutritional content. These spices add distinctive flavors, aromas, and pungency to dishes, with volatile oils contributing to the aroma and flavor, while oleoresins provide the pungency.
- In addition to flavoring and seasoning, spices have a wide range of uses in traditional medicine, pharmaceuticals, nutraceuticals, aromatherapy, preservatives, beverages, natural coloring, perfumes, dental products, cosmetics, and as botanical pesticides. They play a significant economic role in the countries that produce them due to the diverse range of chemicals they contain. The growing demand for nutraceuticals is boosting global consumption of Indian spices, as they are now an essential part of the traditional food sector. Non-traditional applications, including nutraceuticals, now account for around 15% of spice production in India.
- One prominent nutraceutical product in India is Chawanprash, which contains spices like cinnamon, clove, turmeric, saffron, and long pepper. These spices are rich in vitamin C and antioxidants, helping to enhance immunity, improve digestion, and prevent conditions like cough, asthma, fever, heart disease, and voice roughness.
- Spices such as turmeric, red pepper, black pepper, clove, ginger, garlic, coriander, rosemary, saffron, and cinnamon have shown beneficial effects in combating neurodegenerative diseases like Alzheimer's, Parkinson's, multiple sclerosis, brain tumors, and meningitis.
The use of herbs and spices for enhancing beauty dates back to ancient times. Spices like turmeric, for example, are commonly used in skincare. The growing demand for anti-aging products and cosmeceuticals in the health and wellness industry has led to increased use of spices like turmeric, cardamom, clove, aniseed, coriander, basil, saffron, garlic, and sage in beauty and cosmetic products. 20113270805
The application of a 0.5% turmeric extract cream helps regulate sebum production in human skin, making it especially beneficial for individuals with oily skin or those suffering from acne. Saffron (Crocus sativus) is known for its complexion-enhancing properties in skincare. Studies have shown that using 0.3% saffron in creams and lotions can result in brighter, shinier skin. This effect is primarily attributed to the crocin and cicrocrocin compounds in saffron, which help regulate melanin biosynthesis in the skin [25].
Nutraceuticals and Their Role in Diseases
- Cardiovascular diseases (CVD):
Nutraceuticals such as antioxidants, dietary fibers, omega-3 polyunsaturated fatty acids, vitamins, and minerals are commonly used in the prevention and treatment of cardiovascular diseases (CVD). Polyphenols found in grapes have been shown to prevent and manage arterial diseases. Flavonoids, present in onions, vegetables, grapes, red wine, apples, and cherries, help block ACE (angiotensin-converting enzyme) and strengthen small capillaries that deliver oxygen and nutrients to cells. Rice bran has been shown to reduce serum cholesterol levels by lowering LDL (low-density lipoprotein) cholesterol and increasing HDL (high-density lipoprotein) cholesterol, which supports cardiovascular health. A higher LDL/HDL ratio increases the risk of coronary heart disease. Additionally, rice bran contains lutein and zeaxanthin, which enhance vision and reduce the likelihood of cataracts. The essential fatty acids—omega-3, omega-6, omega-9—and folic acid in rice bran also contribute to better eye health. Studies indicate that a low intake of fruits and vegetables is linked to higher mortality rates from CVD [26].
Diet-Related Diseases
In Western countries, the prevalence of diet-related diseases has been steadily rising, driven by the widespread availability of calorie-dense foods and increasingly sedentary lifestyles. Obesity, diabetes, atherosclerosis, and neurodegenerative diseases are key diet-related conditions, all of which are linked by a common underlying factor: low-grade inflammation. Functional foods and nutraceuticals offer a promising therapeutic strategy to prevent or mitigate these diseases, primarily due to their ability to trigger anti-inflammatory responses. Specifically, stimulating intestinal T regulatory cells and maintaining balance in the gut microbiota could play a crucial role in reducing the low-grade inflammation that underpins many diet-related health issues.
Heart Attack and Lung Cancer
Corn contributes to heart health not only through its fiber content but also by providing significant amounts of folate. Folate helps regulate homocysteine, an intermediary in the methylation cycle, which is crucial for proper metabolic function. Elevated homocysteine levels can damage blood vessels, increasing the risk of heart attacks, strokes, and peripheral vascular disease. Research suggests that consuming the full recommended daily value of folate could reduce the incidence of heart attacks by 10%. Additionally, corn contains cryptoxanthin, a natural carotenoid, which has been shown to lower the risk of lung cancer by 27% when consumed regularly.
Diabetes
Ethyl esters of omega-3 fatty acids could offer benefits for diabetic patients. Docosahexaenoic acid (DHA) helps in managing insulin resistance and plays a critical role in neurovisual development. Lipoic acid, a potent antioxidant, is also used in the treatment of diabetic neuropathy. Psyllium-derived dietary fibers have been utilized to help regulate blood glucose levels in diabetic patients and reduce lipid levels in those with hyperlipidemia.
Obesity
Obesity is a major global health issue, characterized by the excessive accumulation of body fat. It is a well-recognized risk factor for numerous health conditions, including angina pectoris, congestive heart failure (CHF), hypertension, hyperlipidemia, respiratory disorders, renal vein thrombosis, osteoarthritis, cancer, and infertility. DOI:
Cancer
Flavonoids, which inhibit the enzymes responsible for estrogen production, can help reduce estrogen-induced cancers. To prevent prostate and breast cancer, a variety of phyto-pharmaceuticals with hormonal activity, known as "phytoestrogens," are recommended. Soy foods, which are rich in isoflavones, as well as curcumin from curry and soy isoflavones, have cancer chemopreventive properties. Lycopene, which accumulates in the skin, testes, adrenal glands, and prostate, offers protective benefits against cancer.
Anti-inflammatory Activities
Curcumin, a polyphenol found in turmeric, is known for its anticancer, antioxidant, and anti-inflammatory properties. Beetroot, cucumber, spinach, and turmeric rhizomes have also been reported to have anti-tumor effects. Gamma-linolenic acid, present in green leafy vegetables, nuts, vegetable oils (like evening primrose oil, blackcurrant seed oil, and hemp seed oil), as well as spirulina, has been used to treat inflammatory and autoimmune conditions. Glucosamine and chondroitin sulfate are commonly used for osteoarthritis, helping to regulate gene expression and the synthesis of PGE2. Cat's claw, a potent anti-inflammatory agent, comes from two species: Uncaria guianensis, traditionally used for wound healing, and Uncaria tomentosa, which has numerous medicinal applications and is commonly found in supplements. Cat's claw is rich in phytochemicals, including alkaloids, glycosides, tannins, flavonoids, sterols, and other compounds [27].
Alzheimer’s Disease
Compounds like ?-carotene, curcumin, lutein, lycopene, and turmerin may offer benefits for specific diseases by counteracting the harmful effects of oxidative stress, mitochondrial dysfunction, and various types of neural degeneration.
Parkinson’s Disease
Vitamin E found in food may help protect against Parkinson's disease, as suggested by Canadian research. Creatine has also shown potential in alleviating some features of Parkinson’s disease, as evidenced by a reduction in clinical symptoms (Brower V, 2005). While preliminary studies on nutritional supplements show promising results, there is not enough scientific evidence at this time to recommend them as a treatment for Parkinson's disease. Patients should be aware that over-the-counter medications may have side effects, interact with other drugs, and be costly.
Osteoarthritis
Osteoarthritis (OA) is the most common form of arthritis in the United States, affecting an estimated 21 million people. In 2004, the total healthcare costs related to arthritis were about 86 billion dollars. Joint pain from OA and other joint disorders can limit physical activity, leading to energy imbalances and weight gain. Excess weight can further aggravate joint stress and worsen symptoms (Kaliora AC et al., 2006). Glucosamine (GLN) and chondroitin sulfate (CS) are commonly used to manage OA symptoms. These nutraceuticals have both nutrient and pharmaceutical properties, potentially regulating gene expression and the production of NO and PGE2, which could explain their anti-inflammatory effects.
Adrenal Dysfunction
Some substances have antioxidant and neuroprotective properties, potentially slowing the progression of dementia and supporting adrenal function.
Adaptogens
Adaptogens are natural herbs known for their general, non-specific effects on the body, helping to improve resilience to stressors without disrupting normal physiological functions. Examples of adaptogens include Eleutherococcus senticosus, Ginkgo biloba, Ocimum sanctum, Panax ginseng, Withania somnifera, and the mushroom Cordyceps sinensis.
Ginkgo biloba has been used in traditional Chinese medicine for thousands of years to treat various health issues, including vertigo, short-term memory loss, and lack of focus or attention. Standardized ginkgo extracts have been utilized for these purposes [28].
- Ocimum sanctum (Holy basil or Tulsi) is widely used in Ayurvedic medicine and has demonstrated antistress properties. In a study by Sembulingham et al., rats were exposed to either acute or chronic noise stress, with and without prior treatment with Ocimum. The rats that received Ocimum pretreatment, regardless of whether they were exposed to acute or chronic noise stress, showed significantly lower levels of corticosterone (Sembulingam K et al., 1997). Below is Table-2, which highlights various nutraceuticals and their applications
Table 1. Physiological properties of dietary fibers and proposed health benefits
|
Physiological property
|
Proposed effect
|
|
|
Soluble dietary fiber
|
Delays gastric emptying and prolonging intestinal phase
|
Contribute to safety.
|
|
Prevent or delays nutrients uptake in small intestine
|
Lower blood cholesterol level.
|
|
Prevent the reabsorption of bile acid
|
Prevents breast cancer.
|
|
Prevent the digestive enzymes from reaching lipid substrates, inhibits enzyme activity
|
Lowers glucose, insulin and lipid level after meal.
|
|
Interaction/binding
|
Binding to bile acids
|
Lower blood cholesterol level
|
Table 2. Common nutrients and their associated health benefits.
|
Nutrients
|
Health benefits
|
|
Fat Soluble Vitamins
Vitamin A
|
Antioxidant, essential, for growth and development, maintains healthy vision, skin and mucous membranes, may aid in the prevention and treatment of certain cancers and in the treatment of certain skin disorders
|
|
Vitamin D
|
Essential for formation of bones and teeth, helps the body absorb and use calcium
|
|
Vitamin E
|
Antioxidant, helps form blood cells, muscles, lung and nerve tissue, boosts the immune system
|
|
Vitamin K
|
Essential for blood clotting
|
|
Water Soluble Vitamins
|
Antioxidant, necessary for healthy bones, gums, teeth and skin, helps in wound healing may
|
|
Vitamin C
|
prevent common cold and attenuate its symptom
|
|
Vitamin B1
|
Helps to convert food in to energy, essential in neurologic functions
|
|
Vitamin B2
|
Helps in energy production and other chemical processes in the body, helps maintain healthy eyes, skin and nerve function
|
|
Vitamin B3
|
Helps to convert food in to energy and maintain proper brain function
|
|
Vitamin B6
|
Helps to produce essential proteins and convert protein in to energy
|
|
Vitamin B12
|
Helps to produce the genetic material of cells, helps with formation of red blood cells, maintenance of central nervous system and synthesize amino acids and is involved in metabolism of fats, protein and carbohydrates
|
|
Folic acid
|
Necessary to produce the genetic materials of cells, essential in first three months of pregnancy for preventing birth defects, helps in red blood cell formation, protects against heart disease
|
|
Pantothenic acid
|
Aids in synthesis of cholesterol, steroids and fatty acids, crucial for intra-neuronal synthesis of acetylcholine
|
|
Minerals
Calcium
|
Essential for building bones and teeth and maintaining bone strength, important in nerve,
muscle and glandular functions
|
|
Iron
|
Helps in energy production, helps to carry and transfer oxygen to tissues
|
|
Magnesium
|
Essential for healthy nerve and muscle function and bone formation, may help prevent premenstrual syndrome (PMS)
|
|
Phosphorous
|
Essential for building strong bones and teeth, helps in formation of genetic material, energy production and storage
|
|
Trace elements
Chromium
|
With insulin helps to convert carbohydrates and fats into energy
|
|
Cobalt
|
Essential component of vitamin B12, but ingested cobalt is metabolized in vivo to form the B12coenzymes
|
|
Copper
|
Essential for hemoglobin and collagen production, healthy functioning of the heart, energy production, absorption of iron from digestive tract
|
|
Iodine
|
Essential for proper functioning of the thyroid
|
|
Selenium
|
Antioxidant, essential for healthy functioning of the heart muscle
|
|
Zinc
|
Essential for cell reproduction, normal growth and development in children, wound healing, production of sperm and testosterone
|
|
Vitamin like compounds Biotin
|
Required for various metabolic functions
|
|
L- Carnitine
|
Oxidation of fatty acids, promotion of certain organic acid excretion and enhancement of the rate of oxidative phosphorylation
|
|
Choline
|
Lipotropic agent used to treat fatty liver and disturbed fat metabolism
|
|
Vitamin F
|
Involved in proper development of various membranes and synthesis of prostaglandins, leukotrienes and various hydroxyfatty acids
|
|
Inositol
|
Lipotropic agent necessary for amino acid transport and movement of potassium and sodium
|
|
Taurine
|
Aids in retinal photoreceptor activity, bile acid conjugation, white blood cell antioxidant activity, CNS neuromodulation, platelet aggregation, cardiac contractility, sperm motility, growth and insulin activity
|
Table 3. Nutraceuticals and their uses
|
Chemical constituents
|
Source
|
Uses
|
|
Carotenoids
|
|
Lycopene
|
Guava, papaya, water melon,
Tomatoes, pink colored grape fruit.
|
They reduces cholesterol levels, antioxidants, protects against cancer
|
|
?-Carotene
|
Vegetables, fruits, oats, Carrots..
|
Antioxidants, protection of cornea against UV light
|
|
Lutein
|
Spinach, corn, avocado, egg yolk
|
Protect eyes against age related muscular degenerations, cataracts, anticancer activity(colon)
|
|
Tocotrienol
|
Palm oil, different grains
|
Improves cardio vascular health, fight against cancer (breast cancer)
|
|
Saponins
|
Beans like soya beans, chickpeas
|
Very effective against colon cancer, reduces cholesterol level
|
|
Polyphenolic Compounds
|
|
Flavonones
|
All citrus fruits
|
Different types of anti-oxidant and anticancer activity
|
|
Flavone
|
Different types of fruits, soya beans, vegetables
|
Different types of anti-oxidant and anti-cancer activity
|
|
Flavonols
|
Broccoli, tea, onions, fruits like apple
|
Antioxidant activity
|
|
Curcumin
|
Turmeric root
|
Strongly anti-inflammatory and strongly antioxidant ,effective anti-clotting agent
|
|
Glucosinolates
|
Cauliflower, cruciferous vegetables
|
Anticancer activity, protect against bladder cancer
|
|
Phytoestrogens
|
|
Isoflavones
|
Legumes, beans like soy beans
|
It Lowers LDL cholesterol, antioxidants, protects against prostate, breast, bowel and other cancers
|
|
Lignans
|
Vegetables, rye and flaxseed
|
Protect against development of cancer like colon and breast cancer
|
|
Dietary fibre
|
|
Soluble fibre
|
Beans like Legumes, cereals like oats,barley, some fibrous fruits
|
They help in maintenance of a healthy digestive tract & have anticancer activity
|
|
Insoluble fibre
|
whole grain foods wheat and cornbran, nuts
|
They help in maintenance of a healthy digestive tract, and have Anticancer (colon) activity
|
|
Sulphides/Thiols
|
Present in Cruciferous vegetables
|
Help in maintenance of healthy immune function
|
|
Fatty acids
|
|
Omega 3 fatty acids
|
Present in salmon and flax seed
|
They are the Potent controllers of the inflammatory processes, help in Maintenance of brain function & Reduce cholesterol disposition.
|
|
Monosaturated fatty acids
|
Present in tree nuts
|
Reduce the risk of coronary heart disease
|
|
Prebiotics/Probiotics
|
Lactobacilli, bifidobacteria present in yogurt, other dairy and nondairy applications
|
They help to improve gastrointestinal health and systematic immunity
|
|
Minerals like zinc, calcium, selenium, copper, potassium
|
Food
|
They are the important constituents of balanced diet
|
|
Polyols sugar alcohols
(xylitol, sorbitol)
|
Present in foods
|
They may reduce the risk of dental caries(cavities)
|
Types of delivery system
Introduction
A vast amount of evidence demonstrates the health benefits and significance of nutraceuticals in both the treatment and prevention of diseases, as well as their crucial role in supporting physiological functions [29] .Evidence supports the use of natural compounds because of their distinctive ability to enhance the body's inherent healing processes [30]. Nutraceuticals are not only used in preventive medicine but are also commonly prescribed and recommended as complementary or alternative treatments for chronic diseases. Some researchers have found promising preliminary data that supports the inclusion of nutraceuticals in clinical practice, recognizing them as powerful adjuncts. Nutraceuticals are not only used in preventive medicine but are also commonly prescribed and recommended as complementary or alternative treatments for chronic diseases. Some researchers have found promising preliminary data that supports the inclusion of nutraceuticals in clinical practice, recognizing them as powerful adjuncts [31]. Supplementing with essential nutrients is an invaluable treatment strategy, as these substances can have a protective impact on the body by influencing the progression of various diseases and preventing conditions before they arise. It has been suggested that simple dietary changes can prevent the development of 35% of all cancers, and the intake of antioxidants has been associated with neuroprotective effects in both in vitro and in vivo studies [32]. Different nutritional strategies have been explored to reduce a patient's disease risk by supplementing with essential nutrients. Dietary proteins like soy, once thought to have no biological effects in humans, have gained attention for their potential to lower serum cholesterol, blood pressure, and body weight. Additionally, peptides found in milk and plant-based proteins have introduced a new approach to treating hypertension, showing effectiveness comparable to Angiotensin I converting enzyme (ACE) inhibitors, but without the associated side effects [33].
