Abstract
When compared to traditional topical preparations, film-forming sprays, also known as spray film forming systems (SFFSs), offer a number of benefits, including consistent drug distribution and dosage, enhanced bioavailability, a decreased risk of irritation, continuous drug release, and accelerated wound healing through moisture control. Polymers and excipients are used in film-forming sprays to improve preparation properties and increase the stability of active ingredients. Different features in films will be produced by each type of polymer and excipient. Therefore, in order to create a more ideal film-forming spray, it is necessary to investigate the many kinds of polymers and excipients as well as their assessment criteria [1]. Research on polymers as film-forming matrices and the use of these sprays for current or prospective medical uses were covered in the chosen literature. Research on polymers as film-forming matrices and the use of these sprays for current or prospective medical applications were covered in the chosen literature. The kinds and amounts of polymers and excipients, types of sprayers, assessments, and crucial factors that affect spray-ability and film properties are all covered in this article. The review comes to the conclusion that topical drug delivery can be optimized by using both natural and synthetic polymers with in situ film or viscoelastic capabilities. [2]
Keywords
film-forming polymer, topical drug delivery, film-forming solution, film-forming matrices, spray film forming systems (SFFSs
Introduction
Wound therapy now uses liquid, semi-solid, and solid dose forms. Sponges, plasters, and films can be applied individually, or in combination with sterile dressing materials (ointments, creams, gels, liniments, and aqueous and non-aqueous solutions). Nonetheless, in certain instances, the requirement for a sterile bandage and the establishment of aseptic conditions for its administration can hamper the patient's ability to self-treat the wound surface. In these situations, spray film-forming systems (SFFSs) can be used as delivery systems to apply an elastic polymer film that is produced in situ and does not impede mobility locally on the wound surface. Since SFFSs are sprayed at a distance, there is no need for the patient to come into direct touch with the wound area, which lowers the possibility of further mechanical injury to contamination and its wound [3]. In contrast to these dosage forms, SFFSs, particularly those based on aqueous film-forming solutions, can be withdrawn without causing harm. In certain instances, the wound can be cleaned by using a focused water stream. [4] To ensure optimal wound healing, one can manipulate the properties of the films that are generated in situ through the use of SFFSs. The films that are created can be water-washable, biodegradable, and transparent, which makes it simple for the patient and doctor to view the wound. The films' variable occlusiveness allows for adequate, but not excessive, moisture retention (water vapour transmission rate), microbiological protection, and the gas exchange [5] that the wound requires. Patients often do not comply with repeated daily applications of dosage regimens, particularly when treating chronic skin conditions. Therefore, there is ongoing interest in the development of sustained delivery methods for topical medications that allow for less frequent dosing for dermatological therapy. The skin is the body's most accessible organ and, because of its low permeability to environmental micro- and macromolecules, functions as a barrier against them. The average adult's skin has a surface area of roughly 2 m2, and it gets around one-third of the blood that circulates throughout the body [6]. Drug delivery via the skin aims to either transdermal drug absorption in the systemic circulation or topical therapy of skin disorders. [7] The topical route is easy to apply through self-administration, has a wide and varied surface, and delivers an option to both hypodermic injection and oral medication delivery. For topical application of pharmaceutical formulations, a variety of drug delivery systems are available, including gel, transdermal patches, lotions, ointments, creams, and sprays. [8] Whether or not they have an immediate or sustained drug release is determined by the drug pharmacokinetic profile. The most prevalent drawback of patches is skin discomfort. Therefore, in cases of persistent illnesses like athlete's foot, ringworm, and candidiasis, repeated use is necessary. Film-forming agents include cellulose derivatives, chitosan, polyvinyl pyrrolidine, polyvinyl alcohol polymethacrylate copolymers, and polyacrylate copolymers. These compositions contain butanol, isopropanol, and ethanol as solvents. [9] To enhance the films that are created, plasticizers like glycerin, propylene glycol, and polyethylene glycol can be added. FFS eliminates instability by generating supersaturated systems right away after skin application. Consequently, in comparison to other transdermal administration forms, it enhances medication penetration via the skin. The modified version of Fick's law of diffusion explains the idea of supersaturation [10].
Fick's law of diffusion is given as:
J= DKCv/h
where,
J?=?rate of drug permeation per unit area of skin per unit time(flux)
D?=?diffusion coefficient of drug
Cv?=?concentration of drug
h?=?thickness of barrier to diffusion
This equation makes it evident that the drug's concentration and the pace at which it permeates the skin are proportionate.
Comparison of topical drug delivery systems [11]
Transdermal patches and semisolid dose forms are separated by FFS. demonstrating the benefits of both systems in the process. The advantages of film-forming methods over patches and ointments are compiled in Table 1. The drug penetration pattern for each of the three systems is shown in Fig. 1. In the case of transdermal patches, the medication is either formulated as a topical patch to penetrate the skin and reach the target tissue for localized action, or it is stored in a reservoir from which the drug release occurs gradually and is absorbed into the capillaries from where it is transported to systemic circulation. Although the systemic administration of medications is restricted for a variety of reasons, pharmaceuticals integrated into semisolids exhibit their activity on the skin's surface or penetrate into skin layers to reach the site of action. Film-forming devices can be used as patches or semisolids, and they can administer substances topically or transdermal as needed.
Table?1. Comparison of topical drug delivery systems 11.
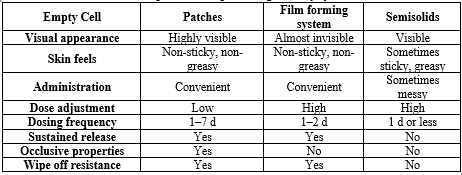
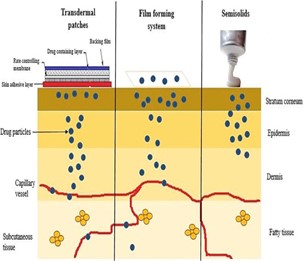
Fig 1: Release profile of the topical and transdermal drug delivery systems [15]
Definition and Mechanism of film-forming spray
An SFFS could be a medication delivery system shaped like a shower arrangement that, by employing the polymer as a network for film arrangement, will form a film when it reaches the intended healing site. Following the film's shape, the medication discharge preparation is similar to a repair, where the medicate is released into a preserved mold using a polymer network 12-13.
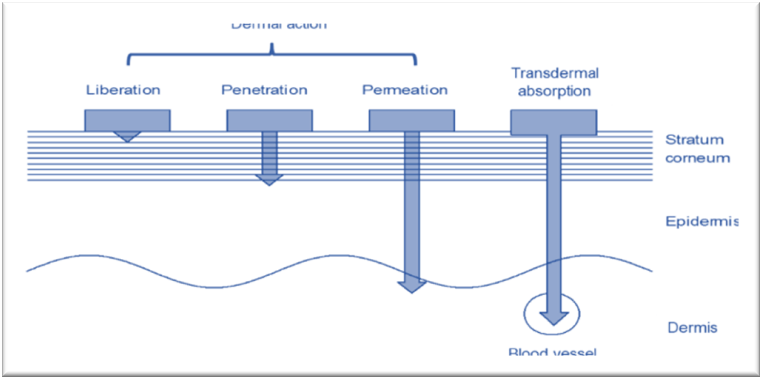
Fig 2: Mechanism of film-forming spray [11]
Various developments have been created during the past few decades to provide effective and efficient spray preparations. A film-forming spray (FFS) is one of them; it is used in many industries, including the food companies, cosmetics, pharmaceuticals, plantations, etc. 14-15 FFS is often made up of polymers, enhancers, and active ingredients soaked in organic solvents. In contrast to other standard topical preparations, a thin, non-sticky film can form that can both increase the drug's permeability and contact time, leading to continuous drug release, and can also inhibit crystallization, increasing the amount of drug accessible to give therapeutic effects. In addition to the film configuration, the disappearance of unstable solvents causes a notable volume loss, which in turn causes a rapid increase in the concentration of dynamic substance within the definition s16-18.
Factors affecting Drug penetration in Dermal Delivery 19
A Physiological factors:
- Thickness of skin.
- Lipid content.
- Regional skin site.
- Density of sweat glands and hair follicles.
- pH of skin.
- Blood flow.
- Skin hydration.
- Inflammation of skin.
B. Physicochemical factors:
- Partition coefficient.
- Molecular weight > 400 Dalton
- Degree of Ionization
- Effect of vehicles
- Skin hydration
- Temperature
- Solubility and Ionization
Film formation mechanisms in SFFSs
Nevertheless, despite the division of these mechanisms, it is clear that they are complementary in some cases, but some take precedence over others. The third mechanism (coalescence-based) is common in tablet coatings. Generally, a water-based dispersion is made and coalescence occurs when the solvent evaporates under the influence of surface-mediated forces. Evaporation-based film formation is associated with high solvent content and low solids content. It is likely that in aerosol spraying the mechanism of film formation relates to coalescence, whereas in spraying via spraying the mechanism is similar to evaporation-based mechanism. The choice of mechanism, and therefore the spraying system, should ideally depend on the nature of the polymers, but to date this has been little described in the literature, and existing methods are more empirical when it
comes to the development of SFFSs. A different mechanism involves exposure to a stimulus or a third-party substance and cross-linking. It is hardly ever used at the current time in the development of SFFSs, but it is common in other areas and can serve as an alternative to the previous two mechanisms with exclusive coating properties 20.
Sprays/solutions
Film forming solutions and sprays is an attractive approach in transdermal dosage form. In this the polymeric solution is applied to the skin as a liquid or sprayed on the skin and forms an almost transparent film by solvent evaporation 21. The film forming sprays/solutions are made up of four main components – drug, solvent systems i.e. volatile and non-volatile vehicles, polymers and penetration enhancers. The non-volatile component present in the solvent system prevents the drug from precipitating in solution when the volatile solvent component evaporates. The non-volatile component is chosen such that it itself partitions rapidly into the stratum corneum and also aids in partitioning of the drug into the stratum corneum, as well as increases drug diffusivity by disrupting the ordered intercellular lipids and enhance permeation. This type of delivery system creates an invisible depot of drug in the stratum corneum from which the drug can be slowly absorbed into the systemic circulation. Thus, a sustained and enhanced permeation of drug across the skin can be achieved following once a day application 22. The formulation preparation involves addition of the polymer to the vehicle and stirring of the solution overnight to ensure complete dissolution of the polymer. Once a clear polymeric solution is obtained other optional excipients such as cross linker or plasticizer are added. After addition of all excipients the solution is stirred for 24?h. For the physical stability of the API, the polymers are chosen such that they function as anti-nucleating agents and crystallization inhibitors which prevent crystallization of drug even after solvent evaporation, e.g. polyvinyl pyrrolidone, polyethylene glycol, hydroxyl propyl methyl cellulose 23-24.

