Abstract
Photodynamic therapy (PDT) is a non-invasive treatment routinely used to treat various types of cancer, including non-neoplastic and bladder. The PDT method uses photosensitive compounds, which are photosensitive compounds that accumulate in pathological tissue, to selectively destroy malignant cells. PDT has been well received by patients and poses no new safety concerns. Bladder cancer is a good candidate for PDT treatment because it spares damaged tissue and is not affected by the chemicals of the treatment. PDT for bladder cancer involves the local action of photosensitizers to produce cytotoxic reactive oxygen species and cause cell death. PDT is a new technology in on curology and deserves much investment in clinical research, especially regarding plant photosensitizers. Natural photosensitizers isolated from plants and other biological sources can be considered as a green route for PDT in cancer treatment.
Keywords
Bladder cancer, PDT (photodynamic therapy), diagnosis, cancers, urothelial carcinoma
Introduction
Background of bladder cancer
Bladder cancer, also known as urinary bladder cancer, is the tenth most common cancer globally. It is becoming more commonplace worldwide, especially in developed countries. It is still the most common cancer in the urinary system [1]. In the lower abdominal cavernous organ, the bladder's main job is storing urine until micturition. The urinary bladder and urinary tract lining, or urothelial cells, are specialized transitional epithelial cells that can hold the urine volume produced by flattening under pressure. Furthermore, the smooth muscle that lines the bladder can contract to push urine out of the urethra and relax to accommodate greater volumes [2]. Exposure to environmental pollutants can result in mutations in the urothelial cells that line the urine system and bladder. Certain substances are eliminated from the urine by the kidneys. The bulk of cancer occurrences, especially in the developed world, are likely caused by these urothelial cells, which are primarily located in the bladder. The most common cause of bladder cancer is direct exposure to chemicals in the workplace and environment, namely tobacco smoke. There may be a rationale for the fourfold gender difference in bladder cancer incidence among men due to their increased exposure to cigarette smoke and occupational dangers. Bladder cancer risk is ranked second only to lung cancer risk after tobacco use [1, 3]. In contrast to other cancers, bladder cancer is rarely discovered by accident during an autopsy. Gross hematuria is painless in 85% of patients with newly diagnosed bladder cancer, and microscopic hematuria is found in almost all individuals [4]. Hematuria is usually sporadic and sometimes accompanied by Valsalva maneuvers. Thus, in addition to a focused history and physical examination, a thorough evaluation of hematuria for bladder cancer includes diagnostic modalities such as upper tract imaging, bladder endoscopies, and urine culture. Bladder cancer is a significant and rising cause of cancer worldwide, especially in developed countries, even though survival rates have increased due to early identification, robotic surgery, and the development of immunotherapy [1]. Bladder cancer patients typically incur greater lifetime treatment costs than those of any other cancer. The total cost of care for each patient varies from $129,000 to $251,000. A projected €4.9 billion in the EU and over $4 billion in the US per year is expected to be spent on directed medical expenses [5]. Consequently, in order to prevent bladder cancer and lessen its impact, a better understanding of its epidemiology and risk factors is crucial.
Background of photodynamic therapy
"Photodynamic therapy (PDT)" is another term for photochemotherapy. To distinguish photosensitized reactions in biology from the physicochemical processes taking place in the emulsions of photographic films, one uses the phrase "photodynamic action" [6]. According to Blum [7], this term should only be used for photochemical processes that involve the consumption of oxygen. Depending on the nature of the primary steps—namely, the initial involvement of radical intermediates that are later scavenged by oxygen or the generation of the highly cytotoxic singlet oxygen 102 by energy transfer from the photoexcited sensitizer—such reactions are also referred to as photosensitized processes of type I and type II. 1O2 was demonstrated to have a narrow radius of action (<0>
Mechanisms of Tumor Destruction
PDT targets the immunological and inflammatory systems of the host, as well as the microvasculature of the tumor bed and normal microvasculature. The proportional relevance of each PDT target for the total tumor response is still unknown, but its actions on all the targets may interact with one another to produce a wide range of responses. Nonetheless, it is evident that the amalgamation of these constituents is necessary for sustained tumor management. Through direct photodamage, PDT exposure to cancers in vivo can lower the number of clonogenic tumor cells; nevertheless, this is insufficient to cure malignancies. Direct photodynamic tumor cell kill was found to be less than 2 logs and, in most cases, less than 1 log in studies [9] conducted in mouse tumor systems using multiple photosensitizers for curative operations. This is significantly less than the 6–8 log reduction needed for tumor cure. Total eradication is predicted to be possible with a high enough light dose for some photosensitizers based on the in vitro irradiation of tumor cells isolated from photosensitized tumors in vivo [10]. Nevertheless, it appears that there are obstacles in the way of realizing the eradication of in vivo PDT treatment. One of these restrictions may be in the uniform