Consuming diets high in fish oil, which is a source of vitamin D and omega-3 fatty acids, has been shown to reduce the incidence of Multiple Sclerosis [34]. Procyanidins from grape seed extract have been effective in reducing cholesterol, pancreatitis, vomiting, and pain [35]. Extracts of bitter melon and cinnamon have been shown to be effective in treating diabetes (Andlauer & Furst, 2002). Initial studies by researchers found that higher blood levels of vitamin E following supplementation were linked to a lower risk of progression to full-blown AIDS in HIV-positive patients [36]. The authors of the EuroSIDA study found that a deficiency in 25-hydroxyvitamin D in HIV-positive patients undergoing antiretroviral (ARV) treatment was independently linked to an increased risk of mortality and AIDS-related events. Therefore, supplementation with these essential micronutrients is crucial for maintaining optimal physiological function and should not be overlooked. It has become evident that traditional pharmacological treatments often fall short when addressing chronic conditions. As a result, further research is needed to identify alternative nutraceutical agents that are both more effective and safer, while also being versatile enough to treat a wider range of patients. Supporting this view, some researchers have pointed out that conventional antidepressants are linked to various side effects, have delayed onset, require high doses for effectiveness, and are successful in treating only 40-60% of patients with Obsessive Compulsive Disorder [37]. In contrast, many nutraceuticals work in a manner similar to antidepressants, offering potential as alternative monotherapies or as supplementary treatments that are more tolerable, with comparable or even enhanced efficacy. In cancer patients, tumorigenesis is a complex process, and the use of multiple agents is widely accepted, despite the significant risk of toxicity to the patient. The question that arises is whether medical research should accept this risk or explore further the untapped benefits that nutraceutical molecules might provide. Some researchers have emphasized that certain nutraceuticals, unlike conventional chemotherapeutic drugs, exhibit multitargeted actions on the cell cycle, with the added advantage of being more affordable and, therefore, more accessible to patients. Therefore, more research should focus on natural agents and active compounds for treating life-threatening conditions.
In recent years, one of the main areas of focus has been optimizing dosage forms and formulations to enhance the bioavailability of promising natural candidates [38].
Many forward-thinking experts suggest that future research should focus on developing co-drugs that combine nutraceuticals with drugs to enhance efficacy, reduce dosage, and significantly alleviate side effects. Most advocates agree that using multiple synergistic nutrients offers a valuable multi-layered approach for clinicians. Some have recommended combining soy derivatives with high doses of the vitamin D3 metabolite, 1.25 dihydroxyvitamin D (calcitriol), as they can help inhibit the metabolic breakdown of calcitriol [39].
- A similar challenge lies in developing formulation strategies that enhance treatment outcomes and disease prevention. Understanding innovative delivery systems and combining various technologies can lead to more advanced formulations. Many bioactive nutraceuticals and their formulation challenges have been recognized and subsequently modified to improve their pharmacokinetic and therapeutic properties when administered to patients. For example, peanuts, which possess a broad range of biological activities, are rapidly metabolized in the liver and intestine, leading to very low oral bioavailability [40].
- Although many nutraceuticals have low inherent oral bioavailability, consumers strongly prefer this method of administration, prompting scientists to focus on improving the delivery mechanisms. Innovative technologies and formulation strategies are crucial to the success of orally administered nutraceuticals, as they help protect these compounds from rapid elimination and degradation while improving solubility and absorption through gastrointestinal tract membranes.
Routes of nutaceuticls
Oral
Oral delivery of nutraceuticals is regarded as the most preferred and acceptable method, as it aligns with the body's natural process of food and nutrient consumption, is non-invasive, and doesn't require special techniques or complex instructions. However, this route can be affected by dietary factors that may either enhance or hinder the bioavailability of nutraceuticals. For instance, lutein absorption significantly increases when consumed with a high-fat meal, and the absorption of lycopene improves when taken alongside beta-carotene [41].
Fat-soluble vitamins are typically absorbed more efficiently when taken with a meal, while fiber can interfere with the oral absorption of certain antioxidants. Nutraceuticals delivered orally include glucosamine, chondroitin, lycopene, resveratrol, coenzyme-Q10, creatine, melatonin, green tea extract, acetyl-L-carnitine, S-adenosylmethionine (SAMe), lipoic acid, dehydroepiandrosterone (DHEA), as well as both water- and fat-soluble vitamins, among others. Despite the claimed benefits of nutraceuticals, many have been found to have low oral bioavailability, with studies showing significant variations in serum levels and inconsistent pharmacokinetics following oral administration. These inconsistencies arise from the harsh conditions in the gastrointestinal tract that the bioactive compounds encounter, including low stomach pH, digestive and metabolic enzymes, and the alkaline pH in the intestines. For instance, resveratrol, a polyphenol found in grapes and peanuts, has a broad range of biological activities but is rapidly metabolized in the liver and intestine, leading to extremely low oral bioavailability [42].
Dermal delivery
Consumers are increasingly focused on quick results and expect skin products to be as "natural" and "organic" as possible, while still offering an all-in-one, innovative solution to meet their health and beauty needs [43]. The antioxidant effects of nutraceutical-derived bioactives may be amplified by applying them both orally and dermally, as seen with lutein [44].
- While oral dosing typically results in higher and more consistent plasma levels, dermal application can lead to the accumulation of nutraceuticals in the skin. This superior reservoir effect, seen after dermal application, is beneficial for long-term storage and the continuous release of nutraceuticals to the body, even after treatment has stopped, due to the skin's unique buffering properties [45].
- The long-term use of dermal products containing nutraceuticals like Coenzyme Q10 (CoQ10) and vitamin C may lead to visible clinical improvements, such as a reduction in wrinkle depth in aging skin [46].
- It is no surprise that an increasing number of patients are seeking treatments to slow down and reverse age-related skin changes, leading to greater demand for the development of natural dermal products. Topically applied nutraceuticals that offer positive cosmetic benefits include coenzyme Q10, genistein, curcumin, N-acetylcysteine, gluconolactone, and fucose-rich sulfated polysaccharides.
The dermal route is a preferred alternative to oral administration as it bypasses the gastrointestinal tract, reducing hepatic and renal inactivation. However, some nutraceuticals may become unstable when applied topically due to exposure to light or heat, leading to photodegradation. Along with modified carrier systems for nutraceutical formulations, several delivery aid mechanisms, such as chemical penetration enhancers, iontophoresis, and sonophoresis, can be used locally to help transport active ingredients into the skin and underlying tissues.
Ophthalmic delivery
Consumers typically prefer and accept nutraceuticals in oral form. However, the optimal method for delivering nutraceuticals in ocular therapy remains topical instillation into the eye. Many nutraceuticals, such as coenzyme Q10, vitamin E, and lutein, when applied ophthalmically, can modify eye diseases due to their antioxidant, anti-inflammatory, and anticataract properties. The success of intraocular treatments depends largely on the residence time and permeability of the topical drop or ointment. However, this success is often hindered by the body's defense mechanisms, which make it challenging to maintain an effective drug concentration at the target site, resulting in low bioavailability of the instilled active compound [47].
The straightforward addition of specific nutraceutical agents, administered locally alongside allopathic treatments, may increase intraocular retention time, offer synergistic clinical benefits, and serve as safer alternatives for long-term ophthalmic therapy.
Liposomal carrier system
Liposomes are spherical microscopic lipid vesicles, typically made from phospholipids, that encapsulate a small amount of the solvent in which they are formed. These tiny particles have been used to modify the pharmacokinetics of various nutraceuticals, including vitamins, enzymes, herbs, and minerals. Key factors influencing their delivery effectiveness include vesicle size, surface charge, lipid concentration, and the composition of nutraceuticals within the liposome. Liposomes have proven to be an excellent delivery system for cosmetics and for treating and preventing skin conditions. The phospholipid membrane of liposomes plays a crucial role in transporting active agents across the stratum corneum, enhancing dermal delivery and the deposition of vitamins into the skin [48]. These systems owe their drug delivery benefits to the similarity between liposomal membranes and biological membranes. This characteristic allows formulations to bypass the skin barrier and helps protect sensitive nutraceuticals from UV damage. Research has shown that the lipid-to-drug ratio, the amount of phospholipid, and the quantity of stabilizer used affect the vesicle size and the deposition of vitamin E acetate in rat skin. In this study, liposomal dermal formulations demonstrated up to a sevenfold greater drug deposition compared to control formulations, highlighting their advantages for delivering nutraceuticals to the skin. Additionally, other researchers developed a surfactant-free liposomal formulation to encapsulate coenzyme Q10 (CoQ10). They used the solvent injection method to create liposomal vesicles smaller than 200 nm, which were found to enhance CoQ10 penetration through the stratum corneum. In addition to dermal formulations, liposomal technology has also been utilized for oral and parenteral administration. Resveratrol, a polyphenol known for its anti-inflammatory, antioxidant, cardioprotective, and cancer-preventive properties, has particularly benefited from liposomal delivery due to its low bioavailability and stability. Incorporating resveratrol into liposomal carrier systems has improved its stability, biological activity, and efficacy, while also enhancing its side-effect profile, enabling oral and intravenous formulations of the compound [49].
Electrospun fiber mats
Researchers have developed electrospun fiber mats for delivering various nutraceuticals. Cellulose acetate (CA) nanofiber mats have been electrospun to serve as carriers for dermal vitamin delivery. When vitamin E (as ?-tocopherol) and vitamin A (as all-trans-retinoic acid) were loaded into the electrospun fiber mats, they demonstrated a gradual increase in the cumulative release of the vitamins over time. Another group created ultra-fine electrospun mats using the same biopolymer (CA) for delivering the popular nutraceutical curcumin. Their study found that the system was non-toxic and effectively delivered curcumin to the skin, offering potential as a topical or transdermal dressing with antioxidant, anti-inflammatory, and anti-tumor properties. The same team also synthesized CA fiber mats containing asiaticoside, the most active compound in Centella asiatica L, known for its wound-healing properties[50].
The ‘herb-loaded’ CA fiber mats, tested for dermal release, achieved a maximum release of 26% of asiaticoside through various pigskin methods. Cytotoxicity tests showed no harmful substances being released to the skin, supporting the potential of these formulations as transdermal wound dressing patches. A vitamin C derivative, ascorbyl palmitate (AP), was incorporated into nanofibrous mats made from poly (?-caprolactone) (PCL) and coated with silver nanoparticles in a solution. The study demonstrated that the stability of vitamin C was enhanced after four months of storage, confirming the success of this drug delivery system (DDS). Furthermore, the presence of AP facilitated the deposition of silver ions onto the mats after immersion in silver nitrate aqueous solutions, enhancing the nutraceutical composition of the delivery system.
Cyclodextrin complexation
Cyclodextrins (CD) and their derivatives are commonly used as carrier compounds to enhance the solubility, stability, permeation, and bioavailability of nutraceuticals in the body. Many vitamins can form complexes with these "cage-like" molecules, which improves their physicochemical properties when interacting with biological membranes. ?-CD is the most widely used cyclodextrin due to its ideal cavity size. 7-Dehydrocholesterol (7-DHC), a precursor vitamin commonly used in cosmetics and pharmaceuticals, is nearly insoluble in water, making it difficult to incorporate into dermal products. Researchers have confirmed the formation of an inclusion complex between hydroxypropyl-?-CD and 7-DHC, which showed improved solubility compared to uncomplexed 7-DHC. Silymarin, a liver-protective nutraceutical, has very low water solubility and bioavailability, requiring innovative formulation strategies for effective oral delivery. When silymarin was combined with ?-CD through a co-precipitation method, the resulting complexes showed improved dissolution rates compared to the nutraceutical alone, as well as a more sustained release profile. GO has strong antioxidant and antimicrobial properties, but its use as a functional food ingredient is hindered by its inherent volatility and poor physicochemical stability. Differential scanning calorimetry (DSC) studies confirmed that the nutraceutical was successfully encapsulated within the CD cavity, protecting it from oxidation and resulting in improved stability of the complex. The GO/?-CD complex enhanced the aqueous solubility of GO and achieved a controlled release rate, as shown by in vitro dissolution tests. Research involving vitamin A loaded in hydroxypropyl-?-CD (HPCD) has shown that cyclodextrins can increase the solubility of a vitamin by as much as 35,000 times [51]. Curcumin is a promising anticancer and antiviral nutraceutical, but its use is limited by its near insolubility in water at neutral and acidic pH levels, as well as its high decomposition at alkaline pH. Some researchers have significantly improved the stability of curcumin under basic conditions and achieved a notable increase in solubility by forming inclusion complexes with cyclodextrins (CD). Other studies have employed 2-hydroxypropyl-?-CD complexation to enhance the oral bioavailability and permeation of curcumin. The extract of Kaempferia parviflora (KP), which contains methoxy flavones with antimicrobial, anti-inflammatory, and anti-allergic properties, also benefited from CD complexation. The KP-2-HP-?-CD formulation demonstrated 3.5 times greater permeation ability and 21–34 times higher bioavailability compared to a standard extract formulation. These findings are continually contributing to the development of oral nutraceutical agents containing CD to improve gastrointestinal (GIT) delivery and stability within the harsh GIT environment [52].
Biodegradable hydrogels
Biodegradable hydrogels have been extensively studied for their ability to carry, protect, and regulate the delivery of various pharmaceutical compounds, including nutraceuticals. Many pharmaceutical polymers are stimuli-responsive, meaning they can degrade or swell depending on changes in the physiological environment, such as variations in temperature, pH, or hydration. For most nutrients, absorption predominantly occurs in the small intestine, and hydrogels can aid in this process by shielding nutraceuticals from degradation or denaturation and ensuring their controlled release. Vitamins, being thermo-labile, are particularly well-suited for inclusion in hydrogels, as the gels offer protection and facilitate targeted absorption in the intestine. In one study, riboflavin was incorporated into soy protein cold-set hydrogels, which effectively protected the vitamin for at least 6 hours from gastric conditions and allowed for pH-dependent release in the intestine [53]. Soy-based gel carriers offer additional benefits, such as enhanced nutritional value from the soy itself, a reduced need for inorganic solvents that could be incompatible with food supplements, and the advantage that the bioactive compounds are not exposed to heat during the gelation process, which could otherwise denature sensitive bioactives. These hydrogel matrices could also be applied to deliver other nutraceutical products. Similarly, milk proteins have demonstrated significant success as nutrition-based hydrogel carriers, due to their optimal structural and physicochemical properties that facilitate the transport of bioactives through the gastrointestinal tract. Milk proteins are cost-effective, widely available, and non-toxic, making them excellent delivery vehicles for transporting a variety of drugs through the gastrointestinal tract. These proteins offer exceptional versatility in terms of the types and number of interactions, functionalities, and bonds they can form with different bioactives, as illustrated. This wide range of possible associations makes many nutraceutical compounds ideal candidates for complexation with milk proteins. Similar to soy proteins, milk proteins also possess favorable gelation properties that help protect bioactives and demonstrate advantageous swelling behavior. Furthermore, these proteins have a superior buffering capacity, providing enhanced protection for sensitive nutraceutical bioactives from acidic environments.