Fig 3: Mechanism of Film Forming Spray [24]

Fig 4: Conventional grafting approaches for wound healing. Autologous skin grafts can be obtained from a biopsy of the patient’s own undamaged skin, and this autograft can be either applied directly over the wound, or expanded by meshing techniques or cell culturing prior to application. On the other hand, skin grafts obtained from another individual as a donor (allograft) or from an animal (xenograft), respectively, can be applied temporarily to cover the patient’s wound [24].
Film-Forming Sprayers
Ordinal Spray
The ordinal spray is a type of spray that does not use unique technology in the spraying process, generally employing a plastic or aluminum container with a dip tube diameter of 1.2 mm and an aperture size of 0.3 mm 25. The average spray angle produced is 78.69–87.39° 26-27. The average amount of film-forming solution that can be sprayed is 0.11–0.35 g or mL 28. The average leakage rate of an ordinal spray container is 0.01–0.03 ). An ordinal spray can be either horizontal or vertical. The 3 K® Horizontal Spray Nozzle (Ursatec, St. Wendel, Germany) has been reported to be able to maintain the sterility of the film-forming solution during storage and use. The spray force of the ordinal spray also varies depending on the type and concentration of the polymer used 30-31. The ordinal spray can also be used for extract preparations 32.
Metered Dose Spray 33-36
The metered dose spray (MDS) is a spray device that can adjust the amount of spray. This tool is generally used to deliver preparations to the systemic compartment via the transdermal or transmucosal route. Therefore, in evaluating a film-forming spray, the spray volume needs to be considered because it is related to the dose of the drug. The spray volume of the MDS can be influenced by the volume available in the bottle, the homogeneity of the particle dispersion, and the position of the container during use. The average amount of FFS that can be sprayed is 90–102 ml. The average spray angle of MDS is 83.51°. The average leakage rate of an MDS container is 0.01–0.02 %.
AERESOLS
Pharmaceutical aerosols are products that contain therapeutically active ingredients which are packed under pressure and are previously released upon activation of an appropriate actuator and valve system. The term “aerosols” was originally accustomed to describe a liquid or solid fine mists particles with a specific size range. The term “aerosols” now refers to pressurized packed products that contain the active ingredient(s) and excipients that are dispersed in a liquefied or compressed gas known as the propellant. It is by the power of this propellant that the contents of the aerosols are expelled from their container [37].
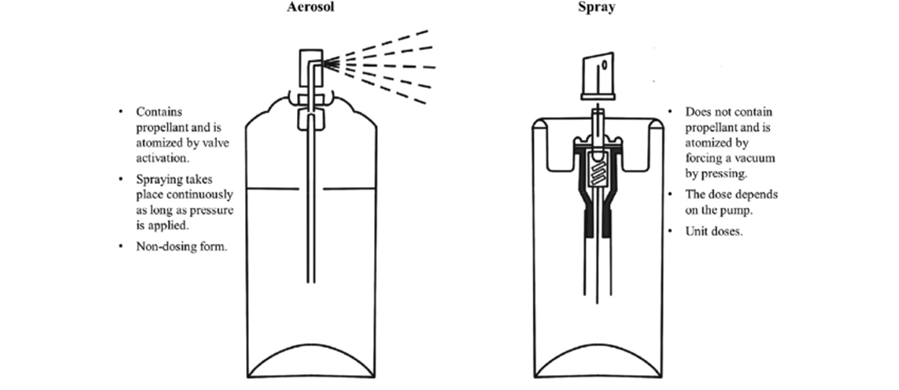
Fig 5: Aerosol Systems [39]
Film forming solutions can be applied with an applicator to the skin and allowed to dry. Film forming spray is manufactured as a metered dose pump dispenser to provide fixed amount of drug and it is sprayed on the topical site to form a film. These systems form a stable fast drying, non-irritating invisible film from which the drug is available for transdermal therapy 38. SFFs creates supersaturated systems immediately after application to the skin, overcoming the problem of instability. Thus, it improves the drug permeation through skin compared to other transdermal dosage forms 39.
Electrostatic Spray 40-43
Electrostatic spray (ES) is used extensively in the agricultural field of pesticide application. ES can improve the deposition efficiency, speed of droplet formation, uniformity of coverage, and reduce the loss to drift. The performance of ES is influenced by the viscosity, surface tension, and electrical resistivity of the solution. A solution cannot be sprayed with ES if the conductivity is not within 10?8-10?5 S/m. The size of the droplets produced by ES ranges from 4–26 µm with an average diameter of 6.3–12 µm.
Ultrasonic Spray 44-46
Ultrasonic spraying technology is indeed a powerful tool for producing thin films and coatings. Ultrasonic Spray Technology Overview:
The ultrasonic spray nozzle is capable of producing droplets with diameters ranging from 1 µm to 10 µm. This fine control allows for the deposition of very thin, uniform films.
The nozzle typically has a diameter of 0.5 mm. This small size contributes to the ability to create very fine droplets.
The resonant frequency of the ultrasonic electrode used is 10 MHz. This high frequency is crucial for generating the small droplet sizes necessary for precise applications.
The nozzle can operate under both low and high pressures, which adds flexibility to its applications and can impact the uniformity and size of the droplets produced.
In the medical industry, ultrasonic spraying is particularly advantageous for producing layer-by-layer (LBL) coatings. This technique ensures superior particle size uniformity compared to traditional LBL spraying methods.
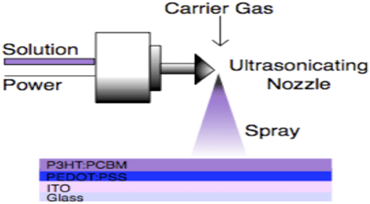
Fig 6: Ultrasonic Spray [47]
Polymer Compatibility 48-50
Ultrasonic spray systems are versatile and can be adapted to work with a variety of polymers, both natural and synthetic. The choice of polymer affects the performance of the spray system, as different polymers may have different viscosities, solubilities, and film-forming properties. For instance:
Natural Polymers:
These might include biopolymers like alginate, chitosan, or cellulose derivatives. They often require careful adjustment of the spray parameters to ensure proper film formation.
Synthetic Polymers:
Examples include polyvinyl alcohol (PVA), polystyrene, or polymethylmethacrylate (PMMA). These materials often have different processing requirements compared to natural polymers.
By matching the specific sprayer specifications to the properties of the polymer being used, it’s possible to achieve optimal results in terms of film quality, consistency, and application efficiency.
Polymers Used in Film Forming Sprays 51-54
Polymers that have thermo-sensitive properties will form a solution at room temperature and turn into a gel when they are exposed to the body temperature, while those that have pH-sensitive properties will form a solution at a certain pH and turn into a gel if the pH of the system changes. Viscoelastic polymers start at a thick consistency but can become elastic when placed under pressure (sprayed) and return to a thick consistency after the pressure is removed.
Natural and Semi-Synthetic Polymers
- Cellulose 55-59
Ethyl cellulose is known for forming films that can be easily washed away with water. Typically, films with optimal characteristics are achieved with ethyl cellulose concentrations between 5.02% and 5.25%, often in combination with Eudragit. Hydroxy propyl methyl cellulose (HPMC) tends to have a slower drying time, but at a concentration of 2%, it produces clear, thin, and smooth films.
Sodium carboxymethylcellulose (Na-CMC) is characterized by its thixotropic flow, meaning it becomes thinner under pressure, making it easier to spray, and then returns to its original consistency. For effective spraying, the maximum concentration of Na-CMC is 2.5%, but the optimal concentration for producing films with excellent adhesion and consistent dosing is 1.5%. Additionally, Na-CMC helps maintain stability and ensures controlled drug release, enhancing its therapeutic efficacy.
- Cyclodextrin 60
Cyclodextrin is recognized for its ability to preserve drug stability by preventing crystal deformation. Additionally, it has a minimal effect on increasing the viscosity of the film-forming solution, making it easier to spray.
- Xanthan Gum 61
Based on research conducted by Shilin Wang, the spray ability of xanthan gum was strongly influenced by its viscosity. The addition of surfactants in the xanthan gum solution decreased the surface tension and reduced the size of the droplet. Interestingly, the viscosity and flow properties were not significantly changed. In addition, the spray angle and coverage area of the xanthan gum solution decreased with increasing xanthan gum concentration.
- Chitosan 62-64
Chitosan shows enhanced conductivity with increased molecular weight, which facilitates its delivery via electrostatic spray. Its viscosity decreases as the degree of deacetylation rises, allowing chitosan to form films with a higher density of smaller droplets, typically between 4 and 27 ?m in diameter. At higher degrees of deacetylation, chitosan becomes more hydrophilic, although this does not directly affect its permeability to water vapor. While its tensile strength increases with greater deacetylation, its elongation tends to decrease. In addition to its film-forming properties, chitosan offers antimicrobial, antioxidant, and mucoadhesive activities, making it a valuable material for topical drug delivery. It also has a relatively high surface tension. Furthermore, the incorporation of PEG 400 can enhance the stability and solubility of drugs when combined with chitosan.
Synthetic Polymers
- Carbopol 65-66
Carbopol exhibits thixotropic flow properties, making it versatile in various applications. It forms an amorphous hydrogel that is particularly beneficial for treating open wounds due to its ability to both donate and absorb moisture. The viscoelastic properties of Carbopol enhance the diffusion coefficient of drugs, improving their efficacy. Combining Carbopol with Poloxamer has been found to be more effective than using Carbopol alone. At a concentration of 0.05%, this polymer blend produces a film with excellent spray ability characterized by favorable spray angle, drying time, and uniform content per spray while ensuring satisfactory drug release. Additionally, Carbopol is known for creating gels that are more resistant to heating, adding to its versatility.
- Eudragit 67,64,68
Eudragit is a versatile group of synthetic polymers available in various types, each serving specific functions. While these polymers are commonly used as additives in tablets to modify drug release, they are also recognized for enhancing drug permeation through the skin, making them popular in topical preparations.
Different types of Eudragit produce distinct film characteristics:
Eudragit EPO, Eudragit E 100, Eudragit S 100, Eudragit RL 100, and Eudragit RS 100 create transparent and shiny films.
Eudragit RSPO and RLPO do not produce transparent films.
Among these, films made from Eudragit EPO, Eudragit E 100, Eudragit RL 100, and Eudragit RS 100 are not washable with water, while Eudragit S100 forms a film that can be removed with water due to its solubility above pH 7. Notably, Eudragit S100 does not cause skin irritation. In in vitro permeability tests, Eudragit RS produced a thicker film on a silicone membrane, likely due to the crystallization of methylphenidate, which could have decreased permeation. However, the methylphenidate penetration was higher with the Eudragit RS film compared to Eudragit E. Films made with Eudragit L100 were found to be ineffective in preventing the crystallization of testosterone. Eudragit RS 100 is noted for its good spray ability, adhesiveness, and flexibility. However, using it at concentrations above 15?n diminish its water washability. In contrast, Eudragit RLPO yields better film performance when used at a concentration of 10.05% in combination with 5.02% ethyl cellulose.