photosensitizer distribution throughout the tumor. Furthermore, Korbelik and Krosl [11] have shown that tumor cells accumulate less photosensitizer and die less frequently the farther they are from the vascular supply. Another element that can limit the direct destruction of tumor cells is the oxygen content of the tissue undergoing photodynamic treatment (PDT). Such restrictions can result from two different processes: PDT's impacts on the tissue microvasculature and the photochemical oxygen consumption that occurs throughout the photodynamic process. Since 1O2 arises from ground-state oxygen, it follows that this process can consume oxygen in the tissue environment. Rapid and substantial reductions in tissue oxygen tensions on illumination of photosensitized tissue were reported. Mathematical modeling supports these findings and demonstrates that the rate of oxygen consumption during photoprint-PDT can be enough to move a fraction of the tumor into very low levels of oxygenation, outpacing the rate of oxygen diffusion from the capillaries and shrinking the radius of oxygenated tissue volume around them. The rates of 1O2 generation and therefore tissue oxygen consumption or depletion are high when both tissue photosensitizer levels and the fluence rate of light are high [12, 13]. The fluence rate can be adjusted downward to slow oxygen consumption sufficiently to facilitate the maintenance of tumor tissue pO2 levels during treatment. Since the reduction of sensitizer levels also reduces the rate of photochemical oxygen consumption, photobleaching of the sensitizer is a significant parameter influencing the rate of tissue oxygen consumption. Fractionated light delivery is an additional strategy for maintaining tissue oxygenation during photodynamic therapy. This has very brief light and dark cycles (of the order of 20–50 seconds) so that oxygen can be replenished during the dark times. Generally, treatment regimens using a low fluence rate or intermittent light show superior effectiveness in delaying tumor regrowth [14, 15, 16] According to early clinical research conducted at the Roswell Park Cancer Institute, patients' oxygen depletion happens during PDT as well. In patients receiving Photofrin (1 mg/kg)-PDT at a light dose rate of 150 mW/cm2, the kinetics for this depletion ranged from very quick (within seconds of light exposure) to slow (>10 minutes of light exposure) and to no effect at all in basal cell carcinoma lesions. During ALA-PDT (20% topical ALA), no oxygen depletion was seen in cutaneous lymphoma lesions; this could be because the effects were too subtle for the interstitial oxygen probe to pick up on. The oxygen supply in the tissue can also be decreased by the harmful effects of PDT on the microvasculature. When photosensitizers such as Photofrin are used at high doses, the effects can be strong enough to restrict the amount of oxygen that the tumor receives during photodynamic therapy. This mechanism loses significance with lower doses of photosensitizers and some second-generation sensitizers, many of which have less of an impact on the vasculature. [17] A significant portion of the previously mentioned data came from research on healthy microvasculature. Damage to the tumor-supplying normal vasculature may considerably impair tumor curability by PDT, as proven by the lack of tumor cures when the normal tissue around the tumor was insulated from PDT light [18]. Reports on the impact of fluence rate on vascular responses further corroborate this. Following PDT, a low-fluence rate therapy may cause normal microvascular perfusion to stop, but a high rate may preserve microvascular patency. Conversely, when the treatment was administered at low or high fluence rates, there were no differences in the effects on either tumor perfusion or oxygenation. Following PDT exposure, these response values were consistently and significantly lower in the tumors. Tumor curability was hindered by high-fluence rate therapy, suggesting that long-term tumor control was negatively impacted by high-fluence rate PDT's protection of the normal vasculature surrounding the tumor. Previous research has indicated that PDT effects on tumors and normal arteries can be qualitatively and quantitatively comparable. The aforementioned recent research, however, appears to highlight significant distinctions between PDT's impact on tumors and normal vasculature. [19,20] Mathematical models have predicted and experimental measurements have demonstrated that dynamic, dose-rate-dependent changes in tissue oxygenation can occur during PDT light delivery. Large intervention and interpatient variability preclude the ability to forecast these effects. The need for more advancements in instrumentation that enable real-time monitoring of the variables—such as blood flow, pO2, photosensitizer tissue concentration, photobleaching rates, and so on—that affect these changes (and therefore PDT dose) is greater than ever. If more is known about the mechanisms underlying PDT-induced vascular damage, it may be possible to use the distinctions between tumor and normal vasculature to improve treatment selectivity and efficacy.PDT targets the immunological and inflammatory systems of the host, as well as the microvasculature of the tumor bed and normal microvasculature. The proportional relevance of each PDT target for the total tumor response is still unknown, but its actions on all the targets may interact with one another to produce a wide range of responses. Nonetheless, it is evident that the amalgamation of these constituents is necessary for sustained tumor management. Through direct photodamage, PDT exposure to cancers in vivo can lower the number of clonogenic tumor cells; nevertheless, this is insufficient to cure malignancies. Direct photodynamic