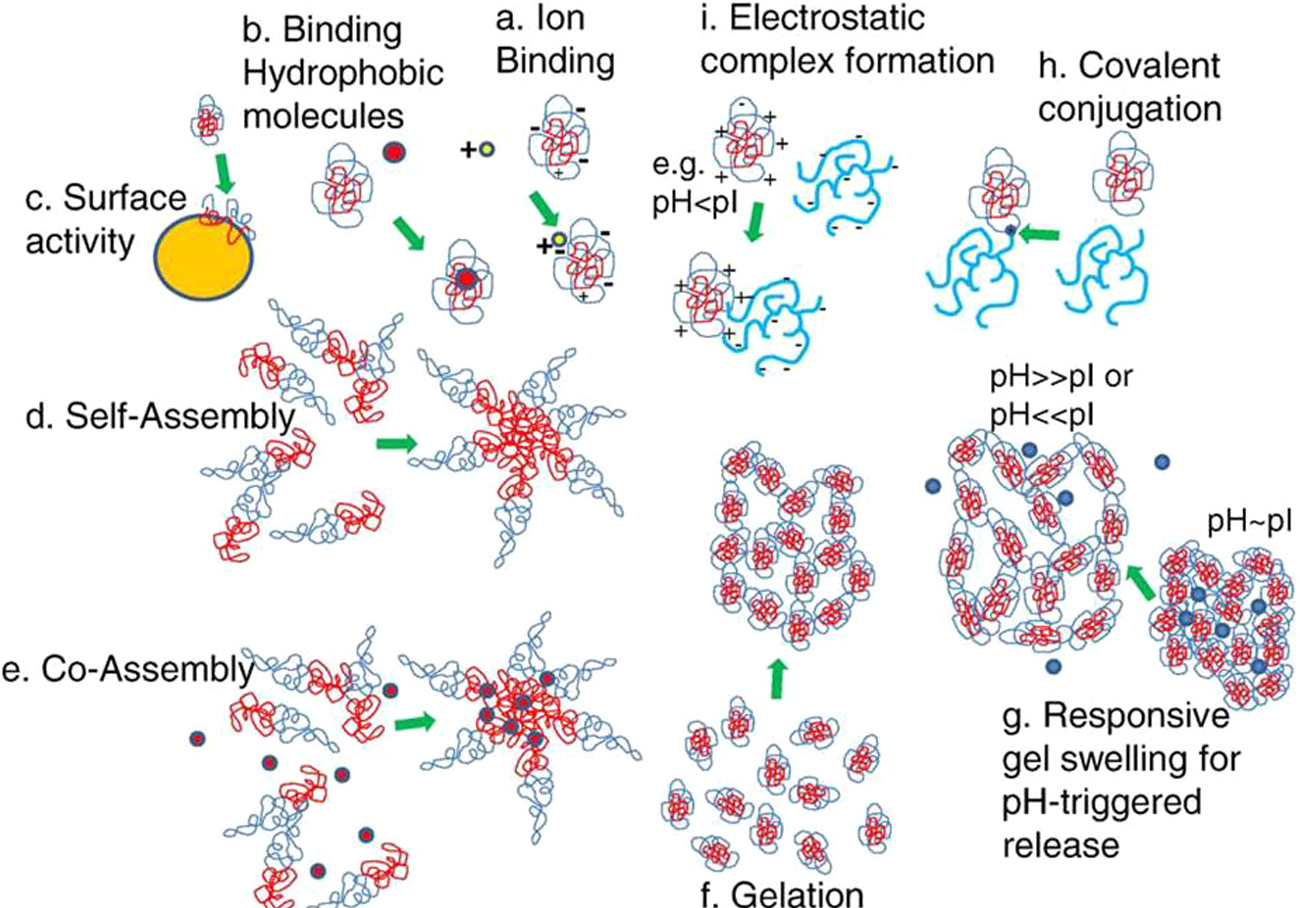
Nanotechnology-based applications for nutraceutical delivery
Nanotechnology has frequently been employed to improve the solubility of poorly soluble nutraceuticals, allowing for their incorporation into various dosage forms. It is also known to enhance bioavailability and enable targeted delivery of bioactives. The application of nanoparticle technology is believed to boost the commercial potential of numerous nutraceuticals.
Nanosuspensions and nanoemulsions
The key factors influencing bioavailability and penetration of a nutraceutical are dissolution velocity and saturation solubility, which are critical for oral and dermal delivery, respectively. By preparing nanosuspensions and nanoemulsions of hydrophobic nutraceuticals with poor solubility, both of these factors can be improved. In one study, researchers used high-pressure homogenization to prepare lutein, a widely used antioxidant, in nanosuspensions with particles around 400 nm in size. The nanosuspension was then lyophilized and incorporated into creams and gels for dermal application, as well as into pellets for oral dosing in hard gelatin capsules. This nanoformulation enhanced penetration due to its improved solubility and larger surface area. The permeation of the lutein nanocrystal formulation across a 0.1 µm synthetic cellulose nitrate membrane was 14 times greater than that of coarse lutein powder. For the oral formulation, the dissolution profiles showed clear improvements in both dissolution and bioavailability.
- In a recent study, the hydrophobic phytochemical 5-hydroxy-6,7,8,4-tetramethoxyflavone (PMF), extracted from sweet orange peels, was incorporated into various oil-in-water nanoemulsions to effectively deliver the compound in dietary supplements [54].
Nanostructured lipid carriers (NLC)
This new generation of lipid "nano-sized" structures, which consist of a lipid matrix with unique nanostructures, offers great potential for optimal nutraceutical delivery through dermal, oral, and topical routes. Nanostructured lipid carriers (NLCs) made from cetyl palmitate and caprylic/capric triacylglycerols were developed to deliver the nutraceutical CoQ10 more effectively to the skin.
The formulated NLCs exhibited excellent physical stability, high entrapment efficiency, and a biphasic release profile for the incorporated CoQ10. The biphasic release pattern was particularly effective, as it initially allowed for rapid CoQ10 saturation of the skin, followed by a controlled and prolonged release phase, ensuring a continuous supply of the vitamin. Scanning electron microscopy (SEM) analysis showed that the particles had an anisometric shape and a size of around 200 nm. Additionally, it was demonstrated that CoQ10-loaded NLC dispersions maintained good long-term physical and chemical stability. PMCID: PMC9367884 After being stored at various temperatures (4°C, 25°C, and 40°C), the entrapped CoQ10 in the NLC carrier system remained above 90% and within the nanosize range for 12 months across all temperature conditions. This further highlights the enhanced stability and robustness of NLCs as an effective dermal delivery system for nutraceuticals [55]. In another study, researchers developed optimized NLCs of lutein for oral delivery, which successfully protected the encapsulated nutraceutical from simulated gastric fluid and provided a sustained release. Similarly, other teams created fish oil-based NLCs for lutein delivery, aiming to develop a drug delivery system (DDS) that not only improved delivery but also offered additional biological benefits due to the omega-3 fatty acid content.
Nanomicelles
- Researchers have recognized the potential of nanovehicles for delivering nutraceuticals. Quercetin, a phytochemical with promising properties, has shown potential for treating neurological diseases, cardiovascular conditions, and cancer, and has even progressed to clinical trial evaluations. However, its poor water solubility has hindered its broader commercialization. To address this, researchers have successfully developed a self-assembled nanomicellar delivery system using the diblock copolymer polyethylene glycol (PEG)-modified phosphatidylethanolamine (PE), which was loaded with quercetin [56].
The quercetin nanomicelles were found to be stable across a pH range from 1.2 to 7.0, demonstrating relatively low toxicity and good tolerance in pre-clinical in vivo studies. The improved solubility offered by these nanomicelles led to a significant boost in the anticancer efficacy of quercetin, with a notable increase in activity at a dose of 30 mg/kg in mice, compared to the control ethanol suspension of quercetin. Similarly, other researchers have employed casein micelles to nanoencapsulate vitamin D2, providing protection against UV degradation [57].
Nanoparticles, nanocapsules and nano-encapsulation.
Many phytonutrients, although proven to provide significant health benefits, face challenges as nutraceutical additives due to their vulnerability to degradation or poor solubility in water. This instability arises when these compounds are exposed to changes in temperature, pH, or oxygen levels. One such antioxidant is epigallocatechin-3-gallate (EGCG), found in green tea, which is widely recognized for its neuroprotective, anti-cancer, and cardiovascular benefits. However, EGCG rapidly degrades in soft drinks, resulting in the formation of degradation products that discolor the beverage and reduce the health benefits of the remaining compound, as most of its activity is lost over time. To address this, co-assembled nanovehicles have been developed to protect and enhance the delivery of EGCG and other antioxidant polyphenols. In one study, researchers used thermally modified ?-lactoglobulin (blg) to form an optimal complex with EGCG, resulting in nano-sized blg-EGCG particles that provided strong protection against oxidation. These particles, which were less than 50 nm in size, also offered ideal transparency, making them suitable for incorporation into clear beverages. Additionally, milk proteins, such as casein, are being explored as natural self-assemblers and co-assemblers to nanoencapsulate various nutraceuticals, including ?-carotene, fatty acids, and vitamin D [58]. The EO-zein nanoparticles resulted in a 14-fold increase in solubility while preserving their antioxidant and antimicrobial properties. Research has also focused on enhancing the stability and delivery of water-soluble vitamins through the development of advanced, miniature delivery systems. One study explored the creation of sodium tripolyphosphate (STPP)-chitosan/vitamin C nanoparticles, aiming to improve the shelf-life and effectiveness of vitamin C delivery [59]. In vivo studies revealed that the mucoadhesive properties of chitosan extended the release time of vitamin C to 12 hours, compared to just 3–4 hours in the control group. The release of vitamin C was also pH-dependent, with a faster release observed in PBS compared to a lower pH environment. Another notable study involved the preparation of solid lipid nanoparticles (SLNs) with the addition of a surfactant. Originally developed for parenteral delivery, this technology has since been adapted for use in dermal formulations. Transdermal delivery of melatonin using SLNs has been shown to result in sustained plasma levels [60].
Solid dispersions
Solid dispersions (SD) represent another strategy used to improve the bioavailability of oral nutraceuticals. In amorphous solid dispersions, a bioactive is molecularly dispersed within a hydrophilic polymer matrix, leading to enhanced solubility and a faster dissolution rate of the active compound. Poorly water-soluble nutraceuticals typically have slow dissolution rates and suboptimal bioavailability when administered via the gastrointestinal tract. Research has focused on developing stable solid dispersions of CoQ10 using the carrier polymer poloxamer 407, along with an adsorbent and recrystallization inhibitor, Aerosil 200. These components were combined in various ratios using the melting method, with the optimal formulation achieved at a 1:5:6 weight ratio [61].
- Dissolution profiles indicated that pure CoQ10 was nearly insoluble, while a physical mixture of CoQ10 and poloxamer 407 showed only a 5% dissolution rate. In contrast, the optimized solid dispersion (SD) formulation with a 1:5 ratio of CoQ10 to poloxamer 407 achieved around 85% dissolution. Additionally, the solubility of CoQ10 was found to improve as the amount of poloxamer 407 increased. The optimized SD formulation exhibited a solubility four times higher than the corresponding physical mixture. Notably, the SD dissolved completely within 15 minutes, and its aqueous solubility was significantly greater than that of the physical mixture. In a related study, the phytochemical resveratrol was combined with various grades of polymers to create solid dispersions, which showed differing types and strengths of interaction between the polymer and the active compound [62].
Self-emulsifying drug delivery systems (SEDDS)
Novel self-emulsifying drug delivery systems (SEDDSs) have been developed and reported by various research teams as a way to improve the oral bioavailability of nutraceuticals. One such advancement, the development of a solid-SEDDS for CoQ10, was found to enhance oral bioavailability by five times [63]. This outcome was the result of the rapid self-emulsification and dispersion of the system in an aqueous medium, which significantly accelerated the dissolution process. Tocotrienols, which belong to the vitamin E family, are known for their various health benefits and are therefore a key area of interest for enhanced formulation. The use of SEDDS (Self-Emulsifying Drug Delivery Systems) to improve the bioavailability of vitamin E following oral administration has been explored [64].
The researchers discovered that when vitamin E was delivered using SEDDS, it resulted in a 2.5–4.5 times greater Cmax, a higher AUC, and a reduced lag time for absorption compared to the non-SEDDS formulation. Recent studies also supported the use of SEDDS, along with high-fat meals, as effective strategies to improve the bioavailability of tocotrienols [65]. Similarly, other researchers developed self-emulsifying pellets of the milk thistle extract, silymarin, using extrusion/spheronization technology. The results showed that the SEDDS released 99% of silymarin within 7 minutes, in contrast to just 13.9% release from the comparator milk thistle dry herbal extract [66]. A self-nanoemulsifying drug delivery system (SNEDDS) was developed using persimmon leaf essential oil (EO), which is known for its antimicrobial, antifungal, and insecticidal properties, encapsulated in zein nanoparticles. These EO-zein nanoparticles demonstrated up to a 14-fold increase in solubility while retaining their antioxidant and antimicrobial effects. Additionally, research has focused on improving the stability and delivery of water-soluble vitamins by creating advanced, miniature delivery vehicles. For example, studies have explored the formulation of sodium tripolyphosphate (STPP)-chitosan/vitamin C nanoparticles to enhance both the shelf-life and delivery efficiency of vitamin C [67].
Microparticulate systems: microparticles, microspheres and microcapsules
Many herbal nutraceuticals possess beneficial antioxidant and antimicrobial properties. Herbs like Fadogia ancylantha (Makoni tea), Melissa officinalis (lemon balm), and Tussilago farfara (coltsfoot) are popular in the nutraceutical market due to their high antioxidant polyphenol content and have been used in traditional beverages. However, these herbs are often found in the form of poorly water-soluble, sticky extracts that have an unpleasant odor and are prone to degradation during storage. In addition to using nanoparticulate complexation and encapsulation methods to protect the polyphenols, researchers have explored alternative strategies to deliver these sensitive, polyphenol-rich extracts to the body. One such innovation involves the creation of a maltodextrin/pectin (M/P) matrix microparticle powder, developed through a spray-drying process.This approach resulted in the encapsulation of the extracts, producing stable powders made up of uniform micronized particles. These powders retained their activity and showed an improved shelf-life during stability testing. Additionally, the M/P matrix effectively masked the unpleasant odor of the extracts and significantly improved their water solubility [68].
Researchers developed microcapsules of lycopene using a spray-drying technique and modified starch (Capsul®). The resulting microcapsules underwent stability testing and were found to offer better protection to the nutraceutical compared to its unencapsulated form. These systems could prove valuable in the production of functional foods and nutraceutical supplements that have challenging physicochemical properties and taste issues. Microspheres are commonly used in cosmetics to prevent incompatibilities between formulation ingredients and to safeguard actives from environmental degradation. For instance, the chemical stability of nylon microspheres makes them ideal for encapsulating both lipophilic and hydrophilic actives, such as vitamin E and ascorbic acid [69]. The addition of vitamin A enhanced the loading efficiency of acyclovir in poly (D, L-lactic-co-glycolic) acid microspheres and improved the release profile of acyclovir during the initial days of the in vitro test. The inclusion of vitamin A in the ophthalmic formulation was justified by the vitamin's antiviral properties and its ability to mitigate the risks associated with intravitreal injections, given its effectiveness in treating vitreoretinal diseases. The microspheres exhibited a consistent release of both acyclovir and vitamin A palmitate over a 49-day period. This approach highlights a beneficial synergistic effect between a nutraceutical agent and a synthetic drug, offering a safer and more effective treatment option compared to current therapies [70].
Particle coatings for protection, site-specific delivery and enhanced efficacy
- Certain delivery systems need to integrate multiple technologies to enhance their effectiveness. An innovative approach for orally delivering nutraceuticals that are sensitive to the stomach environment involved the creation of Chitosan/?-lactoglobulin (CS-blg) core-shell nanoparticles.