- Lutrol 69
Lutrol F-127 exhibits film characteristics and spray patterns similar to those of Carbopol 940 but offers several advantages. It delivers a more consistent dose of the drug in each spray, resulting in a smaller standard deviation. Additionally, Lutrol F-127 films facilitate better drug release compared to those made with Carbopol 940. Importantly, Lutrol F-127 has not been associated with skin irritation.
Excipients Used in Film Forming Sprays
Cross Linkers 70
The inclusion of crosslinkers can significantly influence various properties of polymers, including their elasticity, viscosity, solubility, glass transition temperature, and film stiffness. For instance, using NaCl as a crosslinker in gellan gum impacts the gel's sensitivity to temperature, leading to improved and accelerated film formation. Additionally, NaCl enhances cell encapsulation within gellan gum, making it a valuable additive for applications involving cell-based systems. In the context of film formation for drug delivery systems, plasticizers like polyethylene glycol (PEG) and propylene glycol (PG) play crucial roles. Here’s a breakdown of their functions and effects:
Plasticizer and Stabilizing Agents 71,72
- Plasticizer Functions:
- Maintains Elasticity: Plasticizers help in keeping the film flexible, which prevents cracking.
- Stability of Active Substances: They can stabilize active ingredients within the film.
- Drug Permeation: Both PEG and PG enhance the permeation of drugs through the skin.
- Propylene Glycol (PG):
Role as a Plasticizer:
PG contributes to the film's flexibility and prevents it from becoming brittle.
Role as a Solubiliser:
It helps in dissolving drugs, facilitating their passage through the skin.
Effect on Viscosity:
PG significantly impacts the viscosity of the film-forming solution, which influences the final film's properties.
Crystallization Issue:
When mixed with water and ethanol, PG does not effectively prevent the crystallization of substances like testosterone.
Optimal Concentration:
To enhance drug permeation, PG concentrations should be kept below 5%.
- Polyethylene Glycol (PEG):
Volume per Spray:
Higher concentrations of PEG 400 increase the volume of solution delivered per spray.
Spray Coverage:
Increased PEG concentrations also lead to a larger covered area.
Effect on Vapour Pressure:
PEG, being a non-volatile solvent, decreases the vapor pressure, which affects the spray characteristics.
Permeation Enhancers 73-82
Eutectic blends, like the mixture of camphor and menthol, are powerful tools for enhancing drug permeation. This blend is particularly effective for hydrophobic drugs due to its own hydrophobic nature. When applied, camphor and menthol create a distinctive sensation: a warming effect followed by a gradual cooling sensation. However, it's important to note that this blend can lead to leaching and pore formation in the skin. The camphor-menthol blend has been shown to significantly boost the permeation of antifungal medications such as fluconazole, clotrimazole, and voriconazole when tested in a Franz diffusion cell with nylon membranes. The hydrophobic properties of camphor and menthol facilitate drug permeation by interacting with the lipids in the skin’s outer layer, the stratum corneum. Research by Lu et al. has identified the following order of effectiveness for penetration enhancers in increasing testosterone permeation: azone > isopropyl myristate (IPM) > propylene glycol (PG) > N-methyl-2-pyrrolidone (NMP). For dexketoprofen, the most effective enhancers were found to be lauryl lactate (LA) > IPM > azone > PG. These findings highlight that the effectiveness of penetration enhancers can vary depending on the drug in question. Notably, azone is especially effective for hydrophilic drugs, and its penetration-enhancing ability is further improved when combined with PG.
1. Propylene Glycol (PG)
Role:
Propylene glycol serves as both a plasticizer and a solubilizer. It helps in dissolving the active ingredients, enhancing their ability to permeate through the skin.
Mechanism:
PG increases the fluidity of the film and can disrupt the lipid structure of the stratum corneum, facilitating better drug diffusion.
Applications:
It is used in various topical formulations, including film-forming sprays, to improve drug absorption and enhance skin penetration.
2. Isopropyl Myristate (IPM)
Role:
Isopropyl myristate is an effective penetration enhancer known for its ability to reduce skin barrier resistance.
Mechanism:
IPM works by disrupting the stratum corneum lipids, which increases skin permeability and allows for greater drug penetration.
Applications:
It is commonly used in topical and transdermal formulations, including film-forming sprays, to enhance the delivery of both hydrophobic and hydrophilic drugs.
4. Azone
Role:
Azone significantly increases the permeation of both hydrophobic and hydrophilic drugs, making it versatile for a range of active ingredients used in film-forming sprays. The effective concentration of Azone must be optimized to balance enhanced permeation with potential irritation. Typically, concentrations range from 1% to 5%, depending on the specific formulation and drug.
Mechanism:
Azone disrupts the lipid bilayer structure of the stratum corneum, the outermost layer of the skin. This disruption increases the permeability of the skin, allowing drugs to penetrate more effectively By interacting with the lipids in the skin, Azone creates transient pathways that facilitate the passage of drugs through the skin barrier.
Application:
Azone is used in film-forming sprays for treating conditions like fungal infections, acne, and localized pain. In addition to pharmaceuticals, Azone is sometimes used in cosmetic formulations to improve the delivery of active ingredients.
Solvents 83-87
In film-forming solutions (FFS), a mix of volatile and non-volatile solvents is employed to achieve an optimal drying rate. Striking the right balance is crucial; if the film dries too rapidly and becomes overly rigid, it can hinder the release and penetration of the drug. To ensure efficient film formation, the active ingredient is typically dissolved to saturation within the solvent, which aids in managing the drying process and maintaining the film’s effectiveness. In film-forming sprays, various vehicles (or carriers) are used to deliver and stabilize the active ingredients while forming a uniform film upon application. These vehicles can be categorized into volatile and non-volatile types, each playing a crucial role in the film formation and drug delivery process. Here’s an overview of common vehicles used:
- Volatile Solvents
Purpose:
These solvents evaporate quickly, helping to form a dry film rapidly after application. They help in controlling the drying time and the final texture of the film.
Examples:
Ethanol:
Often used for its fast evaporation rate and ability to dissolve a wide range of substances.
Isopropyl Alcohol:
Known for quick drying and is used to enhance the evaporation process.
Acetone:
Used in some formulations for its rapid evaporation properties.
2. Non-Volatile Solvents
Purpose: These solvents remain in the film after the volatile components have evaporated, contributing to the film’s flexibility, stability, and adhesive properties.
Examples:
Polyethylene Glycol (PEG):
Provides flexibility and helps in solubilizing active ingredients. PEG 400 is commonly used to increase the volume per spray and improve the spray area coverage.
Propylene Glycol (PG):
Acts as both a plasticizer and a solubilizer, improving drug permeation and film flexibility.
Glycerin:
Used for its moisturizing properties and to maintain film elasticity.
3. Combination Vehicles
Purpose:
Blending volatile and non-volatile solvents can optimize the film-forming process, balancing drying time, film flexibility, and drug release.
Examples:
Ethanol and PEG:
Combining these can provide a good balance of quick drying and film flexibility.
Ethanol and PG:
This mix can enhance both the evaporation rate and the permeation of the active ingredient.
4. Specialty Vehicle
Purpose:
These are used to address specific formulation needs or improve the performance of the film.
Examples:
Silicone-Based Solvents:
Used to create films with enhanced water resistance and flexibility.
Hydrocarbons:
Such as alkanes and alkenes, can be used in specialized formulations for their unique drying and film-forming properties. Each vehicle contributes uniquely to the formulation, influencing the drying rate, film properties, and drug delivery efficiency. The choice of vehicles depends on the desired characteristics of the final film and the specific requirements of the active ingredient being used.
Propellants 88-05
Propellants are substances used in aerosol products to create the force needed to expel the product from its container. They are integral to the operation of aerosol systems, helping to deliver the formulation in a controlled and consistent manner. Propellants can be gases or liquids, and their primary function is to provide pressure within the aerosol container, causing the product to be sprayed out. The most common propellants used in skin film-forming sprays include hydrofluoroalkanes (HFAs) for their low environmental impact and effective performance, liquefied gases like propane and butane for their cost-effectiveness and reliable spray characteristics, carbon dioxide for its safety and eco-friendliness, and nitrous oxide for specific formulation needs. The choice of propellant is guided by factors such as compatibility, safety, and environmental considerations.
Key Considerations in Choosing Propellants:
Compatibility:
The propellant must be compatible with the formulation to avoid reactions that could degrade the active ingredients or affect the stability of the spray.
Safety:
Consideration of the propellant's flammability and toxicity is essential. Hydrocarbons (propane, butane) are flammable, while HFAs and CO2 are generally considered safer in terms of flammability.
Environmental Impact:
With increasing awareness of environmental issues, propellants with lower ozone depletion potential and reduced greenhouse gas effects, like HFAs and CO2, are preferred.
Propellants used in these types of sprays:
1. Hydrofluoroalkanes (HFAs)S
Examples:
HFA-134a, HFA-227ea.
Usage:
HFAs are frequently used in pharmaceutical and some cosmetic aerosols due to their low environmental impact, low toxicity, and effective spray performance. They help in creating a fine, consistent mist without affecting the formulation negatively.
2. Liquefied Gases
Examples:
Propane, butane, isobutane.
Usage:
These hydrocarbon propellants are common in a wide range of aerosol products, including personal care items. They are effective at creating a steady spray and are relatively cost-effective. Propane and butane are often used in combination to achieve desired spray characteristics.
3. Carbon Dioxide (CO2)
Usage:
CO2 is used in some film-forming sprays for its non-flammable nature and its ability to create a gentle, consistent spray. It is also considered more environmentally friendly compared to some other propellants.
4. Nitrous Oxide (N2O)
Usage:
Although less common than hydrocarbon propellants, nitrous oxide is sometimes used in specific formulations. It provides a fine spray and is non-flammable, making it suitable for certain applications.
Evaluation of Film-Forming Sprays
1 Ph 98-98
The pH value is measured and adjusted to improve the stability of the active substance or make it suitable for the area of application. For skin pH ranging from 4–6,102 the pH of diabetic wounds ranges from 6.5–8,103 whereas faster healing time for burns occurs below pH 7.32. The pH adjustment of the preparation aims to prevent irritation and changes in the physiological condition of the wound in the healing process. Besides, the pH value of the dosage can also affect drug permeation through the skin based on the degree of ionization.
2 Viscosity 99-100
Each type and concentration variation of the polymer will result in a different viscosity. The viscosity of the film forming solution will affect its spray ability, so this is an important parameter, especially in MDS. Assessment of the Influence Factors on Nasal Spray Droplet Velocity Using Phase-Doppler Anemometry (PDA). Increasing the concentration of the film-forming solution can reduce the coverage area of the spray.
3 Rheological Properties 101-104
Flow testing aims to determine whether a compound is thixotropic or not. A mixture can easily pass through the sprayer nozzle repeatedly if it has these flow properties. This flowing property allows thinning of the film-forming solution as it shifts along past the nozzle (stressed) and returns to its original viscosity after being sprayed (stress is lost).