tumor cell kill was found to be less than 2 logs and, in most cases, less than 1 log in studies [9] conducted in mouse tumor systems using multiple photosensitizers for curative operations. This is significantly less than the 6–8 log reduction needed for tumor cure. Total eradication is predicted to be possible with a high enough light dose for some photosensitizers based on the in vitro irradiation of tumor cells isolated from photosensitized tumors in vivo [10]. Nevertheless, it appears that there are obstacles in the way of realizing the eradication of in vivo PDT treatment. One of these restrictions may be in the uniform photosensitizer distribution throughout the tumor. Furthermore, Korbelik and Krosl [11] have shown that tumor cells accumulate less photosensitizer and die less frequently the farther they are from the vascular supply. Another element that can limit the direct destruction of tumor cells is the oxygen content of the tissue undergoing photodynamic treatment (PDT). Such restrictions can result from two different processes: PDT's impacts on the tissue microvasculature and the photochemical oxygen consumption that occurs throughout the photodynamic process. Since 1O2 arises from ground-state oxygen, it follows that this process can consume oxygen in the tissue environment. Rapid and substantial reductions in tissue oxygen tensions on illumination of photosensitized tissue were reported. Mathematical modeling supports these findings and demonstrates that the rate of oxygen consumption during photoprint-PDT can be enough to move a fraction of the tumor into very low levels of oxygenation, outpacing the rate of oxygen diffusion from the capillaries and shrinking the radius of oxygenated tissue volume around them. The rates of 1O2 generation and therefore tissue oxygen consumption or depletion are high when both tissue photosensitizer levels and the fluence rate of light are high [12, 13]. The fluence rate can be adjusted downward to slow oxygen consumption sufficiently to facilitate the maintenance of tumor tissue pO2 levels during treatment. Since the reduction of sensitizer levels also reduces the rate of photochemical oxygen consumption, photobleaching of the sensitizer is a significant parameter influencing the rate of tissue oxygen consumption. Fractionated light delivery is an additional strategy for maintaining tissue oxygenation during photodynamic therapy. This has very brief light and dark cycles (of the order of 20–50 seconds) so that oxygen can be replenished during the dark times. Generally, treatment regimens using a low fluence rate or intermittent light show superior effectiveness in delaying tumor regrowth [14, 15, 16]. According to early clinical research conducted at the Roswell Park Cancer Institute, patients' oxygen depletion happens during PDT as well. In patients receiving Photofrin (1 mg/kg)-PDT at a light dose rate of 150 mW/cm2, the kinetics for this depletion ranged from very quick (within seconds of light exposure) to slow (>10 minutes of light exposure) and to no effect at all in basal cell carcinoma lesions. During ALA-PDT (20% topical ALA), no oxygen depletion was seen in cutaneous lymphoma lesions; this could be because the effects were too subtle for the interstitial oxygen probe to pick up on. The oxygen supply in the tissue can also be decreased by the harmful effects of PDT on the microvasculature. When photosensitizers such as Photofrin are used at high doses, the effects can be strong enough to restrict the amount of oxygen that the tumor receives during photodynamic therapy. This mechanism loses significance with lower doses of photosensitizers and some second-generation sensitizers, many of which have less of an impact on the vasculature. [17] A significant portion of the previously mentioned data came from research on healthy microvasculature. Damage to the tumor-supplying normal vasculature may considerably impair tumor curability by PDT, as proven by the lack of tumor cures when the normal tissue around the tumor was insulated from PDT light [18]. Reports on the impact of fluence rate on vascular responses further corroborate this. Following PDT, a low-fluence rate therapy may cause normal microvascular perfusion to stop, but a high rate may preserve microvascular patency. Conversely, when the treatment was administered at low or high fluence rates, there were no differences in the effects on either tumor perfusion or oxygenation. Following PDT exposure, these response values were consistently and significantly lower in the tumors. Tumor curability was hindered by high-fluence rate therapy, suggesting that long-term tumor control was negatively impacted by high-fluence rate PDT's protection of the normal vasculature surrounding the tumor. Previous research has indicated that PDT effects on tumors and normal arteries can be qualitatively and quantitatively comparable. The aforementioned recent research, however, appears to highlight significant distinctions between PDT's impact on tumors and normal vasculature. [19,20] Mathematical models have predicted and experimental measurements have demonstrated that dynamic, dose-rate-dependent changes in tissue oxygenation can occur during PDT light delivery. Large intervention and interpatient variability preclude the ability to forecast these effects. The need for more advancements in instrumentation that enable real-time monitoring of the variables—such as blood flow, pO2, photosensitizer tissue concentration, photobleaching rates, and so on—that affect these changes (and therefore PDT dose) is greater than ever. If more is known about the mechanisms underlying PDT-induced vascular damage, it may be possible to use the distinctions between tumor and normal vasculature to improve treatment selectivity and efficacy.