Chitosan, while possessing ideal characteristics as a nutraceutical carrier—such as being biodegradable, biocompatible, mucoadhesive, and versatile for health applications—lacks stability at low pH and rapidly degrades, potentially leading to premature destruction of bioactive compounds. To safeguard both chitosan and the encapsulated nutraceuticals from the acidic conditions of the stomach and pepsin activity, the chitosan nanoparticles were coated with ?-lactoglobulin, a protein known for its strong resistance to pepsin degradation. This carrier system successfully passed through the stomach and reached the intestine:[71]. The researchers created lycopene microcapsules using a spray drying technique with a modified starch (Capsul™). These microcapsules were tested for stability and were found to offer better protection to the nutraceutical compared to its unencapsulated form. Such delivery systems could be valuable in the production of functional foods and nutraceutical supplements that face challenges related to physicochemical properties and taste. Microspheres are commonly used in cosmetics to prevent incompatibility between ingredients and to shield active compounds from environmental degradation. For instance, the chemical stability of nylon microspheres makes them ideal for encapsulating both lipophilic and hydrophilic actives, like vitamin E and ascorbic acid [72]. The addition of vitamin A enhanced the loading efficiency of acyclovir in poly(D,L-lactic-co-glycolic) acid microspheres and improved the acyclovir release profile during the initial days of the in vitro study. The rationale for incorporating vitamin A into the ophthalmic formulation was based on its antiviral properties and its ability to mitigate the inherent risks of intravitreal injections, given vitamin A’s effectiveness in treating vitreoretinal diseases. The microspheres demonstrated a sustained release of both acyclovir and vitamin A palmitate over a 49-day period. This approach showcases an optimal synergistic interaction between a nutraceutical agent and a synthetic drug, offering a safer and more effective treatment option compared to existing therapies.
Particle coatings for protection, site-specific delivery and enhanced efficacy
Certain delivery systems need the integration of multiple technologies to enhance or ensure their effectiveness. An innovative approach for the oral delivery of gastric-sensitive nutraceuticals was the development of Chitosan/?-lactoglobulin (CS-blg) core-shell nanoparticles [73].
- Chitosan possesses ideal properties for use as a nutraceutical carrier—such as being biodegradable, biocompatible, mucoadhesive, and versatile in health applications—yet it is unstable at low pH and degrades quickly, which can lead to the premature breakdown of bioactive compounds. To protect both chitosan and the encapsulated nutraceuticals from the acidic conditions of the stomach and the action of pepsin, the chitosan nanoparticles were coated with ?-lactoglobulin, a protein known for its strong resistance to pepsin degradation. This carrier system successfully passed through the stomach and reached the intestine intact, where pancreatin degraded the ?-lactoglobulin shell, releasing the chitosan nanoparticles. These nanoparticles were then able to enhance the muco-adhesiveness and absorption properties of the entrapped nutraceuticals. This system effectively combined the benefits of two carriers: the polymer chitosan and the milk protein ?-lactoglobulin. In a related development, researchers introduced a novel technology for delivering CoQ10 effectively to the eye as a therapeutic agent for treating cataracts [74].
- The delivery system developed by the researchers consisted of CoQ10-loaded soy phosphatidylcholine (SPC) liposomes, which were coated with trimethyl chitosan (TMC) of varying molecular weights. Liposomes are commonly used in ophthalmic formulations due to their ability to serve as excellent drug reservoirs without affecting vision. However, the additional TMC coating enhanced absorption and improved the stability of the liposomal formulation. The modified system significantly increased corneal retention time compared to a standard 99mTc-DTPA solution. This improvement was attributed to the electrostatic interaction between the positively charged TMC layer around the CoQ10 liposomes and the negatively charged mucous layer of the cornea. Furthermore, a direct correlation was observed between the molecular weight of the TMC coating and the formulation’s precorneal retention time and area under the curve (AUC) values, underscoring the benefit of the coating. Their research also demonstrated that CoQ10, as a nutraceutical, could effectively prevent the onset and progression of cataracts, as confirmed by an in vivo animal study. Without a delivery system like the one developed, however, CoQ10 would not be effective due to its natural instability to light and lipophilicity, which hinder its bioavailability. In other ophthalmic drug delivery systems (DDS), nutraceuticals themselves have also been used as coating materials [75].
Nutraceutical derivatives for improved properties,efficacy and delivery mechanisms
Beyond its high susceptibility to degradation, EGCG has also been shown to have limited efficacy in vivo due to its hydrophilic nature, which leads to poor absorption through cell membranes. Recent studies have focused on esterifying EGCG with docosapentaenoic acid (DPA) and other fatty acids to improve its lipophilicity and enhance its effectiveness [76]. The formulated derivatives demonstrated notable anti-inflammatory, antioxidant, and antiviral properties, making them valuable alternatives to the native EGCG molecule. Chemical modifications of vitamins have led to the creation of new compounds with the potential to address various chronic diseases. In one study, a research team synthesized six novel tocopherol-based derivatives, all of which showed anticancer activity when tested on human MCF-7 and MDA-MB-231 breast cancer cell lines [77].
– Summary of recent formulation approaches for improved iv
Optimization delivery of nutraceuticals
Orally consumed nutraceuticals undergo various physiological and physicochemical changes as they pass through the human gastrointestinal (GI) tract, which reduces the dosage, leading to a smaller amount entering the bloodstream. To address this, many researchers have developed different delivery systems with specific properties that enhance the bioavailability of bioactive compounds and overcome related challenges. Below are several strategies used to design optimal delivery systems for nutraceuticals:
- Protection of Labile Compounds: When nutraceuticals are taken orally, they must be processed by the body, which involves complex physiological and chemical changes. The GI tract, from the mouth to the colon, exposes bioactive ingredients to different environments that alter their chemical structures. These factors include motility, pH variations, enzyme activity, and ionic strength, all of which contribute to the degradation of the nutraceuticals. To improve gastric stability and oral dosing efficiency, delivery systems with protective mechanisms are used to shield sensitive bioactives.
- Extended Gastric Retention Time: The digestive process in the GI tract is dynamic, with nutrients being moved constantly toward their sites of digestion and absorption. If the gastric retention time is too short, nutrients may not be fully absorbed, leading to excessive excretion and reduced therapeutic effectiveness. Delivery systems that slow down the movement of bioactive compounds or increase the viscosity of the formulation can prolong the retention time in the stomach, allowing more bioactive compounds to be absorbed before being discharged.
Increased Aqueous Solubility: For bioactive compounds to be properly absorbed by the intestine, they need to be dissolved or suspended in an aqueous environment. Lipophilic compounds, however, tend to have low solubility in water and often precipitate as clusters. Enhancing the solubility of these compounds can improve their absorption and effectiveness [78].
Large compound clusters obstruct intestinal absorption and are quickly eliminated through excretion, due to their significant particle size. Since poor aqueous solubility is a major factor limiting the absorption of lipophilic compounds, delivery systems that enhance the solubility or dispersion of these ingredients can increase their concentrations at targeted sites in the body.
- Controlled/Delayed Release: Maintaining a steady dosage level in the circulatory system is crucial for ensuring effective therapeutic intervals. Well-designed controlled release mechanisms help maintain consistent release profiles that regulate digestion and absorption processes. These systems are often broken down by enzymatic activity, which releases the compound over time. By incorporating protective layers on the delivery vehicle and selecting materials with varying digestive sustainability, the release times and rates of bioactive compounds can be controlled.
- Facilitation of Lymphatic Uptake: Most compounds are absorbed in the small intestine and enter the bloodstream via the portal vein, where they undergo hepatic metabolism. However, lymphatic uptake is a more efficient method for transporting some highly lipophilic compounds. This route avoids first-pass hepatic metabolism, leading to higher bioavailability. Compounds that associate with lipoproteins in enterocytes are more easily absorbed through the lymphatic system. Using lipid-based delivery vehicles with nanoscale particles has proven effective in enhancing lymphatic uptake of lipophilic compounds.
- Enhancement of Intestinal Permeability: Certain materials can alter the physical barrier function of the intestinal wall. Dietary lipids can influence intestinal membrane fluidity and interact with mucoadhesive polymers. When designing delivery systems, all components can be tailored to support membrane fluidity. For instance, positively charged mucoadhesive polymers like chitosan have been shown to impact intestinal membrane integrity and widen tight junctions, enabling the paracellular absorption of lipophilic compounds.
- Modulation of Metabolic Activity: While absorption is the first barrier to bioavailability, first-pass metabolic processes further reduce the concentration of nutraceuticals in the system. Adding inhibitors of metabolic enzymes to delivery systems can significantly increase bioactive concentrations in the bloodstream. However, because these inhibitors can impair detoxification processes, careful consideration is needed to avoid potential toxicity when using such enzyme inhibitors.
1. Phospholipid-Based Delivery Systems
Phospholipids are a type of lipid with amphipathic properties, meaning they have both hydrophilic (water-attracting) and hydrophobic (water-repelling) parts. Their basic structure consists of a polar phosphate head at one end and two nonpolar fatty acid tails at the other. Due to their amphipathic nature, phospholipids can self-organize into a lipid bilayer, which forms a major component of cell membranes in both animals and plants. Phospholipids are naturally occurring in biological systems, rather than synthetic, making them more compatible with the human body. As a result, delivery systems based on phospholipids are considered more biodegradable and biocompatible. In aqueous environments, phospholipids arrange themselves so that their hydrophilic heads face outward, while their hydrophobic tails are positioned inward. In nonpolar, fat-soluble environments, this arrangement is reversed. This unique property makes phospholipids useful as carrier materials in the human body.
1.1 Phytosomes
Phytosomes, or phospholipid complexes, are popular delivery systems that share similarities in structure and configuration with liposomes. They offer a greater capacity for incorporating nutraceutical compounds due to their stable, chemically bonded structure. Plant extracts can easily bind to phosphatidylcholines, thanks to the presence of terpenoids and flavonoids. As delivery systems, phytosomes have demonstrated superior performance compared to liposomes [79].
It was found that the curcumin-phospholipid complex contributed to the restoration of the liver's glutathione system, thereby aiding in liver protection [80].
Silybin's bioavailability seemed to be improved in dogs when administered with a phytosome complex of phosphatidylcholine. Evodiamine is a bioactive compound derived from the unripe and dried fruit of Evodia rutaecarpa Benth [81].
Evodiamine, along with rutaecarpine (another bioactive compound from the same fruit), has been used to treat diarrhea, headaches, hemorrhages, and gastrointestinal disorders [82].
- The Boswellia genus produces gum resins with unique lipophilic extracts that are highly effective for treating various types of inflammatory diseases [83].
1.2 Liposomes
Liposomes are composed of amphipathic lipid structures arranged in a bilayer, with an aqueous and polar region at the core of the molecule. This bilayer structure allows liposomes to act as carriers for both polar (hydrophilic) and nonpolar (hydrophobic) compounds. They are particularly suitable for transporting polar and hydrophilic substances, as their aqueous regions facilitate the delivery of functional compounds to absorption and digestion sites in the gastrointestinal (GI) tract. For instance, (+)-catechin is known for its strong antioxidant activity and neuroprotective properties, making it beneficial for various age-related cognitive diseases. Liposomes are stable as they pass through the GI tract, making them effective for delivering compounds to digestive and metabolic sites in the body, ultimately ensuring the components reach their target locations [84]. proved that the average diameter of a liposome is 35–70 nm, which is inversely proportional to Tween 80 quantity and zeta potential to be –15 mV. It provides a high capture of (+)-catechin—as high as 80% in its aqueous center. It remains stable in GI fluids and has demonstrated sustained release. In an in vivo study, it enhanced the level of bioactive in the blood. In the same study, the accumulation in the brain of same compound increased by as much as 2.7–2.9 times. In another in vivo study on curcumin [85]. A study conducted on Sprague-Dawley rats using liposome-encapsulated curcumin, with liposome particles measuring 263 nm, found that the bioavailability of curcumin was greater compared to other delivery systems.Another nutraceutical, silymarin, is derived from the seeds of Silybum marianum and is well-known for its effectiveness in treating various liver diseases [86].
1.3 Solid Lipid Nanoparticles
Lipid-based carrier systems are primarily classified into two categories: emulsions and solid lipid nanoparticles (SLNs). Both systems consist of a lipid phase that is dispersed and stabilized in a continuous water phase using surfactants, with the bioactive compound being carried within the lipid phase. The key distinction between SLNs and nanoemulsions is that in SLNs, the lipids are in a solid state, as the name suggests, whereas in nanoemulsions, the lipids are in a liquid state. Nanoemulsions can also exist as water droplets dispersed in a continuous lipid phase, though they are less commonly used. In this chapter, we consider the lipid as the dispersed phase when comparing nanoemulsions and SLNs. Chapter 11 provides a detailed discussion on nanoemulsions as delivery systems. The use of lipid nanoemulsions in parenteral nutrition dates back to the 1950s when vegetable oils were emulsified with phospholipids to enhance the caloric content of formulations. Later efforts aimed to use nanoemulsions as delivery vehicles; however, their application in the pharmaceutical market is currently limited, with only a few drugs utilizing this carrier system due to the constraints on drug delivery. Stabilization and a well-controlled production process are essential for these systems. In the food industry, nanoemulsions are widely used to encapsulate essential oils and flavors for use in beverages and other products. In these applications, the controlled release of flavor compounds upon consumption is not the primary focus; instead, other factors, such as the emulsion's transparency, are more important. The limited stability of liquid oils is partly due to the high mobility of the compounds, which allows them to disperse into the interface, altering the zeta potential (surface charge) of the droplets or disrupting the interfacial layer, thereby compromising the overall stability of the formulation. One approach to reduce the mobility of bioactive compounds is to replace oils with solid lipids. This substitution not only reduces mobility but also allows for the controlled release of encapsulated compounds, as the lipid structure degrades slowly, releasing the active compound gradually. As a result, solid lipid nanoparticles (SLNs) emerged in the early 1990s as an alternative to polymeric nanoparticles and liposomes for emulsion-based delivery systems [87].
|
Synthetic Polymers
|
Natural Polymers
|
|
• Polyester family
|
• Chitosan
|
|
• Polylactic acid
|
• Whey proteins (e.g., ?-lactoglobulin and ?-lactalbumin)
|
|
• Poly(?-caprolactone)
|
• Zein
|
|
• Poly (glycolic acid)
|
• Pectin
|
|
• Approved by FDA as GRAS
|
• Albumin
|
|
• Poly(d,l-lactide-co-glycolide)
|
• Alginic acid
|
|
|
• Xanthan gum
|
2. Emulsion-Based Delivery Approach
Emulsions are mixtures of two or more liquids that typically cannot blend with each other. A common example is oil and water emulsions, where one liquid is dispersed within the other. The liquids are combined in specific proportions to form a homogeneous mixture. In scientific literature, emulsions are often discussed as oral delivery systems that help protect against degradation, enhance solubility, and improve the uptake of nutraceuticals. Emulsions are advantageous because they are easy to prepare, making them suitable for bioactive compounds that have poor absorption and bioavailability due to their chemical properties. Emulsions have been explored as carrier systems for many years, with recent developments focusing on micro- and Nano emulsions. Lipophilic compounds, in particular, are commonly used within the continuous phase, which has low aqueous solubility. Emulsion systems improve bioavailability through various mechanisms, such as enhancing aqueous solubility and protecting against degradation, which in turn increases the absorption of functional components. However, during the oral phase, oil droplets may undergo aggregation [88].
1 Micro- and Nanosized Emulsions
To address size limitations, such as those seen with the bioavailability of dibenzoylmethane, scientists are turning to advanced nanotechnology for emulsions. These emulsions are created with very small particles (nanoparticles), typically less than 100 nm in diameter, and have been shown to enhance the oral efficiency of nutraceutical intake. Nanoemulsions are formed by dispersing immiscible liquids into one another, with the dispersed-phase particle size reduced to a range of 10–100 nm.
Emulsion droplets are naturally thermodynamically unstable and high-energy particles due to the unfavorable energy at their surfaces. This energy penalty becomes more pronounced as the particle size decreases. Unlike conventional emulsions, the formation of nanoemulsions requires additional energy, either through mechanical pressure or by reducing the interfacial tension at the droplet interface. Recent advancements in high-pressure homogenizers, ultrasonicators, and microfluidizers have helped overcome the increased Laplace pressures associated with smaller droplets. To effectively form nanoemulsions, a reduction in interfacial tension is needed when the droplets deform or break apart. Research by Meleson et al. showed that droplet size decreased significantly with higher concentrations of the hydrophilic surfactant sodium dodecyl sulfate, particularly when concentrations exceeded the critical micelle concentration (CMC). Increasing the surfactant concentration requires a higher rate of surfactant adsorption during the emulsification process, reducing droplet size from around 100 nm to less than 20 nm, moving from the upper to the lower end of the nano range.