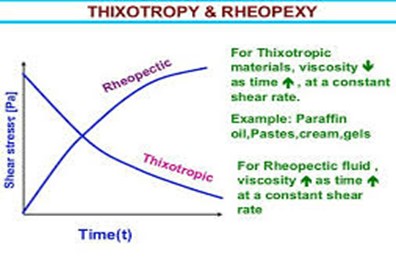
Fig 7: The hysteresis circle, i.e. the area covered by the ascending and descending curves, is characteristic for thixotropic behavior [100]
The nature of this flow can be determined using a rheometer in rotation model with a logarithmic increase in the shear rate of 1–900 s?1 and back again from 900–10 s?1. This test is carried out at room temperature and the storage temperature of the film-forming solution. Testing the flow properties using the oscillation time sweep and amplitude sweep method in various variations of excipient concentration and the temperature range can also be done to find out how the effect of excipient and temperature in the change in gel consistency.
4 Bio adhesive Strength of the Film 105
Measurement of the bio adhesive strength of the film can be done by attaching a film to the surface of the mouse skin (2 x 5 cm). Then, the skin is hydrated with 0.5 mL distilled water. The film is allowed to interact with the tissue surface for 5 minutes.114 The total force (F) to detach the film from the surface of the skin is recorded. The bio adhesive strength (FB) is calculated per unit area (A) of the film.
FB = F/A
5 Water Washability 106
The ease of film wetting is assessed in the dried film. The film is washed with water and assessed in ordinal scale, ie easily washed, moderately washed, and poorly washed.34,40 The ease of sprinkling with water will be useful if the film-forming solutions contact with sensitive areas in the body such as eyes and mouth.
6 Spraying Force 107-110
This test is carried out to find out how much pressure is needed to spray the film-forming solution. The tool that can be used is TA. XT Plus texture analyzer (Stable Micro Systems). Paper soaked with indicator reagents is used, which makes it easy to observe the spray patterns that are formed.81 This depends on the type of solvent and the pH of the film-forming solution. Using a solvent-sensitive paper will clarify the pattern and the spray droplet size distribution. The diameter of the pattern is then measured to determine the area covered and the spray angle.
Spray angle ð = Þ¼ ? tan-11/r
Where ? is the distance of the paper surface from the nozzle and r is the radius of the circle. The distance of the nozzle from the paper is generally around 15 cm.81 The higher the spray angle, the more difficult is it for the film-forming solution to spread when sprayed. An illustration of the spray pattern measurement can be seen in Figure
To measure the covered area, the spray pattern is scanned at 600x600 dpi (Konica Minolta scanner, bizhub c3350). The image is then converted to a binary image using ImageJ software. Then, the percentage of the covered area is calculated using the following equation.
% coverage = (Area covered black pixel X ð X Þ)/(Area original white pixel X ð X Þ) 100%
The particle analysis plugin (ImageJ software) is used to determine the size of the droplets.69 Spraytec® by Malvern (Malvern, UK) can also be used, which works on the principle of laser diffraction.20 Droplet diameter is measured in units of mm, then D10 %, D50% and D90% are determined. The relative span factor (RSF) is then calculated to determine the uniformity of the droplet size distribution using the following equation
RSF = ( D90%-D10%)/(D50%)
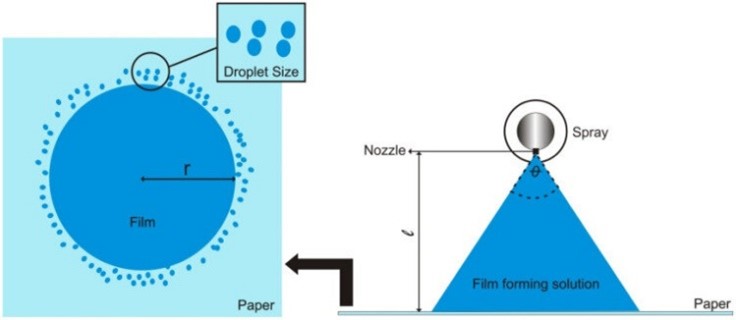
Fig 8: Spray Angle vs %Coverage [108]
8 Permeation Studies 111,112
Permeation Data Analysis The amount of drug that passes through the membrane per unit area to the receptor compartment per unit time is called flux. Flux is expressed in units of mass/area/time. If the dose of the drug is a finite dose, the steady-state flux can be calculated using the following equation
JSS = Q/((AXT))
where Q is the number of drugs that penetrate at time t and A is the area of the membrane exposed to the film-forming solution. The units of JSS is quantity/cm2 /min. The permeation coefficient (Kp) can also be calculated using the following equation.
Kp = Jss/C
where C is the drug concentration in the film-forming solution placed in the donor compartment. The objective in determining the permeation coefficient is to determine the effectiveness of permeation enhancing agents for increasing the permeation of drugs. This can be determined using the enhancement ratio (ER).
ER= (Kp with penetration enhancer)/(Kp without penetration enhancer)
The higher the ER value, the better the effect of penetration enhancers in increasing drug penetration.
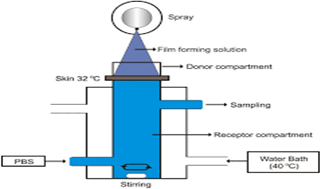
Fig 9: Illustration of ex-vivo permeation testing [109]
9 Stickiness 113
The stickiness of the film formed is determined by pressing cotton wool on the dry film with low pressure. Depending on the quantity of cotton fibers that are retained by the film, the stickiness is rated high if there is dense accumulation of fibers on the film, medium if there is a thin fiber layer on the film and low if there is an occasional or no adherence of fibers. This evaluation parameter is essential, as the formulation should be non-sticky to avoid adherence to the patients’ outfits.
10 Stability Study 114-116
Characteristics commonly tested are changes in particle size, chemical and 3D structures, levels, and therapeutic activity of active substances after storage under various conditions. In some cases, thermal analysis is also conducted to find out whether or not recrystallisation of metastable active substances occurs. The polymer used is an anti-nucleate, which can maintain the drug in the initial crystalline form. In several studies, the content of the drug per spray and its spray pattern as a metered-dose spray will be tested again. It is essential to guarantee the dose during the storage period.
11 Drug Content per Spray and Uniformity 117-119
The dose uniformity of each spray is determined by measuring the weight or volume of each spray, which is then used to obtain the amount of active substance based on its concentration in the film-forming solution. The level of the active substance can also be determined by collecting the sprayed solution, then measuring it instrumentally. To determine how much spray volume comes out, the film-forming solution coming out of the nozzle should not be weighed, but rather the weight of the film-forming solution remaining in the sprayer should be determined. Because the droplets that come out of the spray are so small and easily carried by the wind, not all of it will likely be collected to be weighed. The following equation is used for measuring spray volume:
V = (Wt-Wo)/D
where V is the spray volume, Wt is the weight of the film-forming solution after spraying, Wo is the weight of the film-forming solution beforehand, and D is the specific gravity of the film-forming solution determined using the pycnometer method.20 This test is critical in the use of metered-dose sprays. Drug levels are determined at sprays 5, 10, 20, 30, and 50 by collecting sprays, then measuring them instrumentally.
Applications
Film-forming sprays are versatile tools used in a variety of fields due to their ability to create protective, functional, or aesthetic layers. Here are some of the key applications across different industries:
Medical Applications 120,121
Wound Care:
Film-forming sprays are used to create a protective barrier over wounds, which helps in keeping out contaminants, preventing infection, and promoting faster healing. These sprays often contain biocompatible polymers or other agents that form a flexible, breathable film.
Burns and Skin Protection:
For treating burns or sensitive skin areas, film-forming sprays can create a protective layer that minimizes pain, shields the area from external irritants, and aids in recovery.
Drug Delivery:
Certain film-forming sprays are designed to deliver medications directly to the skin or mucous membranes, allowing for localized treatment of conditions such as fungal infections or chronic skin conditions.
Cosmetic Applications 122-124
Setting Sprays:
In the cosmetics industry, film-forming sprays are used as setting sprays to lock makeup in place, providing a long-lasting finish and helping to prevent smudging or fading.
Moisturizers and Hydrating Sprays:
These sprays create a thin film that helps to lock in moisture, improving skin hydration and providing a barrier against environmental factors like wind and pollution.
Hair Care:
Film-forming sprays are used in hair products to provide hold, shine, and protection. For instance, hair styling sprays can create a flexible, yet strong film that helps maintain a hairstyle.
Industrial Applications 125
Protective Coatings:
In various industrial settings, film-forming sprays are applied to machinery, tools, or surfaces to provide protection against corrosion, wear, and environmental damage. These coatings can enhance the durability and longevity of equipment.
Anti-Fog and Anti-Scratch Films:
Applied to surfaces like goggles, mirrors, and screens, these sprays form a protective film that prevents fogging or scratching, improving visibility and extending the life of the product.
Consumer Products 126
Household Cleaners:
Some cleaning products use film-forming agents to leave behind a protective layer that repels dust, stains, or dirt. This can make cleaning easier and maintain the appearance of surfaces for longer periods.
Fabric Protection:
Film-forming sprays are used on textiles to create water-resistant or stain-resistant layers. This helps to protect fabrics from spills, stains, and other environmental factors.
5. Food Industry 121
Packaging:
Film-forming sprays are used in the food industry to apply coatings to packaging materials, providing barriers to moisture, oxygen, and other factors that can affect food quality and shelf life
Surface Coatings:
In food processing, sprays can be used to apply protective coatings on food products, such as a glaze on fruits or a coating on baked goods to enhance appearance and texture.
Construction 127
Surface Protection:
In construction, film-forming sprays can be used to protect surfaces from damage during construction or renovation processes. They can also be used as primers or bonding agents for various materials.
Regulatory Compliance 128-131
Regulatory compliance for a topical film-forming spray involves several key aspects depending on the region and specific use of the product. Here’s a general overview:
1. Product Classification
Cosmetic or Drug:
Determine whether the spray is classified as a cosmetic, drug, or both. In many jurisdictions, this depends on the intended use of the product. For example, if the spray has therapeutic claims, it might be regulated as a drug rather than a cosmetic.
2. Regulatory Authorities
U.S. (FDA):
In the United States, the FDA regulates cosmetics under the Federal Food, Drug, and Cosmetic Act. If the product makes drug claims (e.g., treating or preventing a disease), it may fall under FDA’s drug regulations.
EU (EMA/Cosmetic Regulation):
In the European Union, the product would be subject to regulations under the European Medicines Agency (EMA) for drugs or the EU Cosmetic Regulation for cosmetics.
Other Regions:
Compliance requirements vary in other regions, such as Health Canada, the Australian Therapeutic Goods Administration (TGA), or similar bodies in other countries.
3. Ingredients
Safety and Efficacy:
Ensure that all ingredients are approved and meet safety standards. For drugs, this may involve proving efficacy through clinical trials.
Labeling:
Ingredient labeling must comply with local regulations, including any restrictions on specific substances.
4. Manufacturing Practices
Good Manufacturing Practices (GMP):
Adhere to GMP guidelines to ensure the product is consistently produced and controlled. This is crucial for both cosmetic and drug products.