MECHANISMS OF PHOTOSENSITIZATION
The idea behind PDD and PDT is that tumor cells are exposed to a photosensitizer (PS). Tumor cells absorb this to a higher degree than do healthy cells. Therefore, in PDD, illumination with a light source of the proper wavelength results in a better ability to identify the tumor, whereas in PDT, it causes the tumor cells to die [21].
Photosensitizers in Bladder Cancer
We have concentrated on PS that are either the most crucial or widely utilized in the treatment of BC for this review.
Using Different Synthetic Photosensitizers
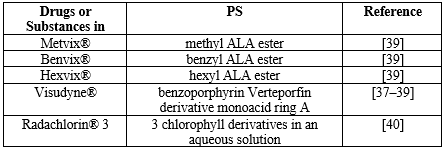
Hemaporphyrins' application in the detection of bladder cancer is among the most significant studies involving the use of a photosensitizer. The patient received an intravenous injection of a hematoporphyrin derivative, which caused the damaged tissue to glow under UV light and take on a distinctive brilliant red color. Tissues that are normal do not glow. Several photosensitizers, both synthetic and natural, are employed in the clinic when PDT is used. The most widely used medical databases list HAL (hexylaminolevulinate) as an ester derivative of ALA (5-aminolevulinic acid). (ALA) is recognized as a precursor in the manufacture of hemo, a porphyrin that occurs naturally. It is well-recognized that hemoglobin, myoglobin, and other hemoproteins contain hemoglobin. PPIX, or protoporphyrin IX, is recognized as a precursor to hemoglobin. Because of the coordination of a paramagnetic ion in the macrocycle's center, which significantly reduces excited state lifetimes, hemem itself is not a photosensitizer [22–29]. On the other hand, hypericin and chlorophyllin are photosensitizers that come from natural sources and work well. Deep tissue penetration can be achieved by photosensitizers by absorbing light with red or far-red wavelengths. the compounds that make up the photosensitizers that are most frequently used in medical offices. Finding naturally occurring chemicals in plants or animals was how the selection process was carried out. An amino acid called ALA and a heme precursor called HAL are found naturally. As a natural green colorant, copper chlorophyllin is permitted. One of the main active ingredients of hypericum, or Saint John's wort, is hypericin, a derivative of anthraquinone. [29, 31] Since there are currently only a few approved PDT medications, such as Photofrin®, Foscan®, and Levulan®, which are mostly used for skin, gynecological, gastrointestinal, and head and neck malignancies, searching for potential novel photosensitizers is an essential first step in PDT studies. PDT has the following benefits: low cost, short treatment duration, minimal toxicity, and minimal invasiveness. Therefore, clinical trials for novel photosensitizers are ongoing. The first patient in a human clinical trial received TLD1433 in March 2017 (ClinicalTrials.gov Identifier: NCT03053635). Because it can specifically trigger apoptosis in cancer cells, tumor necrosis factor-related apoptosis-inducing ligand (TRAIL) is a prospective candidate for anticancer therapy. However, not every tumor cell responds to TRAIL. Anticancer drugs have the ability to sensitize TRAIL-resistant cancer cells to TRAIL-induced apoptosis. The effect of ALA-mediated PDT enhances TRAIL's cytotoxic action on bladder transitional carcinoma cells. The data obtained indicate that treating bladder cancer cells with both TRAIL and PDT together could serve as the foundation for a novel approach to inducing cytotoxicity. It is necessary to develop better diagnostic and treatment techniques with the goal of lowering bladder cancer recurrence and progression rates. Publications from the present day include details on a wide range of novel diagnostic techniques, including photoacoustic imaging, UV autofluorescence microscopy, confocal laser endoscopy, Raman spectroscopy, and molecular imaging. Due to the fact that these techniques primarily make use of physical phenomena that are inert to the human body, they do not demonstrate any appreciable side effects and are well accepted by patients. To put it simply, one of the most prevalent malignancies in young men today is