Mason et al. note the following key points:
- The surfactants used in nanoemulsions should quickly adsorb to the surface from the solvents, typically liquid in nature. This can be achieved by using surfactants at concentrations above their CMC.
- The surfactants must not form liquid crystals within the solvents, as the adsorption rates in these structures are generally very slow.
If these conditions are met, certain food-grade surfactants, such as lecithin, diglycerides, long-chain monosaccharides, and proteins, are excluded due to their slower adsorption rates. Interestingly, literature suggests that nanoemulsions created with lecithin, proteins, and other crystal-forming liquids or lipids often result in droplets larger than 100 nm, contrary to the smaller droplets produced with highly hydrophilic amphiphiles [89].
2 Semisolid Organogels
A new method involves the use of semisolid, hydrophobic, nonpolar gels that offer good physical stability and are more efficient at incorporating nutraceuticals. Gels are semisolid preparations containing both solid and liquid components. The solid part forms a network structure that holds the liquid phase in a stationary state. Depending on the nature of the liquid phase, it can either be polar or nonpolar. When the liquid phase is polar, the gel is referred to as a hydrogel, whereas if the phase is nonpolar, it is called an organogel. The solid components of these gels are known as gelators. The resistance to mobility of the liquid phase within the networked solid structure is due to the interfacial tension between the solid and liquid components. Organogelators, such as sunflower oil, Tween, and Span, include an aqueous phase that forms fiber-like structures, creating a network. Organogels produced using this system are typically not crystalline or glassy and are usually thermoreversible. The polar phase in organogels can include mineral oils, vegetable oils, or organic solvents. Recent research on organogels has grown due to the ease of production and their greater stability. They do not cause irritation and offer enhanced stability. Additionally, organogels are transparent, which allows for the use of various spectroscopic techniques to detect structural changes. Organogels have significant potential as effective skin moisturizers and have appealing cosmetic properties. With relatively low spreadability issues, many organogels are used as delivery systems in skin applications for cosmetics and other substances. In one study, a mixture of Tween 80 and Span 80 was used to create organogels with fluid-filled structures, designed for transdermal drug delivery. These gels transformed into liquids with lower viscosities at temperatures above 40°C and regained their higher viscosities upon cooling, enabling them to solubilize various guest molecules.
Organogels are widely used in nutraceuticals, cosmetics, pharmaceuticals, food products, and more. Their potential has expanded as the topical route has become an easier method for drug delivery. The simple manufacturing process also makes organogels more cost-effective for commercialization. Furthermore, organogels could be used to treat long-term diseases, such as osteoarthritis, once the appropriate drugs are incorporated. Additionally, due to the harmful effects of ultraviolet radiation on the skin, organogels may provide an alternative delivery system for anticancer agents [90].
3. Self-Emulsifying Drug Delivery System Approach
A self-emulsifying drug delivery system (SEDDS) does not contain an aqueous phase in its composition, making it an incomplete emulsion that can maintain an isotropic structure when exposed to aqueous environments. Essentially, a SEDDS is a mixture of cosolvents, oils, and emulsifiers or surfactants, such as Tween 80 or Tween 20. SEDDS can exist in both liquid and solid forms. Typically, they are administered as liquids since many of the excipients used in SEDDS are not solid at room temperature. However, due to the benefits of solid dosages, solid-state SEDDS have been the subject of extensive research. These solid versions are often found to be more effective alternatives to traditional liquid SEDDS. When in contact with water, they can emulsify into stable mixtures. The particle sizes of SEDDS generally range from 100 to 300 nm. Once in the gastrointestinal tract, SEDDS typically convert into emulsions, making them efficient and stable delivery systems for nutraceuticals. These systems help maintain the stability of nutraceutical compounds within the human body. While water-soluble compounds may not be as effectively incorporated into SEDDS, these systems are excellent carriers for less water-soluble compounds, such as curcumin, due to their isotropic nature [91].
4 Chitosan-Based Delivery Systems
Chitosan is produced by deacetylating chitin in the presence of a base. It is a cationic copolymer composed of N-acetyl-d-glucosamine and d-glucosamine. Chitosan is derived from the shells of certain insects, which are primarily made of chitin. In fact, this class of insects is one of the most abundant on Earth, making chitin and chitosan the second-most prevalent polysaccharides in nature. Being derived from a natural source, chitosan is highly biocompatible and biodegradable. When it comes to oral bioavailability, factors like gastric stability and membrane transport efficiency play a crucial role, and when these factors are not ideal, chitosan-based delivery systems offer a viable solution. These systems are particularly effective at enhancing the absorption of various compounds. Due to its safe nature and its ability to improve permeability, along with its favorable physical and chemical properties, chitosan is considered an ideal material for designing ocular nutraceutical delivery systems. Chitosan-based delivery methods for ophthalmic nutraceuticals include hydrogels, coated colloidal systems, and nanoparticles. These carriers improve the biodistribution and retention of nutraceuticals when applied to the eye. By utilizing its in situ gelling properties, chitosan can be delivered to the ocular surface in a liquid form, which then gels upon application. This approach allows for prolonged ocular retention and enhanced therapeutic effectiveness.
4 Chitosan-Based Delivery Systems
Chitosan is produced by deacetylating chitin in the presence of a base. It is a cationic copolymer composed of N-acetyl-d-glucosamine and d-glucosamine. Chitosan is derived from the shells of certain insects, which are primarily made of chitin. In fact, this class of insects is one of the most abundant on Earth, making chitin and chitosan the second-most prevalent polysaccharides in nature. Being derived from a natural source, chitosan is highly biocompatible and biodegradable. When it comes to oral bioavailability, factors like gastric stability and membrane transport efficiency play a crucial role, and when these factors are not ideal, chitosan-based delivery systems offer a viable solution. These systems are particularly effective at enhancing the absorption of various compounds. Due to its safe nature and its ability to improve permeability, along with its favorable physical and chemical properties, chitosan is considered an ideal material for designing ocular nutraceutical delivery systems. Chitosan-based delivery methods for ophthalmic nutraceuticals include hydrogels, coated colloidal systems, and nanoparticles. These carriers improve the biodistribution and retention of nutraceuticals when applied to the eye. By utilizing its in situ gelling properties, chitosan can be delivered to the ocular surface in a liquid form, which then gels upon application. This approach allows for prolonged ocular retention and enhanced therapeutic effectiveness [92].
5. Nanodispersions
Camptothecin, a bioactive compound derived from Camptotheca acuminate, is known for its anticancer properties but has low bioavailability. However, when combined with a solid lipid nanoparticle (SLN) emulsion with a size of approximately 196.8 nm, it demonstrates improved stability and enhanced bioavailability. In these delivery systems, the particles are suspended in a solution with minimal stabilizers and no carriers. Research indicates that camptothecin maintains its stability and bioavailability, making it an effective and convenient method for the oral delivery of drugs and nutraceuticals [93].
6.Suspension-Based Delivery Systems
Suspension-based delivery systems are commonly used in dermal applications of bioactive compounds, such as in creams or gels, for hydrophobic nutraceuticals, thereby enhancing their effectiveness. A recent advancement in nutraceutical science involves integrating nanoscience, leading to the development of nanosuspensions. In this approach, nanotechnology is used to improve the solubility of compounds and manage their hydrophobic nature by homogenizing the material under high pressure to achieve a particle size of around 400 nm. The resulting material is then lyophilized and formed into pellets for oral use or incorporated into creams for dermal applications. This method has been shown to enhance solubility and surface area [94].
7. Nanostructured Lipid Carriers
Nanostructured lipid carriers (NLCs) are a technique that combines lipid-based delivery systems with nanosized particles. This method uses lipid-based nanoscale molecules, along with a nanostructure, to deliver bioactive compounds via various routes, such as oral or dermal. It enhances delivery efficiency, improves entrapment, and offers a dual-phase controlled release mechanism, allowing for more precise control over the targeted delivery of compounds. NLCs improve encapsulation efficiency, nutraceutical loading, and the physical stability of the formulation. This technique is particularly valuable for enhancing chemical stability, bioavailability, and the controlled release of lipophilic compounds in foods. NLCs protect the bioactive compounds from degradation by confining them within the solid matrix of the particle. [95].
8. Micelle-Based Delivery Systems
Micelles have been utilized to enhance the delivery of nutraceuticals, although they may have limitations, such as higher costs. Examples of micelles include casein micelles and phosphatidylethanolamine (PE), which is derived from polyethylene glycol (PEG) and used to load nutraceuticals for delivery. PEG is a widely trusted hydrophilic polymeric material commonly used for surface modification of nutraceutical carriers and for forming the corona in therapeutic micelles. It has been shown to effectively protect biologically active molecules and other particulate delivery systems, acting as a steric stabilizer. PEG is inexpensive, has low toxicity, and is approved for internal use by regulatory authorities, along with its PE conjugates. Additionally, other hydrophilic polymers have been identified that can serve as micelle-forming blocks. These polymers are effective as steric protectors when they are hydrophilic and flexible, and with sufficient surface density, they can form micelles after modification with a hydrophobic block [96].
10 Encapsulation of Bioactives in Electrospun Fibers
Enrichment and fortification are commonly used to enhance the nutritional value of food. However, the nutrients and bioactives added to food often interact with other components in the food matrix, leading to reduced bioefficiency and bioavailability. Furthermore, many of these nutrients and bioactive compounds are naturally unstable and may possess undesirable sensory or organoleptic properties, which can negatively affect consumer acceptance. Therefore, these materials need to be protected during their transport and delivery to the human body. Several studies have demonstrated the effectiveness of electrospun fibers for this purpose. For instance, EGCG, a polyphenol found in green tea with known health benefits (such as treating heart disease and cancer), was encapsulated in electrospun zein fibers. A 20% weight ratio of EGCG was dissolved in a 20% zein solution in aqueous ethanol (70:30 ethanol to water), which was then electrospun at 12 kV using a 10 cm spinneret and collector [97].
Formulations and Challenges Involved
Developing a high-quality nutraceutical formulation that ensures physical and chemical stability, safety, technological feasibility, and cost-effectiveness presents several challenges. Unlike well-defined drug molecules, botanicals are complex substances containing a variety of chemical components, often including multiple classes of compounds in a single product. Many of these botanicals are sensitive to factors like heat, light, oxygen, alkaline pH, and high humidity. They typically exhibit poor flow properties, low bulk density, and inconsistent particle size distribution. Therefore, successfully creating a nutraceutical formulation requires a deep understanding of the physicochemical properties of the ingredients, the use of appropriate manufacturing techniques, careful selection of excipients, and the inclusion of suitable overages based on critical stability assessments [98].
Challenges associated with different dosage forms
Strategies to address formulation difficulties
Selection of appropriate excipients
1.Challenges in Formulating Nutraceuticals and Dietary Supplements
Formulating nutraceuticals can be challenging due to issues such as poor aqueous solubility, high melting points, and the chemical instability of active ingredients. For instance, omega-3 fatty acids, carotenoids, oil-soluble vitamins, and curcumin have significant nutritional benefits but are not easily soluble. One potential solution is to develop novel delivery systems for these compounds, though this can increase the cost. Therefore, efforts are required to make these formulations more affordable. DOI:10.1016/B978-0-85709-124-6.50002-0
Another challenge in formulating nutraceuticals is their high melting points. Compounds like phytosterols, fatty alcohols, and carotenoids, for example, have high melting points that can cause formulation instability. One possible solution is to create solid dispersions or dissolve these compounds in appropriate solvents, then introduce them into food as suspended nanocrystals. However, this approach can result in reduced stability, shorter shelf life, undesirable appearance, unpleasant odor, and taste, which can negatively impact market value and consumer demand. Therefore, there is a need to develop cost-effective technologies to overcome these issues [99]. Chemical instability presents another challenge. For instance, omega-3 fatty acid-rich oils like fish oil, flaxseed oil, and cod liver oil, as well as carotenoids, lycopene, and curcumin, all face stability issues. The degree of chemical degradation is influenced by the composition of the bioactive compound, environmental factors such as temperature, pH, and pressure, as well as the presence of metals or other oxidation-promoting substances. To protect these compounds from degradation, the development of nanoscale products is crucial [100]. Further, in the case of the development of probiotics, special, selective bacterial strains are necessary. A current challenge is the selection of the proper strain followed by their incorporation in foods. Application of any mismatching bacterial or toxic cultures, in addition to their negligent handling, can cause disastrous consequences. Apart from these challenges, other considerations are solid dosage formulation and process design for drug products and nutrition products that are similar, but the purpose and regulatory requirements may differ [101]. Lastly, a challenge in formulating nutraceuticals and dietary supplements is creating dosage forms that are appropriate for different segments of the aging population, particularly older adults and children. This is due to difficulties with swallowing solid dosage forms, such as tablets or capsules (dysphagia). As a solution, advanced dosage forms like orodispersible tablets, fast-dissolving films, and easy-to-swallow gels, commonly used in pharmaceutical applications, should be considered for the administration of nutraceuticals and dietary supplements [102].
2. Approaches to Address Formulation Challenges
One common approach to overcoming formulation challenges is the isolation or preparation of concentrates from natural sources. This method is beneficial because many herbal nutraceuticals require large doses per daily serving. Additionally, multiple active ingredients can be found in various sources. However, there can be significant variations in the compression and flow properties of active ingredients within a single dosage form, as well as differences in their heat and moisture sensitivity. This can lead to substantial stability issues, with numerous opportunities for ingredient interactions. Various extraction methods, including microwave-assisted extraction, countercurrent extraction, maceration, percolation, and Soxhlet extraction, are employed. Natural bioactive compounds used in these processes may come from plant extracts, herbal concentrates, fruit and vegetable concentrates, and fungal or microbial materials, which are used as feedstock for bio-fermentation processes. The concentrates may also include excipients like spray-dried carriers used in their production [103]. Another promising approach is modification in delivery systems. Novel Drug Delivery
Systems have been used to modify the properties of various compounds to produce new generations of drug compounds. They also play a role in the food industry and in various kinds of nutritional supplements. Various formulations of supplements prepared by nanotechnology, called nano formulations, that have increased bioavailability, reduced side effects and protect the active ingredients against the process of degradation, have been reported [104]. Polyphenols, as nutraceuticals derived from dietary sources, have shown significant benefits in alleviating symptoms of various diseases, as demonstrated in preclinical and clinical studies. However, one major drawback is their low bioavailability, which is due to limited absorption in the upper gastrointestinal tract. This is primarily caused by their hydrophobic nature, their presence in polymeric or glycosylated forms in foods, and their strong binding with food matrices. These factors result in minimal bio-accessibility of polyphenols within the body. To address this issue, research and development techniques involving food processing, along with nanoformulations, enzymatic treatments, and probiotic combination therapies, have proven effective in improving the bioavailability of polyphenols [105].
- Polyphenols, when derived from dietary sources as nutraceuticals, have shown significant benefits in alleviating disease symptoms in both preclinical and clinical studies. However, a major limitation is their low bioavailability, primarily due to poor absorption in the upper gastrointestinal tract. This is caused by factors such as their hydrophobic nature, their occurrence in polymeric or glycosylated forms in food, and their strong binding to food matrices. These factors reduce the bio-accessibility of polyphenols in the body. To address this challenge, food processing research, nanoformulations, enzymatic treatments, and probiotic combination therapies have been found to be effective solutions [105].
3.1Liposomes and nanoemulsions,
also known as bilayer phospholipid vesicles, hold significant promise in the nutraceutical industry due to their ability to encapsulate both hydrophilic and lipophilic substances. This dual encapsulation promotes synergistic effects and aids in protecting sensitive bioactive compounds, enhancing bioavailability, ensuring controlled release, and improving storage stability. Nanoliposomes, in particular, have unique characteristics that make them effective for disease prevention and health promotion. A notable example is lipid-based nanocarriers, such as nanophytosomes, which facilitate the delivery of botanical nutraceuticals. These carriers show potential for use in developing innovative functional beverages and food products. A study demonstrated that rutin, when complexed in phytosome form with phosphatidylcholine (PC) in a 1:3 molar ratio, exhibited increased chemical and physical stability. The resulting phytosomes, with a particle size of less than 100 nm and 99% encapsulation efficiency, successfully masked the undesirable properties of rutin.