Quality Control:
Implement robust quality control procedures to ensure product safety and effectiveness.
5. Labeling and Claims
Accuracy:
Labels must accurately reflect the product's intended use, benefits, and any other claims. Misleading claims can result in regulatory action.
Instructions for Use:
Include clear instructions for use to ensure safe and effective application.
6. Testing and Documentation
Clinical Testing:
For drug claims, you may need clinical trials to support the efficacy and safety of the product.
Stability Testing:
Ensure that the product maintains its quality and effectiveness over its shelf life.
7. Registration and Approval
Pre-market Approval:
Some regions require pre-market approval or registration before the product can be sold. This could involve submitting data on safety, efficacy, and labeling to the regulatory authority.
8. Post-market Surveillance
Adverse Event Reporting:
Implement a system for monitoring and reporting adverse events or complaints related to the product.
Compliance Audits:
Regular audits to ensure ongoing compliance with regulatory requirements.
Good Manufacturing Practices (GMP): Regardless of classification, ensure adherence to GMP standards to maintain product quality and safety. Each region has specific requirements, so it’s important to consult local regulations and possibly work with regulatory consultants to ensure full compliance.
Environmental Impact: 132
The environmental impact of the spray formulation and its components should be considered, including factors like biodegradability and potential for VOC emissions.
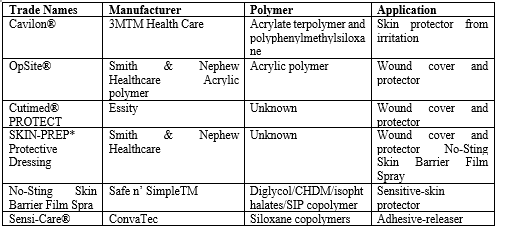
TABLE – Products of Film Forming System 140
Advances In Spray Products and its Technologies 133-139
ARTISS® is another fibrin sealant by Baxter, used to adhere autologous skin grafts to the wound beds in burns and to adhere tissue flaps during facial rhytidectomy surgery, but, in contrast to TISSEEL®, ARTISS® is not employed as an adjunct to hemostasis. Earnshaw used ARTISS® in 50 patients undergoing lateral selective neck dissections, showing a significant reduction in the length of the hospital stay of the patients, in the drain retention time, and in the total volume drained. Moreover, they reported an easier use of ARTISS® in comparison to TISSEEL®
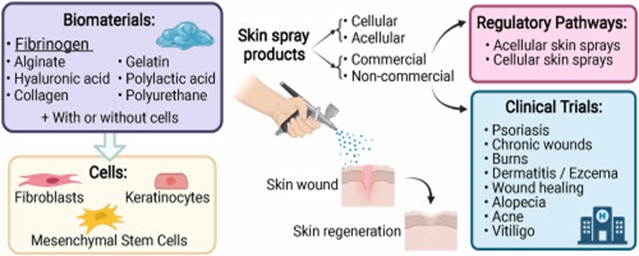
Fig 10: Effectiveness of skin spray and Application Pathway [137]
EVICEL®
by Johnson & Johnson is a fibrin sealant very similar to TISSEEL®, also derived from human pooled plasma, used as an adjunct to hemostasis for use in patients undergoing surgery, with the difference that its airless spray accessory does not need an external gas source, reducing setup time.
Reddy et al. (2017)
conducted an observational prospective study to evaluate the efficiency of EVICEL® for the adherence of skin grafts. They reported better hemostasis and graft adhesion, and a reduction in surgical time.
Vivostat®
has different products, with a system that prepares autologous fibrin sealant or platelet rich fibrin (PRF) from the patient's own blood without requiring additional thrombin and without using cryoprecipitation.
The fibrin is shown to act as a hemostatic, as well as a glue for graft fixations in burn surgery or to adhere cells to the burn wound. Vivostat® PRF has also been successfully used to treat diabetic foot ulcers, venous ulcers, and pressure ulcers The Spraypen is a sterile, disposable, hand-held device that delivers the fibrin sealant or PRF solution to the tissue; but besides the Spraypen, the Vivostat® system offers different types of applicators (e.g. the Endoscopic Applicator). Vivostat® also has a Co-Delivery system that makes it possible to co-deliver a desired substance, such as a cell suspension, along with Vivostat® Fibrin Sealant or Vivostat® PRF. Studies using Vivostat® fibrin sealant reported that its rate of polymerization is significantly faster than that of other sealants that require thrombin, allowing immediate adhesion of cells onto the wound; and since this system produces completely autologous fibrin, any risk of disease transmission is eliminated
REFERENCES
- Renata C. V., Tatiele, K., Silvia, G. S., & Adriana, R. (2016). Drug transport across skin. Drug delivery across physiological barriers. Taylor and Francis Group LLC, 132-134
- Umar, A. K., Butarbutar, M. E. T., Sriwidodo, S., & Wathoni, N. (2020). Film-Forming Sprays for Topical Drug Delivery Drug Design Development and Therapy, Volume 14, 2909–2925. https://doi.org/10.2147/dddt.s256666
- Radhakrishnan, A., Kuppusamy, G., & Karri, V. V. S. R. (2018). Spray bandage strategy in topical drug delivery. Journal of Drug Delivery Science and Technology, 43, 113–121. https://doi.org/10.1016/j.jddst.2017.09.018
- [4]. Alven, S., Peter, S., Mbese, Z., & Aderibigbe, B. A. (2022). Polymer-Based Wound Dressing Materials Loaded with Bioactive Agents: Potential Materials for the Treatment of Diabetic Wounds. Polymers, 14(4), 724. https://doi.org/10.3390/polym14040724
- [5]. Frederiksen, K., Guy, R. H., & Petersson, K. (2015). The potential of polymeric film-forming systems as sustained delivery platforms for topical drugs. Expert Opinion on Drug Delivery, 13(3), 349–360. https://doi.org/10.1517/17425247.2016.1124412
- Technologies Therapeutics. (n.d.). Crescita Therapeutic. http://www.crescitatherapeutics.com/technology/durapeel/
- [Prausnitz, M. R., & Langer, R. (2008). Transdermal drug delivery. Nature Biotechnology, 26(11), 1261–1268. https://doi.org/10.1038/nbt.1504
- Garvie-Cook, H., Frederiksen, K., Petersson, K., Guy, R. H., & Gordeev, S. N. (2015). Biophysical elucidation of the mechanism of enhanced drug release and topical delivery from polymeric film-forming systems. Journal of Controlled Release, 212, 103–112. https://doi.org/10.1016/j.jconrel.2015.06.015
- Touitou, E., Natsheh, H., & Zailer, J. (2023). Film Forming Systems for Delivery of Active Molecules into and across the Skin. Pharmaceutics, 15(2), 397. https://doi.org/10.3390/pharmaceutics15020397
- Madan, J., Iyer, A., Jyothi, V. S. S., Agrawal, A., Khatri, D., Srivastava, S., & Singh, S. (2021b). Does skin permeation kinetics influence efficacy of topical dermal drug delivery system?: Assessment, prediction, utilization, and integration of chitosan biomacromolecule for augmenting topical dermal drug delivery in skin. Journal of Advanced Pharmaceutical Technology Amp Research, 12(4), 345. https://doi.org/10.4103/japtr.japtr_82_21
- Fu, Y., & Kao, W. J. (2010). Drug release kinetics and transport mechanisms of non-degradable and degradable polymeric delivery systems. Expert Opinion on Drug Delivery, 7(4),429–444. https://doi.org/10.1517/17425241003602259
- Soni, A., Dua, J., & Prasad, D. (2022). Article Reviewing Transdermal Drug Delivery System. Journal of Drug Delivery and Therapeutics, 12(1), 176–180. https://doi.org/10.22270/jddt.v12i1.5159
- Madhuri D. Sahane, Maya Y. Gaikwad, Divya S. Gaikar, Spray: Film Forming System Promising As Transdermal Drug Delivery System, Int. J. of Pharm. Sci., 2024, Vol 2, Issue 4, 294-309. https://doi.org/10.5281/zenodo.10931631
- Pharma Excipients | all about excipients & inactive ingredients. (2024, July 18). Pharma Excipients. https://www.pharmaexcipients.com/
- Umar, A. K., Butarbutar, M. E. T., Sriwidodo, S., & Wathoni, N. (2020b).
Film-Forming Sprays for Topical Drug Delivery
Drug Design Development and Therapy, Volume 14, 2909–2925. https://doi.org/10.2147/dddt.s256666
- Kim, D. S., Kim, J. S., & Lee, M. C. (2014). Thin film forming technique based on hybrid spray coating using electrostatic force and air pressure. Japanese Journal of Applied Physics, 53(5S3), 05HC08. https://doi.org/10.7567/jjap.53.05hc08
- Tran, T. T. D., & Tran, P. H. L. (2019). Controlled Release Film Forming Systems in Drug Delivery: The Potential for Efficient Drug Delivery. Pharmaceutics, 11(6), 290. https://doi.org/10.3390/pharmaceutics11060290
- Bakshi, A., Bajaj, A., Malhotra, G., Madan, M., & Amrutiya, N. (2008). A novel metered dose transdermal spray formulation for oxybutynin. Indian Journal of Pharmaceutical Sciences, 70(6), 733. https://doi.org/10.4103/0250-474x.49094
- Physiological and physicochemical considerations are taken into account for drug absorption in topical areas. ORCID. (n.d.). https://orcid.org/0000-0001-7568-9912
- Bakhrushina, E. O., Shumkova, M. M., Sergienko, F. S., Novozhilova, E. V., & Demina, N. B. (2023). Spray film-forming systems as promising topical in situ systems: A Review. Saudi Pharmaceutical Journal, 31(1), 154–169. https://doi.org/10.1016/j.jsps.2022.11.014
- Kathe, K., & Kathpalia, H. (2017). Film forming systems for topical and transdermal drug delivery. Asian Journal of Pharmaceutical Sciences, 12(6), 487–497. https://doi.org/10.1016/j.ajps.2017.07.004
- Lu, W., Luo, H., Wu, Y., Zhu, Z., & Wang, H. (2013). Preparation and characterization of a metered dose transdermal spray for testosterone. Acta Pharmaceutica Sinica B, 3(6), 392–399. https://doi.org/10.1016/j.apsb.2013.10.003
- Nature, T. (2020, February 20). RECRUTEMENT. Cosmétiques Pour Professionnels En Marque Blanche - Tech Nature. http://www.tech-nature.com/
- Scientific Image and Illustration Software | BioRender. (n.d.). https://www.biorender.com/
- Yilmaz, E. G., Ece, E., Erdem, Ö., E?, I., & Inci, F. (2023). A sustainable solution to skin diseases: ecofriendly transdermal patches. Pharmaceutics, 15(2), 579.