3.2. Lipid-Based Carriers
Lipid-based formulations, such as nanocapsules and micronized carriers, are promising options for improving the controlled release, solubility, and bioavailability of phenolic compounds. For instance, ?-Car nanocapsules (greater than 300 nm) demonstrated excellent physical stability, showing minimal changes during storage. This indicates their potential for widespread use in functional foods, beverages, and nutraceutical products [106].
3.3. Polysaccharide Matrices
These matrices are designed to undergo multiple enzymatic interactions, ensuring degradation at specific locations within the large and small intestines. When used as nanoparticle coatings, they can effectively delay the uncontrolled release of bioactive compounds until the coating is exposed in the targeted environment where it is intended to release. Such coated nanoparticles could be utilized to target various parts of the gastrointestinal tract, enhancing oral bioavailability [107].
3.Excipient Selection
Another approach to addressing nutraceutical formulation challenges is adjusting formulation parameters through the careful selection of excipients. For nutritional products, the final formula must be resilient enough to accommodate the varying physical properties of natural ingredients within a complex mixture. Both materials and manufacturers must comply with internal quality standards, safety specifications, and performance criteria. What works well in one formulation may not function the same in another. The functionality of excipients can only be accurately evaluated in the context of a specific formulation and manufacturing process. In natural product formulations, the performance of excipients is greatly influenced by the intricate interaction of multiple active ingredient characteristics. At times, excipients that seem identical may not perform the same way [108].
4. Safety and Quality Control of Nutraceuticals
Nutraceuticals are commonly consumed as supplements and are available over-the-counter, making their safety a major concern, as unsafe products can have harmful or even fatal effects. The most frequent issues include contamination, adulteration (either accidental or intentional), or misleading labeling. To identify adulteration, three detection methods can be employed: (1) identifying the presence of an undeclared substance, (2) detecting deviations in the normal content levels of a component, and (3) recognizing profiles that are unlikely to occur naturally [109]. Adulteration can occur either unintentionally or intentionally. Unintentional adulteration may happen under various conditions, such as during different growth stages of plants, the formulation and manufacturing processes of nutraceuticals, or while storing the products. Contamination with substances like fertilizers, heavy metals, or microbial agents may occur during these stages. Additionally, adulteration may involve synthetic drugs, substitute species, dust, pollen, insects, rodents, parasites, microbes, fungi, mold, toxins, and heavy metals. Such contamination can lead to infections or serious health issues like gastritis, liver damage, and even life-threatening conditions. To prevent this, strict quality control measures for raw materials and finished products are essential. These controls can be guided by specifications in relevant monographs and should include checks for the stability of active ingredients and microbiological safety [110]. Intentional adulteration in supplements or herbal remedies can lead to severe harmful effects. This type of adulteration typically involves the use of undeclared synthetic compounds, often with the aim of modifying the pharmacological response for economic gain. The sourcing of nutraceuticals from plants is frequently limited, and the process of extracting these compounds is both time-consuming and expensive [111].
Below are some examples of adulteration cases.
(a)Ibutramine hydrochloride monohydrate, a drug that works by inhibiting serotonergic and noradrenergic reuptake, is commonly used as an anti-obesity agent and often found as an adulterant. In a study of twenty-two dietary supplement samples in China, eleven were contaminated with phenolphthalein, N-mono-desmethylsibutramine, and sibutramine. Similarly, in another study of fifteen samples in China, four were found to contain sibutramine and N-di-des methyl sibutramine [112].
(b) Fenfluramine, a drug commonly used as an adulterant in Chinese traditional medicines, was found in several slimming products. It led to primary pulmonary hypertension and valvular heart disease. As a result, this drug was removed from the market in 1997[113].
(c) morphological The use of morphological substitutes is another common form of adulteration that can lead to significant health risks. For instance, Panax ginseng (also known as "Asian" or "Korean ginseng") is a traditional medicine, but it has been found to be adulterated with roots from Panax quinquefolius L. (American ginseng) and Eleutherococcus senticosus Maxim (Siberian ginseng), which may result in health issues [114]. (e) Other examples of species with physical similarities include Anthemis nobilis L., which is commonly known as chamomile, and Matricaria chamomilla L. (Asteraceae), both of which are recognized as therapeutic plants in the European Pharmacopoeia. Additionally, several other species within the Asteraceae family, such as Tanacetum parthenium (L.) Sch. Bip., Tanacetum cinerariifolium (Trevir.) Schultz Bip., Tripleurospermum callosum (Boiss. et Heldr.) E. Hossain, Bellis perennis L., and Leucanthemum vulgare L., also share physical similarities. However, the pharmacological effects vary depending on their specific phytoconstituents [115].
(D) Other examples of species with similar physical characteristics include the flower Anthemis nobilis L., commonly known as chamomile, and Matricaria chamomilla L. (Asteraceae), both of which are recognized as therapeutic plants in the European Pharmacopoeia. Several other species in the Asteraceae family, such as Tanacetum parthenium (L.) Sch. Bip., Tanacetum cinerariifolium (Trevir.) Schultz Bip., Tripleurospermum callosum (Boiss. et Heldr.) E. Hossain, Bellis perennis L., and Leucanthemum vulgare L., also share these similarities. Additionally, the pharmacological effects can vary depending on the specific phytoconstituents present [116].
5. Formulation Challenges
- Drug interactions occur when the activity of one active ingredient is influenced by the presence of other substances. These interactions can be classified as food–drug or drug–drug interactions. Such interactions may either enhance, reduce, or trigger side effects in the pharmacological response [117].
- Garlic (allicin) has properties that lower blood pressure, reduce cholesterol, act as an anti-inflammatory, and possess antibacterial and antifungal effects. However, when taken alongside anticoagulants like warfarin, it may increase the risk of bleeding. If combined with hypoglycemic drugs such as insulin or glipizide, it could cause hypoglycemia. Additionally, when used with protease inhibitors like indinavir or saquinavir, garlic may reduce their blood levels and effectiveness [118].
Ginger is widely used to treat various stomach issues, including gas, motion sickness, diarrhea, nausea (as an anti-emetic), and loss of appetite. It is also used for pain relief from arthritis, menstrual pain, and upper respiratory infections such as coughs and bronchitis. However, when taken with anticoagulants, ginger may increase the risk of bleeding. If consumed with hypoglycemic drugs like insulin or glipizide, it may lead to hypoglycemia. Additionally, when used alongside calcium channel blockers, ginger could potentially lower their effects or cause irregular heartbeats [119]. Green tea (polyphenols) enhances mental alertness and cognitive function. It is also used to treat various medical conditions, including Crohn's disease, Parkinson's disease, cardiovascular issues, diabetes, hypotension, chronic fatigue syndrome (CFS), tooth decay, kidney stones, and skin conditions. However, when taken with stimulant medications, it can lead to harmful effects, such as increased heart rate and blood pressure. Additionally, using green tea alongside bortezomib (Velcade) may reduce its effectiveness in treating certain cancers. Green tea may also decrease the effectiveness of warfarin [120]. The leaf extract of Ginkgo biloba is beneficial for treating Alzheimer's disease, other types of dementia, Raynaud’s syndrome, peripheral vascular disease, vertigo, dizziness, premenstrual syndrome (PMS), and for enhancing color vision in individuals with diabetes. However, when taken with anticoagulants or NSAIDs, it may increase the risk of bleeding. Additionally, when combined with anticonvulsants, Ginkgo biloba may reduce their effectiveness in preventing seizures [121].
One serious case of intentional adulteration involves the use of peanut skin extract in various grape products. Grape seed-based supplements are known for their high content of bioactive polyphenols and are used to prevent cardiovascular and neurodegenerative diseases. Peanut skin is used as an adulterant because it is readily available, inexpensive, and a high-volume byproduct compared to grape products. However, it poses a potential allergen risk, which can lead to significant health concerns. This was highlighted in a study where tested products contained no detectable grape seed extract, only peanut skin as the adulterant [122].
Future Direction
Nutraceuticals are promising options for preventing or treating inflammatory bowel disease (IBD) because they can help regulate the overactive immune response targeting key signaling pathways involved in IBD development. Moreover, growing evidence suggests that nutraceuticals may have therapeutic benefits for IBD by influencing the gut microbiota. Microbial metabolites from food, such as short-chain amino acids, play a significant role in modulating immune responses within the gut, maintaining microbial balance, and preserving mucosal integrity. Due to poor nutrient absorption and loss of appetite, IBD patients often exclude certain foods, leading to nutritional deficiencies like anemia and bone pain. Nutraceuticals can serve as supplements to improve the disease's prognosis. While most nutraceuticals are derived from natural foods, further research is required to determine their safe dosages and potential side effects. A long-term intervention study is needed to assess the relationship between treatment dosage and disease severity or outcomes. Clinical studies should also investigate possible negative interactions between nutraceuticals and medications. Additionally, combining nutraceuticals with drug treatments or using multiple nutraceuticals together could offer synergistic therapeutic benefits for IBD patients. The limitations of current drug therapies, coupled with the rising incidence of IBD spreading from Western countries to East Asia and other westernized regions, have sparked extensive research focused on developing alternative treatments based on natural substances that are both highly effective and safe. In the future, a coordinated effort to identify and address the environmental and dietary risk factors for IBD will be a key priority [123]. Since diet plays a significant role in the development of IBD, there is a strong need for a comprehensive dietary approach that eliminates certain foods while incorporating components that address the root causes of the disease. The manipulation of the gut microbiome using probiotic bacteria has become an intriguing area of research for IBD management. However, current evidence supporting the use of probiotics for chronic metabolic conditions remains limited. Recently, "designer probiotics," which involve genetically engineered bacteria with targeted functions, have gained attention as an innovative treatment strategy [124]. In recent years, "specific targeting," which enables nutrients or bioactive compounds to reach and focus on the precise site of inflammation to deliver their effects, has gained significant attention in IBD research and is expected to play a crucial role in future studies [125]. The development of advanced cell models that replicate the GI tract is seen as a promising area of research for finding natural alternative treatments for IBD. Crucially, gaining a comprehensive and accurate understanding of the pathogenic mechanisms of IBD is essential, as it will enable researchers to identify effective treatments using both current and potential natural therapeutic agents. As a result, future research will focus on creating more efficient therapeutic strategies for IBD, utilizing health-functional materials that can specifically target the affected areas and address the underlying pathogenic mechanisms of the disease.
CONCLUSION
In conclusion, nutraceuticals hold great promise as natural alternatives for the prevention and treatment of various health conditions, including inflammatory bowel disease (IBD). With their potential to target underlying causes, enhance immune responses, and regulate intestinal microflora, nutraceuticals offer an exciting avenue for therapeutic interventions. However, further research is necessary to fully understand their efficacy, safety, and optimal dosage. Advances in cell models, the development of targeted delivery systems, and the integration of nutraceuticals with conventional drug therapies could pave the way for more effective treatments in the future. By leveraging the benefits of nutraceuticals alongside modern scientific approaches, we can develop safer, more sustainable treatments for IBD and other chronic health conditions.
REFERENCES
- M. M. Berger et al., “Clinical, immune and metabolic effects of trace elementsupplements in burns: a double-blind placebo-controlled trial,” Clinical Nutrition, vol. 15, no. 2, pp. 94–96, Apr. 1996, doi: 10.1016/S0261-5614(96)80029-X.
- D. Bagchi, H. G. Preuss, and J. P. Kehrer, “Nutraceutical and functional food industries: aspects on safety and regulatory requirements,” Toxicol Lett, vol. 150, no. 1, pp. 1–2, Apr. 2004, doi: 10.1016/j.toxlet.2004.01.011.
- C. Ramaa, A. Shirode, A. Mundada, and V. Kadam, “Nutraceuticals - An Emerging Era in the Treatment and Prevention of Cardiovascular Diseases,” Curr Pharm Biotechnol, vol. 7, no. 1, pp. 15–23, Feb. 2006, doi: 10.2174/138920106775789647.
- H. K. Biesalski, “Nutraceuticals: the link between nutrition and medicine,” J Toxicol Cutaneous Ocul Toxicol, vol. 21, no. 1–2, pp. 9–30, Jan. 2002, doi: 10.1081/CUS-120004324.
- S. Ross, “Functional foods: the Food and Drug Administration perspective,” Am J Clin Nutr, vol. 71, no. 6, pp. 1735S-1738S, Jun. 2000, doi: 10.1093/ajcn/71.6.1735S.
- V. Brower, “Nutraceuticals: Poised for a healthy slice of the healthcare market?,” Nat Biotechnol, vol. 16, no. 8, pp. 728–731, Aug. 1998, doi: 10.1038/nbt0898-728.
- S. Mali, S. Rathod, N. Kale, and N. Shinde, “Overview of Nutraceuticals,” Asian Journal of Pharmaceutical Research, pp. 61–70, Mar. 2022, doi: 10.52711/2231-5691.2022.00010.
- M. B. Roberfroid, “Global view on functional foods: European perspectives,” British Journal of Nutrition, vol. 88, no. S2, pp. S133–S138, Nov. 2002, doi: 10.1079/BJN2002677.
- R. A. Gibson and M. Makrides, “n?3 Polyunsaturated fatty acid requirements of term infants,” Am J Clin Nutr, vol. 71, no. 1, pp. 251S-255S, Jan. 2000, doi: 10.1093/ajcn/71.1.251S.
- M. Whitman, “Understanding the perceived need for complementary and alternative nutraceuticals: lifestyle issues.,” Clin J Oncol Nurs, vol. 5, no. 5, pp. 190–4, 2001.
- J. Hathcock, “Dietary Supplements: How They Are Used and Regulated,” J Nutr, vol. 131, no. 3, pp. 1114S-1117S, Mar. 2001, doi: 10.1093/jn/131.3.1114S.
- T. Shimizu, “Health claims on functional foods: the Japanese regulations and an international comparison,” Nutr Res Rev, vol. 16, no. 2, pp. 241–252, Dec. 2003, doi: 10.1079/NRR200363.
- D. K. Heyland, “In Search of the Magic Nutraceutical: Problems with Current Approaches,” J Nutr, vol. 131, no. 9, pp. 2591S-2595S, Sep. 2001, doi: 10.1093/jn/131.9.2591S.
- B. Patwardhan, D. Warude, P. Pushpangadan, and N. Bhatt, “Ayurveda and Traditional Chinese Medicine: A Comparative Overview,” Evidence-Based Complementary and Alternative Medicine, vol. 2, no. 4, pp. 465–473, Jan. 2005, doi: 10.1093/ecam/neh140.
- N. M. Childs, “Nutraceutical Industry Trends,” Journal of Nutraceuticals, Functional & Medical Foods, vol. 2, no. 1, pp. 73–85, Jan. 2000, doi: 10.1300/J133v02n01_07.
- G. M. Binder, “Pharmaceutical provisions of the FDA modernization act of 1997: one year and counting,” Curr Opin Biotechnol, vol. 10, no. 3, pp. 303–306, Jun. 1999, doi: 10.1016/S0958-1669(99)80054-8.
- N.-S. Kwak and D. J. Jukes, “Functional foods. Part 1: the development of a regulatory concept,” Food Control, vol. 12, no. 2, pp. 99–107, Mar. 2001, doi: 10.1016/S0956-7135(00)00028-1.
- S. Rahmania and S. Somanadhapai, “Nutraceuticals and Vitamins: A Comprehensive Guide for Naturopaths and Allied Health Professionals,” in Disease and Health Research: New Insights Vol. 2, BP International, 2024, pp. 32–52. doi: 10.9734/bpi/dhrni/v2/1126.
- D. Bagchi, “Nutraceuticals and functional foods regulations in the United States and around the world,” Toxicology, vol. 221, no. 1, pp. 1–3, Apr. 2006, doi: 10.1016/j.tox.2006.01.001.
- G. R. Gibson and M. B. Roberfroid, “Dietary Modulation of the Human Colonic Microbiota: Introducing the Concept of Prebiotics,” J Nutr, vol. 125, no. 6, pp. 1401–1412, Jun. 1995, doi: 10.1093/jn/125.6.1401.
- R. A. Jacob, “The integrated antioxidant system,” Nutrition Research, vol. 15, no. 5, pp. 755–766, May 1995, doi: 10.1016/0271-5317(95)00041-G.
- M. Ndiaye, T. Chataigneau, M. Chataigneau, and V. B. Schini?Kerth, “Red wine polyphenols induce EDHF?mediated relaxations in porcine coronary arteries through the redox?sensitive activation of the PI3?kinase/Akt pathway,” Br J Pharmacol, vol. 142, no. 7, pp. 1131–1136, Aug. 2004, doi: 10.1038/sj.bjp.0705774.
- L. Wang et al., “Grape seed proanthocyanidins attenuate vascular smooth muscle cell proliferation via blocking phosphatidylinositol 3?kinase?dependent signaling pathways,” J Cell Physiol, vol. 223, no. 3, pp. 713–726, Jun. 2010, doi: 10.1002/jcp.22080.
- M. Ndiaye, T. Chataigneau, M. Chataigneau, and V. B. Schini?Kerth, “Red wine polyphenols induce EDHF?mediated relaxations in porcine coronary arteries through the redox?sensitive activation of the PI3?kinase/Akt pathway,” Br J Pharmacol, vol. 142, no. 7, pp. 1131–1136, Aug. 2004, doi: 10.1038/sj.bjp.0705774.
- T. H. Rissanen et al., “Low Intake of Fruits, Berries and Vegetables Is Associated with Excess Mortality in Men: the Kuopio Ischaemic Heart Disease Risk Factor (KIHD) Study,” J Nutr, vol. 133, no. 1, pp. 199–204, Jan. 2003, doi: 10.1093/jn/133.1.199.
- N. J. Temple and K. Kaiser Gladwin, “Fruit, vegetables, and the prevention of cancer,” Nutrition, vol. 19, no. 5, pp. 467–470, May 2003, doi: 10.1016/S0899-9007(02)01037-7.
- S. A. Balch, C. B. McKenney, and D. L. Auld, “Evaluation of Gamma-linolenic Acid Composition of Evening Primrose (Oenothera) Species Native to Texas,” HortScience, vol. 38, no. 4, pp. 595–598, Jul. 2003, doi: 10.21273/HORTSCI.38.4.595.
- K. Sembulingam, P. Sembulingam, and A. Namasivayam, “Effect of Ocimum sanctum Linn on noise induced changes in plasma corticosterone level.,” Indian J Physiol Pharmacol, vol. 41, no. 2, pp. 139–43, Apr. 1997.
- J. Bernal, J. A. Mendiola, E. Ibáñez, and A. Cifuentes, “Advanced analysis of nutraceuticals,” J Pharm Biomed Anal, vol. 55, no. 4, pp. 758–774, Jun. 2011, doi: 10.1016/j.jpba.2010.11.033.
- R. Blaylock and J. Maroon, “Natural plant products and extracts that reduce immunoexcitotoxicity-associated neurodegeneration and promote repair within the central nervous system,” Surg Neurol Int, vol. 3, no. 1, p. 19, 2012, doi: 10.4103/2152-7806.92935.
- D. Gianfrilli et al., “Propionyl-L-carnitine, L-arginine and niacin in sexual medicine: a nutraceutical approach to erectile dysfunction,” Andrologia, vol. 44, pp. 600–604, May 2012, doi: 10.1111/j.1439-0272.2011.01234.x.
- S. C. Gupta, J. H. Kim, S. Prasad, and B. B. Aggarwal, “Regulation of survival, proliferation, invasion, angiogenesis, and metastasis of tumor cells through modulation of inflammatory pathways by nutraceuticals,” Cancer and Metastasis Reviews, vol. 29, no. 3, pp. 405–434, Sep. 2010, doi: 10.1007/s10555-010-9235-2.
- C. R. Sirtori, C. Galli, J. W. Anderson, and A. Arnoldi, “Nutritional and nutraceutical approaches to dyslipidemia and atherosclerosis prevention: Focus on dietary proteins,” Atherosclerosis, vol. 203, no. 1, pp. 8–17, Mar. 2009, doi: 10.1016/j.atherosclerosis.2008.06.019.
- D. A. Fernandes de Abreu, V. Landel, and F. Féron, “Seasonal, gestational and postnatal influences on multiple sclerosis: The beneficial role of a vitamin D supplementation during early life,” J Neurol Sci, vol. 311, no. 1–2, pp. 64–68, Dec. 2011, doi: 10.1016/j.jns.2011.08.044.
- J. C. Espín, M. T. García-Conesa, and F. A. Tomás-Barberán, “Nutraceuticals: Facts and fiction,” Phytochemistry, vol. 68, no. 22–24, pp. 2986–3008, Nov. 2007, doi: 10.1016/j.phytochem.2007.09.014.
- A. M. Tang, N. M. H. Graham, R. D. Semba, and A. J. Saah, “Association between serum vitamin A and E levels and HIV-1 disease progression,” AIDS, vol. 11, no. 5, pp. 613–620, Apr. 1997, doi: 10.1097/00002030-199705000-00009.
- D. A. Camfield, J. Sarris, and M. Berk, “Nutraceuticals in the treatment of Obsessive Compulsive Disorder (OCD): A review of mechanistic and clinical evidence,” Prog Neuropsychopharmacol Biol Psychiatry, vol. 35, no. 4, pp. 887–895, Jun. 2011, doi: 10.1016/j.pnpbp.2011.02.011.
- E. Acosta, “Bioavailability of nanoparticles in nutrient and nutraceutical delivery,” Curr Opin Colloid Interface Sci, vol. 14, no. 1, pp. 3–15, Feb. 2009, doi: 10.1016/j.cocis.2008.01.002.
- G. G. Schwartz, “Vitamin D and Intervention Trials in Prostate Cancer: From Theory to Therapy,” Ann Epidemiol, vol. 19, no. 2, pp. 96–102, Feb. 2009, doi: 10.1016/j.annepidem.2008.03.007.
- T. Walle, F. Hsieh, M. H. DeLegge, J. E. Oatis, and U. K. Walle, “High Absorption But Very Low Bioavailability Of Oral Resveratrol In Humans,” Drug Metabolism and Disposition, vol. 32, no. 12, pp. 1377–1382, Dec. 2004, doi: 10.1124/dmd.104.000885.
- A. Alves-Rodrigues and A. Shao, “The science behind lutein,” Toxicol Lett, vol. 150, no. 1, pp. 57–83, Apr. 2004, doi: 10.1016/j.toxlet.2003.10.031.
- T. Walle, F. Hsieh, M. H. DeLegge, J. E. Oatis, and U. K. Walle, “High Absorption But Very Low Bioavailability Of Oral Resveratrol In Humans,” Drug Metabolism and Disposition, vol. 32, no. 12, pp. 1377–1382, Dec. 2004, doi: 10.1124/dmd.104.000885.
- T. Walle, F. Hsieh, M. H. DeLegge, J. E. Oatis, and U. K. Walle, “High Absorption But Very Low Bioavailability Of Oral Resveratrol In Humans,” Drug Metabolism and Disposition, vol. 32, no. 12, pp. 1377–1382, Dec. 2004, doi: 10.1124/dmd.104.000885.
- P. Palombo et al., “Beneficial Long-Term Effects of Combined Oral/Topical Antioxidant Treatment with the Carotenoids Lutein and Zeaxanthin on Human Skin: A Double-Blind, Placebo-Controlled Study,” Skin Pharmacol Physiol, vol. 20, no. 4, pp. 199–210, 2007, doi: 10.1159/000101807.
- M. C. Meinke, M. E. Darvin, H. Vollert, and J. Lademann, “Bioavailability of natural carotenoids in human skin compared to blood,” European Journal of Pharmaceutics and Biopharmaceutics, vol. 76, no. 2, pp. 269–274, Oct. 2010, doi: 10.1016/j.ejpb.2010.06.004.
- J. H. Rabe, A. J. Mamelak, P. J. S. McElgunn, W. L. Morison, and D. N. Sauder, “Photoaging: Mechanisms and repair,” J Am Acad Dermatol, vol. 55, no. 1, pp. 1–19, Jul. 2006, doi: 10.1016/j.jaad.2005.05.010.
- M. de la Fuente, M. Raviña, P. Paolicelli, A. Sanchez, B. Seijo, and M. J. Alonso, “Chitosan-based nanostructures: A delivery platform for ocular therapeutics,” Adv Drug Deliv Rev, vol. 62, no. 1, pp. 100–117, Jan. 2010, doi: 10.1016/j.addr.2009.11.026.
- C. Martínez-Sancho, R. Herrero-Vanrell, and S. Negro, “Vitamin A palmitate and aciclovir biodegradable microspheres for intraocular sustained release,” Int J Pharm, vol. 326, no. 1–2, pp. 100–106, Dec. 2006, doi: 10.1016/j.ijpharm.2006.07.010.
- A. Amri, J. C. Chaumeil, S. Sfar, and C. Charrueau, “Administration of resveratrol: What formulation solutions to bioavailability limitations?,” Journal of Controlled Release, vol. 158, no. 2, pp. 182–193, Mar. 2012, doi: 10.1016/j.jconrel.2011.09.083.
- O. Suwantong, P. Opanasopit, U. Ruktanonchai, and P. Supaphol, “Electrospun cellulose acetate fiber mats containing curcumin and release characteristic of the herbal substance,” Polymer (Guildf), vol. 48, no. 26, pp. 7546–7557, Dec. 2007, doi: 10.1016/j.polymer.2007.11.019.
- M. Gonnet, L. Lethuaut, and F. Boury, “New trends in encapsulation of liposoluble vitamins,” Journal of Controlled Release, vol. 146, no. 3, pp. 276–290, Sep. 2010, doi: 10.1016/j.jconrel.2010.01.037.
- C. Mekjaruskul, Y.-T. Yang, M. G. D. Leed, M. P. Sadgrove, M. Jay, and B. Sripanidkulchai, “Novel formulation strategies for enhancing oral delivery of methoxyflavones in Kaempferia parviflora by SMEDDS or complexation with 2-hydroxypropyl-?-cyclodextrin,” Int J Pharm, vol. 445, no. 1–2, pp. 1–11, Mar. 2013, doi: 10.1016/j.ijpharm.2013.01.052.
- K. B. Chien, E. J. Chung, and R. N. Shah, “Investigation of soy protein hydrogels for biomedical applications: Materials characterization, drug release, and biocompatibility,” J Biomater Appl, vol. 28, no. 7, pp. 1085–1096, Mar. 2014, doi: 10.1177/0885328213497413.
- Y. Li, J. Zheng, H. Xiao, and D. J. McClements, “Nanoemulsion-based delivery systems for poorly water-soluble bioactive compounds: Influence of formulation parameters on polymethoxyflavone crystallization,” Food Hydrocoll, vol. 27, no. 2, pp. 517–528, Jun. 2012, doi: 10.1016/j.foodhyd.2011.08.017.
- V. B. Junyaprasert, V. Teeranachaideekul, E. B. Souto, P. Boonme, and R. H. Müller, “Q10-loaded NLC versus nanoemulsions: Stability, rheology and in vitro skin permeation,” Int J Pharm, vol. 377, no. 1–2, pp. 207–214, Jul. 2009, doi: 10.1016/j.ijpharm.2009.05.020.
- G. Chiu, Tan, Liu, Chang, and Lim, “Perorally active nanomicellar formulation of quercetin in the treatment of lung cancer,” Int J Nanomedicine, p. 651, Feb. 2012, doi: 10.2147/IJN.S26538.
- E. SEMO, E. KESSELMAN, D. DANINO, and Y. LIVNEY, “Casein micelle as a natural nano-capsular vehicle for nutraceuticals,” Food Hydrocoll, vol. 21, no. 5–6, pp. 936–942, Jul. 2007, doi: 10.1016/j.foodhyd.2006.09.006.
- A. Shpigelman, G. Israeli, and Y. D. Livney, “Thermally-induced protein–polyphenol co-assemblies: beta lactoglobulin-based nanocomplexes as protective nanovehicles for EGCG,” Food Hydrocoll, vol. 24, no. 8, pp. 735–743, Nov. 2010, doi: 10.1016/j.foodhyd.2010.03.015.
- A. Alishahi et al., “Shelf life and delivery enhancement of vitamin C using chitosan nanoparticles,” Food Chem, vol. 126, no. 3, pp. 935–940, Jun. 2011, doi: 10.1016/j.foodchem.2010.11.086.
- K. K. Kandimalla, R. J. Babu, and M. Singh, “Biphasic flux profiles of melatonin: The Yin–Yang of transdermal permeation enhancement mediated by fatty alcohol enhancers,” J Pharm Sci, vol. 99, no. 1, pp. 209–218, Jan. 2010, doi: 10.1002/jps.21812.
- P. R. Nepal, H.-K. Han, and H.-K. Choi, “Enhancement of solubility and dissolution of Coenzyme Q10 using solid dispersion formulation,” Int J Pharm, vol. 383, no. 1–2, pp. 147–153, Jan. 2010, doi: 10.1016/j.ijpharm.2009.09.031.
- L. A. Wegiel, L. J. Mauer, K. J. Edgar, and L. S. Taylor, “Crystallization of Amorphous Solid Dispersions of Resveratrol during Preparation and Storage—Impact of Different Polymers,” J Pharm Sci, vol. 102, no. 1, pp. 171–184, Jan. 2013, doi: 10.1002/jps.23358.
- S. Onoue et al., “Novel solid self-emulsifying drug delivery system of coenzyme Q10 with improved photochemical and pharmacokinetic behaviors,” European Journal of Pharmaceutical Sciences, vol. 46, no. 5, pp. 492–499, Aug. 2012, doi: 10.1016/j.ejps.2012.03.015.
- S. P. Yap and K. H. Yuen, “Influence of lipolysis and droplet size on tocotrienol absorption from self-emulsifying formulations,” Int J Pharm, vol. 281, no. 1–2, pp. 67–78, Aug. 2004, doi: 10.1016/j.ijpharm.2004.05.015.
- J. Frank, X. W. D. Chin, C. Schrader, G. P. Eckert, and G. Rimbach, “Do tocotrienols have potential as neuroprotective dietary factors?,” Ageing Res Rev, vol. 11, no. 1, pp. 163–180, Jan. 2012, doi: 10.1016/j.arr.2011.06.006.
- T. Iosio et al., “Oral bioavailability of silymarin phytocomplex formulated as self-emulsifying pellets,” Phytomedicine, vol. 18, no. 6, pp. 505–512, Apr. 2011, doi: 10.1016/j.phymed.2010.10.012.
- A. Alishahi et al., “Shelf life and delivery enhancement of vitamin C using chitosan nanoparticles,” Food Chem, vol. 126, no. 3, pp. 935–940, Jun. 2011, doi: 10.1016/j.foodchem.2010.11.086.
- F. Sansone, T. Mencherini, P. Picerno, M. d’Amore, R. P. Aquino, and M. R. Lauro, “Maltodextrin/pectin microparticles by spray drying as carrier for nutraceutical extracts,” J Food Eng, vol. 105, no. 3, pp. 468–476, Aug. 2011, doi: 10.1016/j.jfoodeng.2011.03.004.
- V. B. Patravale and S. D. Mandawgade, “Novel cosmetic delivery systems: an application update,” Int J Cosmet Sci, vol. 30, no. 1, pp. 19–33, Feb. 2008, doi: 10.1111/j.1468-2494.2008.00416.x.
- C. Martínez-Sancho, R. Herrero-Vanrell, and S. Negro, “Vitamin A palmitate and aciclovir biodegradable microspheres for intraocular sustained release,” Int J Pharm, vol. 326, no. 1–2, pp. 100–106, Dec. 2006, doi: 10.1016/j.ijpharm.2006.07.010.
- F. Sansone, T. Mencherini, P. Picerno, M. d’Amore, R. P. Aquino, and M. R. Lauro, “Maltodextrin/pectin microparticles by spray drying as carrier for nutraceutical extracts,” J Food Eng, vol. 105, no. 3, pp. 468–476, Aug. 2011, doi: 10.1016/j.jfoodeng.2011.03.004.
- C. Martínez-Sancho, R. Herrero-Vanrell, and S. Negro, “Vitamin A palmitate and aciclovir biodegradable microspheres for intraocular sustained release,” Int J Pharm, vol. 326, no. 1–2, pp. 100–106, Dec. 2006, doi: 10.1016/j.ijpharm.2006.07.010.
- W. Chen, S. K. Park, W. Yu, A. Xiong, B. G. Sanders, and K. Kline, “Synthesis and screening of novel vitamin E derivatives for anticancer functions,” Eur J Med Chem, vol. 58, pp. 72–83, Dec. 2012, doi: 10.1016/j.ejmech.2012.09.045.
- J. Zhang and S. Wang, “Topical use of Coenzyme Q10-loaded liposomes coated with trimethyl chitosan: Tolerance, precorneal retention and anti-cataract effect,” Int J Pharm, vol. 372, no. 1–2, pp. 66–75, May 2009, doi: 10.1016/j.ijpharm.2009.01.001.