- Mori NM, Patel P, Sheth NR, Rathod LV, Ashara KC. Fabrication and characterization of film-forming voriconazole transdermal spray for the treatment of fungal infection. Bull Fac Pharmacy Cairo Univ. 2017;55(1):41–51. doi: 10.1016/j.bfopcu.2017.01.001
- Litmanovich, A. D., Podbel’skiy, V. V., & Kudryavtsev, Y. V. (2011). Lateral ordering during self-organization of statistical multiblock copolymers. Polymer Science Series A, 53(10), 993–1001. https://doi.org/10.1134/s0965545x11100087
- Hakim, M., Walia, H., Rafiq, M., Grannell, T., Cartabuke, R. S., & Tobias, J. D. (2016). Oxymetazoline Metered Dose Spray: Factors Affecting Delivery Volume. The Journal of Pediatric Pharmacology and Therapeutics, 21(3), 247–251. https://doi.org/10.5863/1551-6776-21.3.247
- Geh, K., Stelzl, A., Gröne, A., Wagner, L., Förster, B., & Winter, G. (2019b). Development of a sprayable hydrogel formulation for the skin application of therapeutic antibodies. European Journal of Pharmaceutics and Biopharmaceutics, 142, 123–132. https://doi.org/10.1016/j.ejpb.2019.06.015
- Mirankó, M., Tóth, J., Fodor-Kardos, A., Móricz, K., Szenes-Nagy, A. B., Gácsi, A., Spaits, T., Gyenis, J., & Feczkó, T. (2022). Topical Formulation of Nano Spray-Dried Levocetirizine Dihydrochloride against Allergic Edema. Pharmaceutics, 14(12), 2577. https://doi.org/10.3390/pharmaceutics14122577
- Pawar, N., Parmar, A., Bahmani, K., Mishra, D., Malik, R., Minocha, N., Jalwal, P., & Pawar, R. (2021). Formulation, Optimization and Evaluation of Non-aerosol Topical Spray of Lidocaine for Pain Management. International Journal of Pharmaceutical Investigation, 11(4), 414–419. https://doi.org/10.5530/ijpi.2021.4.74
- Party, P., Bartos, C., Farkas, Á., Szabó-Révész, P., & Ambrus, R. (2021). Formulation and in vitro and in silico characterization of “nano-in-micro” dry powder inhalers containing meloxicam. Pharmaceutics, 13(2), 211.
- Woodcock, A., Janson, C., Rees, J., Frith, L., Löfdahl, M., Moore, A., ... & Leather, D. (2022). Effects of switching from a metered dose inhaler to a dry powder inhaler on climate emissions and asthma control: post-hoc analysis. Thorax, 77(12), 1187-1192.
- Widyastiwi, W., Nurilsyam, T., Roseno, M., & Lhaksmiwati, I. F. (2021). Correlation of Metered Dose Inhaler Use Technique and Asthma Control Level in Asthma Patients at a Hospital in Bandung, West Java, Indonesia. Jurnal Farmasi Galenika (Galenika Journal of Pharmacy)(e-Journal), 7(3), 221-230.
- Shahid, S., Ahmed, F., Shahnaz, G., Saqlain, M., Ans, M., Sana, A., ... & Mubarak, N. (2022). The impact of theoretical and practical guidance regarding metered dose inhaler technique on asthma patients. Journal of Young Pharmacists, 14(3), 327.
- Steiropoulos, P., Bakakos, P., Hatziagorou, E., Katsaounou, P., Loukides, S., Papaioannou, A., ... & Kostikas10, K. (2021). The present and future of inhalation therapy for the management of obstructive airway diseases: Emphasis on pressurized metered-dose inhalers. Pneumon, 34(4), 24.
- Drug Delivery Systems. (2020). In Elsevier eBooks. https://doi.org/10.1016/c2017-0-01074-1
- Gohel and Nagori developed a fluconazole spray containing ethyl cellulose and Eudragit RS 100 as film formers. Gohel, M. C., & Nagori, S. A. (2009). Fabrication of Modified Transport Fluconazole Transdermal Spray Containing Ethyl Cellulose and Eudragit® RS100 as Film Formers. AAPS PharmSciTech, 10(2), 684–691. https://doi.org/10.1208/s12249-009-9256-8
- Rehman, K., & Zulfakar, M. H. (2013). Recent advances in gel technologies for topical and transdermal drug delivery. Drug Development and Industrial Pharmacy, 40(4), 433–440. https://doi.org/10.3109/03639045.2013.828219
- Zhao, D., Cooper, S., Chima, P., Wang, G., Zhang, L., Sun, B., ... & Lan, Y. (2024). Development and Characterization of a Contact-Charging Electrostatic Spray UAV System. Agriculture, 14(3), 467.
- Umar, A. K., Butarbutar, M., Sriwidodo, S., & Wathoni, N. (2020). Film-forming sprays for topical drug delivery. Drug Design, Development and Therapy, 2909-2925.
- Godse, K., Dethe, G., Sawant, S., Sharma, A., Pereira, R., Ghate, S., ... & Patel, K. (2024). Clinical Evaluation of the Safety and Tolerability of Film-Forming Sprays in Patients with Psoriasis and Eczema. Cureus, 16(3).
- Krysiak, Z. J., & Stachewicz, U. (2023). Electrospun fibers as carriers for topical drug delivery and release in skin bandages and patches for atopic dermatitis treatment. Wiley Interdisciplinary Reviews: Nanomedicine and Nanobiotechnology, 15(1), e1829.
- Nan, Y., Zhang, H., Zheng, J., Yang, K., & Ge, Y. (2023). Low-volume precision spray for plant pest control using profile variable rate spraying and ultrasonic detection. Frontiers in Plant Science, 13. https://doi.org/10.3389/fpls.2022.1042769
- Shalunov, A., Khmelev, V., Terentiev, S., Nesterov, V., & Genne, D. (2024). The Development and Analysis of a Multistage Spraying Method for Liquids in an Ultrasonic Field. Applied Sciences, 14(2), 796. https://doi.org/10.3390/app1402079
- Workie, A. B., Ningsih, H. S., & Shih, S. J. (2023). An comprehensive review on the spray pyrolysis technique: Historical context, operational factors, classifications, and product applications. Journal of Analytical and Applied Pyrolysis, 170, 105915. https://doi.org/10.1016/j.jaap.2023.105915
- Steirer, K. X. (2009). Advancing spray deposition for low-cost solar cell production. SPIE Newsroom. https://doi.org/10.1117/2.1200903.1555
- Spisni, G., Massaglia, G., Pirri, C. F., Bianco, S., & Quaglio, M. (2023). Nanostructured Layer Based on Intrinsically Conductive Polymers for Optimising Carbon Electrodes’ Surface: Electrospray and Ultrasonic Spray Coating. Materials Proceedings, 14(1), 53.
- Alves, T. F., Morsink, M., Batain, F., Chaud, M. V., Almeida, T., Fernandes, D. A., ... & Severino, P. (2020). Applications of natural, semi-synthetic, and synthetic polymers in cosmetic formulations. Cosmetics, 7(4), 75.
- Xu, R., Fang, Y., Zhang, Z., Cao, Y., Yan, Y., Gan, L., ... & Zhou, G. (2023). Recent advances in biodegradable and biocompatible synthetic polymers used in skin wound healing. Materials, 16(15), 5459.
- Zarrintaj, P., Jouyandeh, M., Ganjali, M. R., Hadavand, B. S., Mozafari, M., Sheiko, S. S., Vatankhah-Varnoosfaderani, M., Gutiérrez, T. J., & Saeb, M. R. (2019). Thermo-sensitive polymers in medicine: A review. European Polymer Journal, 117, 402–423. https://doi.org/10.1016/j.eurpolymj.2019.05.024
- Sánchez-Moreno, P., De Vicente, J., Nardecchia, S., Marchal, J. A., & Boulaiz, H. (2018). Thermo-Sensitive Nanomaterials: Recent Advance in Synthesis and Biomedical Applications. Nanomaterials, 8(11), 935. https://doi.org/10.3390/nano8110935
- Mutalabisin, M. F., Chatterjee, B., & Jaffri, J. M. (2018). PH Responsive Polymers in Drug Delivery. Research Journal of Pharmacy and Technology, 11(11), 5115. https://doi.org/10.5958/0974-360x.2018.00934.4
- Mohamed, F., Flämig, M., Hofmann, M., Heymann, L., Willner, L., Fatkullin, N., Aksel, N., & Rössler, E. A. (2018). Scaling analysis of the viscoelastic response of linear polymers. The Journal of Chemical Physics, 149(4). https://doi.org/10.1063/1.5038643
- Anjali, P. N., Bosco, S. J. D., Zainab, S., & Sunooj, K. V. (2024). Cellulose and Cellulose Derivative-Based Films. In Polysaccharide Based Films for Food Packaging: Fundamentals, Properties and Applications (pp. 65-94). Singapore: Springer Nature Singapore.
- Akhtar, S., Gupta, A. K., & Kumar, H. (2024). Manufacturing and Applications of Science, 20(4), 384-400.
- Anjali, P. N., Bosco, S. J. D., Zainab, S., & Sunooj, K. V. (2024). Cellulose and Cellulose Derivative-Based Films. In Polysaccharide Based Films for Food Packaging: Fundamentals, Properties and Applications (pp. 65-94). Singapore: Springer Nature Singapore.
- Liang, C., Meng, S., Wang, Y., Xie, X., Zhang, Z., & Cheng, D. (2023). Preparation and activity of sodium carboxymethyl cellulose (CMC-Na) and Metarhizium rileyi ZHKUMR1 composite membrane. International Journal of Biological Macromolecules, 253, 126858.
- Alves, P., Luzio, D., de Sá, K., Correia, I., & Ferreira, P. (2024). Preparation of Gel Forming Polymer-Based Sprays for First Aid Care of Skin Injuries. Gels, 10(5), 297.
- Novatski, A., Ribeiro, M. A., Camilo, A., Lenzi, E. K., Urban, A. M., Schoeffel, A., ... & Farago, P. V. (2023). Chlorhexidine/?-cyclodextrin inclusion complexes by freeze-and spray-drying: Characterization and behavior in aqueous system. Journal of Applied Physics, 133(3).
- Jadav, M., Pooja, D., Adams, D. J., & Kulhari, H. (2023). Advances in xanthan gum-based systems for the delivery of therapeutic agents. Pharmaceutics, 15(2), 402.
- Sriwidodo, N., Subroto, N. T., Maksum, N. I. P., Subarnas, N. A., Kesumawardhany, N. B., Lestari, N. D. M. D. D., & Umar, N. a. K. (2020). Preparation and Optimization of Chitosan-hEGF Nanoparticle Using Ionic Gelation Method Stabilized by Polyethylene Glycol (PEG) for Wound Healing Therapy. International Journal of Research in Pharmaceutical Sciences, 11(1), 1220–1230. https://doi.org/10.26452/ijrps.v11i1.1962
- Deng, W., Zheng, H., Zhu, Z., Deng, Y., Shi, Y., Wang, D., & Zhong, Y. (2023). Effect of Surfactant Formula on the Film Forming Capacity, Wettability, and Preservation Properties of Electrically Sprayed Sodium Alginate Coats. Foods, 12(11), 2197.