- C.-C. Peng, J. Kim, and A. Chauhan, “Extended delivery of hydrophilic drugs from silicone-hydrogel contact lenses containing Vitamin E diffusion barriers,” Biomaterials, vol. 31, no. 14, pp. 4032–4047, May 2010, doi: 10.1016/j.biomaterials.2010.01.113.
- Y. Zhong, Y.-S. Chiou, M.-H. Pan, and F. Shahidi, “Anti-inflammatory activity of lipophilic epigallocatechin gallate (EGCG) derivatives in LPS-stimulated murine macrophages,” Food Chem, vol. 134, no. 2, pp. 742–748, Sep. 2012, doi: 10.1016/j.foodchem.2012.02.172.
- P. Chantarawaratit, P. Sangvanich, W. Banlunara, K. Soontornvipart, and P. Thunyakitpisal, “Acemannan sponges stimulate alveolar bone, cementum and periodontal ligament regeneration in a canine class II furcation defect model,” J Periodontal Res, vol. 49, no. 2, pp. 164–178, Apr. 2014, doi: 10.1111/jre.12090.
- Y. Li, J. Zheng, H. Xiao, and D. J. McClements, “Nanoemulsion-based delivery systems for poorly water-soluble bioactive compounds: Influence of formulation parameters on polymethoxyflavone crystallization,” Food Hydrocoll, vol. 27, no. 2, pp. 517–528, Jun. 2012, doi: 10.1016/j.foodhyd.2011.08.017.
- K. Maiti, K. Mukherjee, A. Gantait, B. P. Saha, and P. K. Mukherjee, “Curcumin–phospholipid complex: Preparation, therapeutic evaluation and pharmacokinetic study in rats,” Int J Pharm, vol. 330, no. 1–2, pp. 155–163, Feb. 2007, doi: 10.1016/j.ijpharm.2006.09.025.
- C. R. FILBURN, R. KETTENACKER, and D. W. GRIFFIN, “Bioavailability of a silybin–phosphatidylcholine complex in dogs,” J Vet Pharmacol Ther, vol. 30, no. 2, pp. 132–138, Apr. 2007, doi: 10.1111/j.1365-2885.2007.00834.x.
- J. F. Rebhun, S. J. Roloff, R. A. Velliquette, and S. R. Missler, “Identification of evodiamine as the bioactive compound in evodia (Evodia rutaecarpa Benth.) fruit extract that activates human peroxisome proliferator-activated receptor gamma (PPAR?),” Fitoterapia, vol. 101, pp. 57–63, Mar. 2015, doi: 10.1016/j.fitote.2014.12.009.
- S. H. Lee et al., “Progress in the Studies on Rutaecarpine,” Molecules, vol. 13, no. 2, pp. 272–300, Feb. 2008, doi: 10.3390/molecules13020272.
- A. Henkel et al., “Boswellic acids target the human immune system-modulating antimicrobial peptide LL-37,” Pharmacol Res, vol. 102, pp. 53–60, Dec. 2015, doi: 10.1016/j.phrs.2015.09.002.
- Z.-Y. Cai et al., “Bioavailability of Tea Catechins and Its Improvement.,” Molecules, vol. 23, no. 9, Sep. 2018, doi: 10.3390/molecules23092346.
- M. Takahashi, S. Uechi, K. Takara, Y. Asikin, and K. Wada, “Evaluation of an Oral Carrier System in Rats: Bioavailability and Antioxidant Properties of Liposome-Encapsulated Curcumin,” J Agric Food Chem, vol. 57, no. 19, pp. 9141–9146, Oct. 2009, doi: 10.1021/jf9013923.
- N. Dixit, S. Baboota, K. Kohli, S. Ahmad, and J. Ali, “Silymarin: A review of pharmacological aspects and bioavailability enhancement approaches,” Indian J Pharmacol, vol. 39, no. 4, p. 172, 2007, doi: 10.4103/0253-7613.36534.
- R. Müller, “Solid lipid nanoparticles (SLN) for controlled drug delivery – a review of the state of the art,” European Journal of Pharmaceutics and Biopharmaceutics, vol. 50, no. 1, pp. 161–177, Jul. 2000, doi: 10.1016/S0939-6411(00)00087-4.
- C. Qian, E. A. Decker, H. Xiao, and D. J. McClements, “Nanoemulsion delivery systems: Influence of carrier oil on ?-carotene bioaccessibility,” Food Chem, vol. 135, no. 3, pp. 1440–1447, Dec. 2012, doi: 10.1016/j.foodchem.2012.06.047.
- S. M. Jafari, E. Assadpoor, B. Bhandari, and Y. He, “Nano-particle encapsulation of fish oil by spray drying,” Food Research International, vol. 41, no. 2, pp. 172–183, Jan. 2008, doi: 10.1016/j.foodres.2007.11.002.
- H. Yu and Q. Huang, “Improving the Oral Bioavailability of Curcumin Using Novel Organogel-Based Nanoemulsions,” J Agric Food Chem, vol. 60, no. 21, pp. 5373–5379, May 2012, doi: 10.1021/jf300609p.
- V. S. Ipar, A. Dsouza, and P. V. Devarajan, “Enhancing Curcumin Oral Bioavailability Through Nanoformulations,” Eur J Drug Metab Pharmacokinet, vol. 44, no. 4, pp. 459–480, Aug. 2019, doi: 10.1007/s13318-019-00545-z.
- A. Bernkop-Schnürch and S. Dünnhaupt, “Chitosan-based drug delivery systems,” European Journal of Pharmaceutics and Biopharmaceutics, vol. 81, no. 3, pp. 463–469, Aug. 2012, doi: 10.1016/j.ejpb.2012.04.007.
- R. Shegokar and R. H. Müller, “Nanocrystals: Industrially feasible multifunctional formulation technology for poorly soluble actives,” Int J Pharm, vol. 399, no. 1–2, pp. 129–139, Oct. 2010, doi: 10.1016/j.ijpharm.2010.07.044.
- K. Mitri, R. Shegokar, S. Gohla, C. Anselmi, and R. H. Müller, “Lutein nanocrystals as antioxidant formulation for oral and dermal delivery,” Int J Pharm, vol. 420, no. 1, pp. 141–146, Nov. 2011, doi: 10.1016/j.ijpharm.2011.08.026.
- F. Tamjidi, M. Shahedi, J. Varshosaz, and A. Nasirpour, “Nanostructured lipid carriers (NLC): A potential delivery system for bioactive food molecules,” Innovative Food Science & Emerging Technologies, vol. 19, pp. 29–43, Jul. 2013, doi: 10.1016/j.ifset.2013.03.002.
- E. SEMO, E. KESSELMAN, D. DANINO, and Y. LIVNEY, “Casein micelle as a natural nano-capsular vehicle for nutraceuticals,” Food Hydrocoll, vol. 21, no. 5–6, pp. 936–942, Jul. 2007, doi: 10.1016/j.foodhyd.2006.09.006.
- H. Li, X. Zhao, Y. Ma, G. Zhai, L. Li, and H. Lou, “Enhancement of gastrointestinal absorption of quercetin by solid lipid nanoparticles,” Journal of Controlled Release, vol. 133, no. 3, pp. 238–244, Feb. 2009, doi: 10.1016/j.jconrel.2008.10.002.
- S. Sut, V. Baldan, M. Faggian, G. Peron, and S. Dall`Acqua, “Nutraceuticals, A New Challenge for Medicinal Chemistry,” Curr Med Chem, vol. 23, no. 28, pp. 3198–3223, Oct. 2016, doi: 10.2174/0929867323666160615104837.
- M. Alexander, A. Acero Lopez, Y. Fang, and M. Corredig, “Incorporation of phytosterols in soy phospholipids nanoliposomes: Encapsulation efficiency and stability,” LWT, vol. 47, no. 2, pp. 427–436, Jul. 2012, doi: 10.1016/j.lwt.2012.01.041.
- T. Ghorbanzade, S. M. Jafari, S. Akhavan, and R. Hadavi, “Nano-encapsulation of fish oil in nano-liposomes and its application in fortification of yogurt,” Food Chem, vol. 216, pp. 146–152, Feb. 2017, doi: 10.1016/j.foodchem.2016.08.022.
- R. Temmerman, B. Pot, G. Huys, and J. Swings, “Identification and antibiotic susceptibility of bacterial isolates from probiotic products,” Int J Food Microbiol, vol. 81, no. 1, pp. 1–10, Feb. 2003, doi: 10.1016/S0168-1605(02)00162-9.
- K. Huanbutta, P. Sriamornsak, I. Singh, and T. Sangnim, “Manufacture of 2D-Printed Precision Drug-Loaded Orodispersible Film Prepared from Tamarind Seed Gum Substrate,” Applied Sciences, vol. 11, no. 13, p. 5852, Jun. 2021, doi: 10.3390/app11135852.
- L.-M. Papaspyridi, N. Aligiannis, P. Christakopoulos, A.-L. Skaltsounis, and N. Fokialakis, “Production of bioactive metabolites with pharmaceutical and nutraceutical interest by submerged fermentation of Pleurotus ostreatus in a batch stirred tank bioreactor,” Procedia Food Sci, vol. 1, pp. 1746–1752, 2011, doi: 10.1016/j.profoo.2011.09.257.
- J. Jampilek, J. Kos, and K. Kralova, “Potential of Nanomaterial Applications in Dietary Supplements and Foods for Special Medical Purposes.,” Nanomaterials (Basel), vol. 9, no. 2, Feb. 2019, doi: 10.3390/nano9020296.
- F. Polia, M. Pastor-Belda, A. Martínez-Blázquez, M.-N. Horcajada, F. A. Tomás-Barberán, and R. García-Villalba, “Technological and Biotechnological Processes To Enhance the Bioavailability of Dietary (Poly)phenols in Humans,” J Agric Food Chem, vol. 70, no. 7, pp. 2092–2107, Feb. 2022, doi: 10.1021/acs.jafc.1c07198.
- A. Babazadeh, B. Ghanbarzadeh, and H. Hamishehkar, “Phosphatidylcholine-rutin complex as a potential nanocarrier for food applications,” J Funct Foods, vol. 33, pp. 134–141, Jun. 2017, doi: 10.1016/j.jff.2017.03.038.
- A. Babazadeh, B. Ghanbarzadeh, and H. Hamishehkar, “Phosphatidylcholine-rutin complex as a potential nanocarrier for food applications,” J Funct Foods, vol. 33, pp. 134–141, Jun. 2017, doi: 10.1016/j.jff.2017.03.038.
- S. Sharma, “An overview of Natural Superdisintegrants,” Research Journal of Topical and Cosmetic Sciences, pp. 113–124, Dec. 2021, doi: 10.52711/2321-5844.2021.00016.
- B. Sasikumar, “Advances in adulteration and authenticity testing of turmeric (Curcuma longa L.),” Journal of Spices and Aromatic Crops, pp. 96–105, Jan. 1970, doi: 10.25081/josac.2019.v28.i2.6072.
- S. Bansal, A. Singh, M. Mangal, A. K. Mangal, and S. Kumar, “Food adulteration: Sources, health risks, and detection methods,” Crit Rev Food Sci Nutr, vol. 57, no. 6, pp. 1174–1189, Apr. 2017, doi: 10.1080/10408398.2014.967834.
- L. R. Aballay, A. R. Eynard, M. del P. Díaz, A. Navarro, and S. E. Muñoz, “Overweight and obesity: a review of their relationship to metabolic syndrome, cardiovascular disease, and cancer in South America,” Nutr Rev, vol. 71, no. 3, pp. 168–179, Mar. 2013, doi: 10.1111/j.1753-4887.2012.00533.x.
- D. Wang, R. Man, M. Shu, H. Liu, Y. Gao, and F. Luan, “Detection of sibutramine and phenolphthalein in functional foods using capillary electrophoresis,” Analytical Methods, vol. 8, no. 3, pp. 621–626, 2016, doi: 10.1039/C5AY02973B.
- R. Pratiwi, R. H. F. Dipadharma, I. J. Prayugo, and O. A. Layandro, “Recent Analytical Method for Detection of Chemical Adulterants in Herbal Medicine,” Molecules, vol. 26, no. 21, p. 6606, Oct. 2021, doi: 10.3390/molecules26216606.
- S.-H. Baek, O.-N. Bae, and J.-H. Park, “Recent Methodology in Ginseng Analysis,” J Ginseng Res, vol. 36, no. 2, pp. 119–134, Apr. 2012, doi: 10.5142/jgr.2012.36.2.119.
- C. Formisano, S. Delfine, F. Oliviero, G. C. Tenore, D. Rigano, and F. Senatore, “Correlation among environmental factors, chemical composition and antioxidative properties of essential oil and extracts of chamomile (Matricaria chamomilla L.) collected in Molise (South-central Italy),” Ind Crops Prod, vol. 63, pp. 256–263, Jan. 2015, doi: 10.1016/j.indcrop.2014.09.042.
- C. Formisano, S. Delfine, F. Oliviero, G. C. Tenore, D. Rigano, and F. Senatore, “Correlation among environmental factors, chemical composition and antioxidative properties of essential oil and extracts of chamomile (Matricaria chamomilla L.) collected in Molise (South-central Italy),” Ind Crops Prod, vol. 63, pp. 256–263, Jan. 2015, doi: 10.1016/j.indcrop.2014.09.042.
- M.-K. Choi and I.-S. Song, “Pharmacokinetic Drug–Drug Interactions and Herb–Drug Interactions,” Pharmaceutics, vol. 13, no. 5, p. 610, Apr. 2021, doi: 10.3390/pharmaceutics13050610.
- P. Riekkinen, M. Riekkinen, J. Sirviö, R. Miettinen, and P. Riekkinen, “Comparison of the effects of acute and chronic ibotenic and quisqualic acid nucleus basalis lesioning,” Brain Res Bull, vol. 27, no. 2, pp. 199–206, Aug. 1991, doi: 10.1016/0361-9230(91)90068-U.
- S. Ahmad et al., “Indian Medicinal Plants and Formulations and Their Potential Against COVID-19–Preclinical and Clinical Research,” Front Pharmacol, vol. 11, Mar. 2021, doi: 10.3389/fphar.2020.578970.
- W. Aidi Wannes and B. Marzouk, “Research progress of Tunisian medicinal plants used for acute diabetes,” Journal of Acute Disease, vol. 5, no. 5, pp. 357–363, Sep. 2016, doi: 10.1016/j.joad.2016.08.001.
- N. Kandiah et al., “Treatment of dementia and mild cognitive impairment with or without cerebrovascular disease: Expert consensus on the use of Ginkgo biloba extract, EGb 761 ®,” CNS Neurosci Ther, vol. 25, no. 2, pp. 288–298, Feb. 2019, doi: 10.1111/cns.13095.
- T. S. Villani, W. Reichert, M. G. Ferruzzi, G. M. Pasinetti, J. E. Simon, and Q. Wu, “Chemical investigation of commercial grape seed derived products to assess quality and detect adulteration,” Food Chem, vol. 170, pp. 271–280, Mar. 2015, doi: 10.1016/j.foodchem.2014.08.084.
- R. K. Harwansh, S. Chauhan, R. Deshmukh, and R. Mazumder, “Recent Insight into Herbal Bioactives-based Novel Approaches for Chronic Intestinal Inflammatory Disorders Therapy,” Curr Pharm Biotechnol, vol. 25, no. 14, pp. 1835–1857, Oct. 2024, doi: 10.2174/0113892010282432231222060355.
- R. G. Kerry, G. Das, U. Golla, M. del Pilar Rodriguez-Torres, H.-S. Shin, and J. K. Patra, “Engineered Probiotic and Prebiotic Nutraceutical Supplementations in Combating Non-communicable Disorders: A Review,” Curr Pharm Biotechnol, vol. 23, no. 1, pp. 72–97, Jan. 2022, doi: 10.2174/1389201021666201013153142.
- K. C. Baral, R. Bajracharya, S. H. Lee, and H.-K. Han, “Advancements in the Pharmaceutical Applications of Probiotics: Dosage Forms and Formulation Technology.,” Int J Nanomedicine, vol. 16, pp. 7535–7556, 2021, doi: 10.2147/IJN.S337427.


 Pooja Sharma *
Pooja Sharma *

 10.5281/zenodo.14739482
10.5281/zenodo.14739482