- Soujith, N. B., & Jawahar, N. (2023). Terbinafine HCl Film-Forming Spray for the Treatment of Topical Fungal Infections. Indian Journal of Pharmaceutical Education and Research, 57(1), S85-97.
- Sharma, R., & Rana, V. (2021). QbD steered fabrication of Pullulan-Terminalia catappa-Carbopol® 971P film forming gel for improved rheological, textural and biopharmaceutical aspects. International Journal of Biological Macromolecules, 193, 1301-1312.
- Saurabh, A., Deshmukh, D., Nath, S., Agarwal, D., Vivek, K., & Kabiraj, L. (2022). Impingement atomization of carbopol gels. AIAA Journal, 60(11), 6463-6472.
- Panneerselvam, K., & Somaskanthan, S. (2024). Fungal Infections: Effect of Eudragit RL-100 Based Miconazole Film Forming Spray. International Journal of Pharmaceutical Investigation, 14(3), 928–934. https://doi.org/10.5530/ijpi.14.3.102
- Jyothi, V. G. S., Pawar, J., Fernandes, V., Kumar, R., Singh, C., Singh, S. B., Madan, J., & Khatri, D. K. (2022). Film forming topical dermal spray of meloxicam attenuated pain and inflammation in carrageenan-induced paw oedema in Sprague Dawley rats. Journal of Drug Delivery Science and Technology, 70, 103195. https://doi.org/10.1016/j.jddst.2022.103195
- Phaechamud, T., & Choncheewa, C. E. (2016). Double-layered matrix of shellac wax-lutrol in controlled dual drug release. AAPS PharmSciTech, 17, 1326-1335.
- Pilicheva, B., Uzunova, Y., Bodurov, I., Viraneva, A., Exner, G., Sotirov, S., Yovcheva, T., & Marudova, M. (2020). Layer-by-layer self-assembly films for buccal drug delivery: The effect of polymer cross-linking. Journal of Drug Delivery Science and Technology, 59, 101897. https://doi.org/10.1016/j.jddst.2020.101 897
- Montilla?Buitrago, C. E., Gómez?López, R. A., Solanilla?Duque, J. F., Serna?Cock, L., & Villada?Castillo, H. S. (2021). Effect of Plasticizers on Properties, Retrogradation, and Processing of Extrusion?Obtained Thermoplastic Starch: A Review. Starch - Stärke, 73(9–10). https://doi.org/10.1002/star.202100060
- Khalkhali, Z., & Rothstein, J. P. (2020). Characterization of the cold spray deposition of a wide variety of polymeric powders. Surface and Coatings Technology, 383, 125251. https://doi.org/10.1016/j.surfcoat.2019.125251
- Ková?ik, A., Kope?ná, M., & Vávrová, K. (2020). Permeation enhancers in transdermal drug delivery: benefits and limitations. Expert Opinion on Drug Delivery, 17(2), 145–155. https://doi.org/10.1080/17425247.2020.1713087
- Ghasemiyeh, P., & Mohammadi-Samani, S. (2020). Potential of Nanoparticles as Permeation Enhancers and Targeted Delivery Options for Skin: Advantages and Disadvantages. "Drug Design, Development and Therapy, 14, 3271–3289. https://doi.org/10.2147/DDDT.S264648
- Patel, B. A. (2024). PERMEATION ENHANCEMENT AND ADVANCED STRATEGIES: A COMPREHENSIVE REVIEW OF IMPROVED TOPICAL DRUG DELIVERY. International Research Journal of Modernization in Engineering Technology and Science, 6(05), 6691-702.
- Sun, Y., Liu, C., Ren, S., Zhang, Y., Ruan, J., & Fang, L. (2023). Combination of ion-pair strategy and chemical enhancers for design of dexmedetomidine long-acting patches: Dual action mechanism induced longer controlled release and better delivery efficiency. European Journal of Pharmaceutics and Biopharmaceutics, 183, 47–60. https://doi.org/10.1016/j.ejpb.2022.12.014
- Bani, K. S., & Bhardwaj, K. (2021). Topical Drug Delivery Therapeutics, Drug Absorption and Penetration Enhancement Techniques. Journal of Drug Delivery and Therapeutics, 11(4), 105–110. https://doi.org/10.22270/jddt.v11i4.4864
- ClinicalTrials.gov. (n.d.). https://www.clinicaltrials.gov/
- Carrer, V., Alonso, C., Pont, M., Zanuy, M., Córdoba, M., Espinosa, S., Barba, C., Oliver, M. A., Martí, M., & Coderch, L. (2019). Effect of propylene glycol on the skin penetration of drugs. Archives of Dermatological Research, 312(5), 337–352. https://doi.org/10.1007/s00403-019-02017-5
- Boztepe, T., Castro, G. R., & León, I. E. (2021). Lipid, polymeric, inorganic-based drug delivery applications for platinum-based anticancer drugs. International Journal of Pharmaceutics, 605, 120788. https://doi.org/10.1016/j.ijpharm.2021.120788
- Chen, H. L., Cai, C. C., Ma, J. Y., Yu, M. L., Zhao, M. H., Guo, J. B., & Xu, H. (2018). Effect of the Dispersion States of Azone in Hydroalcoholic Gels on Its Transdermal Permeation Enhancement Efficacy. Journal of Pharmaceutical Sciences, 107(7), 1879–1885. https://doi.org/10.1016/j.xphs.2018.02.020
- Chen, Y., Quan, P., Liu, X., Wang, M., & Fang, L. (2014). Novel chemical permeation enhancers for transdermal drug delivery. Asian Journal of Pharmaceutical Sciences, 9(2), 51–64. https://doi.org/10.1016/j.ajps.2014.01.001
- Kurek, M., Descours, E., Galic, K., Voilley, A., & Debeaufort, F. (2012). How composition and process parameters affect volatile active compounds in biopolymer films. Carbohydrate Polymers, 88(2), 646–656. https://doi.org/10.1016/j.carbpol.2012.01.012
- Lombardi, L., Roig-Sanchez, S., Bapat, A., & Frostad, J. M. (2023b). Nonaqueous foam stabilization mechanisms in the presence of volatile solvents. Journal of Colloid and Interface Science, 648, 46–55. https://doi.org/10.1016/j.jcis.2023.05.156
- Lorenz, G., & Kandelbauer, A. (2022). Silicones. In Elsevier eBooks (pp. 659–677). https://doi.org/10.1016/b978-0-12-821632-3.00019-1
- Handbook of Odors in Plastic Materials. (2023). In Elsevier eBooks. https://doi.org/10.1016/c2022-0-02103-6
- Shah, N., Hussain, M., Rehan, T., Khan, A., & Khan, Z. U. (2022). Overview of Polyethylene Glycol-based Materials with a Special Focus on Core-Shell Particles for Drug Delivery Application. Current Pharmaceutical Design, 28(5), 352–367. https://doi.org/10.2174/1381612827666210910104333
- Bolt, H. M. (2024). Propane. In Elsevier eBooks (pp. 929–932). https://doi.org/10.1016/b978-0-12-824315-2.00221-9
- Dandekar, A. A. (2023, May 23). EFFECT OF ENHANCEMENT TECHNOLOGIES ON TRANSDERMAL DELIVERY OF SINGLE AND CO-ADMINISTERED MOLECULES ACROSS NORMAL AND COMPROMISED SKIN. http://hdl.handle.net/10898/13709
- Sharpe, E. E. V. (1956). Aerosol Spraying. Transactions of the IMF, 34(1), 407–419. https://doi.org/10.1080/00202967.1956.11869736
- Wang, H., Leal, J., Ordoubadi, M., Minootan, Z., Lachacz, K., Carrigy, N., Lechuga-Ballesteros, D., & Vehring, R. (2024). Understanding the performance of pressurized metered dose inhalers formulated with low Global warming potential propellants. Aerosol Science and Technology, 58(2), 115–133. https://doi.org/10.1080/02786826.2023.2296930
- Shin, H., Barletta, B., Yoonessi, L., Meinardi, S., Leu, S., Radom?Aizik, S., Randhawa, I., Nussbaum, E., Blake, D. R., & Cooper, D. M. (2015). Quantification of Aerosol Hydrofluoroalkane HFA?134a Elimination in the Exhaled Human Breath Following Inhaled Corticosteroids Administration. Clinical and Translational Science, 8(5), 445–450. https://doi.org/10.1111/cts.12305
- Sun, L., Yu, S., & Liu, D. (2024). Characterization of Aerosol and CO2 Co-Emissions around Power Plants through Satellite-Based Synergistic Observations. Remote Sensing, 16(9), 1609. https://doi.org/10.3390/rs16091609
- Allan, J., Cameron, J., & Bruno, J. (2022). A Systematic Review of Recreational Nitrous Oxide Use: Implications for Policy, Service Delivery and Individuals. International Journal of Environmental Research and Public Health, 19(18), 11567. https://doi.org/10.3390/ijerph191811567
- Shen, L., Yao, Z., Deng, S., Wang, S., Mao, C., & Wang, J. (2023). Solid propellants: A first?principles study of nitrous oxide generation from A3. Propellants Explosives Pyrotechnics, 48(10). https://doi.org/10.1002/prep.202300103
- Vávrová, K., Lorencová, K., Klimentová, J., Novotný, J., Holý, A., & Hrabálek, A. (2008). Transdermal and dermal delivery of adefovir: Effects of pH and permeation enhancers. European Journal of Pharmaceutics and Biopharmaceutics, 69(2), 597–604. https://doi.org/10.1016/j.ejpb.2007.12.005
- Pennington, A., Ratcliffe, J., Wilson, C., & Hardy, J. (1988). The influence of solution viscosity on nasal spray deposition and clearance. International Journal of Pharmaceutics, 43(3), 221–224. https://doi.org/10.1016/0378-5173(88)90277-3
- Ali, S. M., & Yosipovitch, G. (2013). Skin pH: From Basic SciencE to Basic Skin Care. Acta Dermato Venereologica, 93(3), 261–267. https://doi.org/10.2340/00015555-1531
- AAPS PharmSciTech, 12(1), 337–343. https://doi.org/10.1208/s12249-011-9594-1
- Zhong, Y., Zhuang, C., Gu, W., & Zhao, Y. (2019). Effect of molecular weight on the properties of chitosan films prepared using electrostatic spraying technique. Carbohydrate Polymers, 212, 197–205. https://doi.org/10.1016/j.carbpol.2019.02.048
- Ovarlez, G. (2012). Introduction to the rheometry of complex suspensions. In Elsevier eBooks (pp. 23–62). https://doi.org/10.1533/9780857095282.1.23
- Lee, C. H., Moturi, V., & Lee, Y. (2009). Thixotropic property in pharmaceutical formulations. Journal of Controlled Release, 136(2), 88–98. https://doi.org/10.1016/j.jconrel.2009.02.013
- Geh, K., Stelzl, A., Gröne, A., Wagner, L., Förster, B., & Winter, G. (2019). Development of a sprayable hydrogel formulation for the skin application of therapeutic antibodies. European Journal of Pharmaceutics and Biopharmaceutics, 142, 123–132. https://doi.org/10.1016/j.ejpb.2019.06.015
- Grip, J., Engstad, R. E., Skjæveland, I., Škalko-Basnet, N., & Holsæter, A. M. (2017). Sprayable Carbopol hydrogel with soluble beta-1,3/1,6-glucan as an active ingredient for wound healing – Development and in-vivo evaluation. European Journal of Pharmaceutical Sciences, 107, 24–31. https://doi.org/10.1016/j.ejps.2017.06.029
- Kenechukwu, F. C., Attama, A. A., Ibezim, E. C., Nnamani, P. O., Umeyor, C. E., Uronnachi, E. M., Gugu, T. H., Momoh, M. A., Ofokansi, K. C., & Akpa, P. A. (2018). Surface-modified mucoadhesive microgels as a controlled release system for miconazole nitrate to improve localized treatment of vulvovaginal candidiasis. European Journal of Pharmaceutical Sciences, 111, 358–375. https://doi.org/10.1016/j.ejps.2017.10.002
- Bácskay, I., Hosszú, Z., Budai, I., Ujhelyi, Z., Fehér, P., Kósa, D., Haimhoffer, D., & Pet?, G. (2023). Formulation and Evaluation of Transdermal Patches Containing BGP-15. Pharmaceutics, 16(1), 36. https://doi.org/10.3390/pharmaceutics16010036
- Lin, Z., Xie, J., Tian, S., Wang, X., Sun, W., & Mo, X. (2023). Research and experiment of electrostatic spraying system for agricultural plant protection unmanned vehicle. Frontiers in Ecology and Evolution, 11. https://doi.org/10.3389/fevo.2023.1138180
- Fefekos, A. G., Gupta, M., Mahade, S., Björklund, S., & Joshi, S. (2023). Effect of spray angle and substrate material on formation mechanisms and properties of HVAF sprayed coatings. Surface and Coatings Technology, 452, 129115. https://doi.org/10.1016/j.surfcoat.2022.129115
- Smith, R., Ruben, C., Pradhan, O., Brogden, N., & Fiegel, J. (2023). Spray coverage analysis of topical sprays formed by cold thermoreversible hydrogels. Drug Development and Industrial Pharmacy, 49(7), 456–466. https://doi.org/10.1080/03639045.2023.2229919
- Chen, C., Li, S., Wu, X., Wang, Y., & Kang, F. (2022). Analysis of droplet size uniformity and selection of spray parameters based on the biological optimum particle size theory. Environmental Research, 204, 112076. https://doi.org/10.1016/j.envres.2021.112076
- Gohel, M. C., & Nagori, S. A. (2009b). Fabrication of Modified Transport Fluconazole Transdermal Spray Containing Ethyl Cellulose and Eudragit® RS100 as Film Formers. AAPS PharmSciTech, 10(2), 684–691. https://doi.org/10.1208/s12249-009-9256-8
- Nnamani, P. O., Kenechukwu, F. C., Dibua, E. U., Ogbonna, C. C., Momoh, M. A., Olisemeka, A. U., Obumneme, A. C., & Attama, A. A. (2013). Formulation, characterization and ex-vivo permeation studies on gentamicin-loaded transdermal patches based on PURASORB polymers. Scientific Research and Essays, 8(22), 973–982. https://doi.org/10.5897/sre2013.5379
- Ranade, S., Bajaj, A., Londhe, V., Babul, N., & Kao, D. (2017). Fabrication of topical metered dose film forming sprays for pain management. European Journal of Pharmaceutical Sciences, 100, 132–141. https://doi.org/10.1016/j.ejps.2017.01.004
- Nnamani, O. (2013). Characterization and controlled release of gentamicin from novel hydrogels based on Poloxamer 407 and polyacrylic acids. African Journal of Pharmacy and Pharmacology, 7(36), 2540–2552. https://doi.org/10.5897/ajpp2013.3803
- Nnamani, P. O., Kenechukwu, F. C., Dibua, E. U., Ogbonna, C. C., Monemeh, U. L., & Attama, A. A. (2013). Transdermal microgels of gentamicin. European Journal of Pharmaceutics and Biopharmaceutics, 84(2), 345–354. https://doi.org/10.1016/j.ejpb.2012.11.015
- Ter Horst, B., Moakes, R., Chouhan, G., Williams, R., Moiemen, N., & Grover, L. (2019). A gellan-based fluid gel carrier to enhance topical spray delivery. Acta Biomaterialia, 89, 166–179. https://doi.org/10.1016/j.actbio.2019.03.036
- Sipos, Bence, et al. “Spray-dried indomethacin-loaded polymeric micelles for the improvement of intestinal drug release and permeability.” European Journal of Pharmaceutical Sciences, vol. 174, July 2022, p. 106200. https://doi.org/10.1016/j.ejps.2022.106200.
- “Spray-dried indomethacin-loaded polymeric micelles for the improvement of intestinal drug release and permeability.” European Journal of Pharmaceutical Sciences, vol. 174, July 2022, p. 106200. https://doi.org/10.1016/j.ejps.2022.106200.
- Party, Petra, et al. “Formulation and In Vitro and In Silico Characterization of ‘Nano-in-Micro’ Dry Powder Inhalers Containing Meloxicam.” Pharmaceutics, vol. 13, no. 2, Feb. 2021, p. 211. https://doi.org/10.3390/pharmaceutics13020211.
- Amante, C., Neagu, M., Falcone, G., Russo, P., Aquino, R. P., Nicolais, L., & Del Gaudio, P. (2024). Hyaluronate loaded advanced wound dressing in form of in situ forming hydrogel powders: Formulation, characterization, and therapeutic potential. International Journal of Biological Macromolecules, 274, 133192.
- Zhang, H., Lin, X., Cao, X., Wang, Y., Wang, J., & Zhao, Y. (2024). Developing natural polymers for skin wound healing. Bioactive Materials, 33, 355-376.
- Salsabila, E., Wulandari, F., & Rohana, E. (2024). Formulation and SPF Value Evaluation of Sunscreen Spray Gel Containing Lime Peel Extract (Citrus aurantifolia). Pharmacy & Pharmaceutical Sciences Journal/Jurnal Farmasi Dan Ilmu Kefarmasian Indonesia, 11(1).
- Neytal, P., Maji, N., & Maji, S. (2024). Advances in Cosmetic Products Towards a New Future. In Preserving Health, Preserving Earth: The Path to Sustainable Healthcare (pp. 193-214). Cham: Springer Nature Switzerland.
- Waller, I., & Tahon, A. (2024). Unleashing Cosmetic Creativity with Multifunctionality. SOFW Journal (English version), 150(6).
- Tewari, K., Thapliyal, D., Bhargava, C. K., Verma, S., Mehra, A., Rana, S., ... & Arya, R. K. (2024). Innovative Coating Methods for the Industrial Applications. Functional Coatings: Innovations and Challenges, 23-50.
- Lin, S., Zhang, Y., Shao, L., & Lau, C. H. (2024). Spray-assisted assembly of thin-film composite membranes in one process. Advanced Membranes, 4, 100080.
- Chen, L., Teng, J., Liu, G., Zheng, Y., Cui, X., & Zhou, Z. (2024). Effect of high humidity on the initial mechanical properties and failure modes of thin spray-on liners (TSLs). Tunnelling and Underground Space Technology, 145, 105617.
- Peerzada Gh, J., Sinclair, B. J., Perinbarajan, G. K., Dutta, R., Shekhawat, R., Saikia, N., ... & Mossa, A. T. (2023). An overview on smart and active edible coatings: safety and regulations. European Food Research and Technology, 249(8), 1935-1952.
- Shinde, S., Ghonge, M., & Kathpalia, H. (2023). Recent updates on oral and dermal film-based formulations and their applications. Current Drug Delivery, 20(4), 335-349.
- FDA Error. (n.d.-b). FDA. https://www.fda.gov/inspections-compliance-enforcement-and-criminal-investigations/inspection-guides/topical-drug-products-794
- García-Arieta, A., Gordon, J., Gwaza, L., Merino, V., & Mangas-Sanjuan, V. (2023). Regulatory Requirements for the Development of Second-Entry Semisolid Topical Products in the European Union. Pharmaceutics, 15(2), 601. https://doi.org/10.3390/pharmaceutics15020601
- K??la, D., Gökmen, G. G., Evrendilek, G. A., Akan, T., Vl?ko, T., Kulawik, P., ... & Ozogul, F. (2023). Recent developments in antimicrobial surface coatings: Various deposition techniques with nanosized particles, their application and environmental concerns. Trends in Food Science & Technology, 135, 144-172.
- “ARTISS.” Baxter Specialty Site, advancedsurgery.baxter.com/products/artiss.
- Earnshaw, Charles H., et al. “An audit of fifty patients receiving ArtissTM fibrin sealant in lateral selective neck dissections.” Clinical Otolaryngology, vol. 45, no. 2, Dec. 2019, pp. 264–67. https://doi.org/10.1111/coa.13485.
- Steinmetz, C. H. D., Haq, F. U., Rehman, S. U., Romman, M., & Pal, M. (2021). Somatic and Psychological Consequences of Covid-19 on Healthcare Professionals and Its Effects on Healthcare Delivery in a Teaching Hospital in Khyber Pakhtunkhwa, Pakistan. International Journal of Medical Science and Health Research, 05(01), 120–144. https://doi.org/10.51505/ijmshr.2021.5113
- Reddy, Konda Sireesha, et al. “Effectiveness of fibrin glue in adherence of skin graft.” Journal of Cutaneous and Aesthetic Surgery, vol. 10, no. 2, Jan. 2017, p. 72. https://doi.org/10.4103/jcas.jcas_100_16.
- Park, Ye Ri, et al. “NF-?B signaling is key in the wound healing processes of silk fibroin.” Acta Biomaterialia, vol. 67, Feb. 2018, pp. 183–95. https://doi.org/10.1016/j.actbio.2017.12.006.
- Gerlach, Jörg C., et al. “Innovative Regenerative Medicine Approaches to Skin Cell-Based Therapy for Patients with Burn Injuries.” Elsevier eBooks, 2008, pp. 1298–321. https://doi.org/10.1016/b978-012369410-2.50078-4.
- Aronson, J. K., Heneghan, C., & Ferner, R. E. (2019). Medical Devices: Definition, Classification, and Regulatory Implications. Drug Safety, 43(2), 83–93. https://doi.org/10.1007/s40264-019-00878-3
Montero, Andrés, et al. “Hyaluronic acid-fibrin hydrogels show improved mechanical stability in dermo-epidermal skin substitutes.” Materials Science and Engineering C, vol. 128, Sept. 2021, p. 112352. https://doi.org/10.1016/j.msec.2021.112352


 Fatema Chinwala*
Fatema Chinwala*













 10.5281/zenodo.13647309
10.5281/zenodo.13647309