transitional cell carcinoma (TCC) of the bladder. As with other malignancies, TCC is treatable when detected early enough. Based on contemporary scientific breakthroughs, such as PDT, there has been a significant development in urological illness treatment techniques in recent years. Examining the impact of the ALA-mediated photodynamic effect on colon cancer cells' secretory activity (MIF, MCP-1) in vitro under both normoxic and hypoxic settings was the study's main objective. When ALA was given orally to individuals undergoing transurethral excision of a bladder tumor, it was linked to hypotension during general anesthesia. In a dosage-dependent way, verteporfin (VP) can stop the proliferation and invasion of bladder cancer cells. Purlin chlorin, a tin etiopurpurin, is used to treat psoriasis and other non-malignant illnesses. It has been the subject of Phase II clinical trials for cutaneous metastatic breast cancer and Kaposi's sarcoma in AIDS patients. According to reports, purlytin localizes in the skin and causes a photoreaction 7–14 days after treatment. The PS in question is meta-tetrahydroxyphenyl chlorin (mTHPC), and PDT exhibits a high tumoricidal depth of 10 mm when photoactivated at higher wavelengths (650–660 nm). Studying the photodynamic properties of mTHPC in liposomal (water-soluble) and solvent-based formulations (Foscan and Foslip) was done using two biliary cancer cell lines (BDC, bile duct cancer cells, and GBC, gall bladder cancer) in an in vitro model system [32–38]. Table 1 presents current photosensitizers subjected to bladder cancer PDT [37–40].
Table 1. PS subjected to bladder cancer PDT.
5-Aminolevulinic Acid (ALA or Levlan)
An endogenous metabolite that is normally produced in the mitochondria is called aminolevulinic acid (5-amino-4-oxopentanoic acid, or ALA for short). At a wavelength of 635 nm, absorption occurs. Upon conjugation, eight ALA molecules create the naturally occurring photosensitizing protoporphyrin IX (PpIX). ALA-PDT is superior to conventional cancer treatments in several ways. It provides re-treatment options and lowers long-term morbidity [33, 41]. Treatment with Protoporphyrin IX (PDT) involves over-accumulation of the fluorescent material in the tumor cells. The cancer cells are damaged by the reactive oxygen created during the visible red or green light irradiation process. PDT using 5-aminolevulinic acid has less of an impact on the surrounding healthy tissues and cells. It is just somewhat invasive since protoporphyrin IX is not accumulating. PDT of superficial bladder cancer has great potential when ALA-induced photosensitization occurs, according to Bachor et al.'s data [49]. By using PDT in patients with bladder dysplasia and early-stage cancer, Kriegmair et al.'s study demonstrated extraordinarily high sensitivity, which they attributed to the use of 5-ALA. The bladder was directly filled with ALA solution in patients undergoing a scheduled bladder biopsy. Compared to traditional white-light cystoscopy, which has a sensitivity of 72.7%, this method has a substantially higher sensitivity of 96.9%. [42]. Anesthesia is not used for PDT with ALA, and it is painless. Unlike radiation therapy, local lesions are treated with low energy levels and are often applied. Considered to be a novel, minimally invasive procedure, it operates on a different principle than earlier approaches. Actually, PDT combined with ALA has been clinically proven to be effective in treating bladder cancer, namely in cases of in situ resistant bladder cancer. PDT, or photodynamic therapy, in conjunction with ALA, is a promising new therapeutic approach for a variety of cancer types. It is based on the same molecular characteristics of malignancies. [43].
Herbal Photosensitizers
There are a lot of chemicals with photosensitizing properties on the market. Recent years have seen a large number of studies published on PDT; however, natural extracts of medicinal plants and their components have received comparatively little attention. In comparison to artificial chemicals, herbal plants and their extracts are natural and regarded as "green" materials. This article discusses the function of natural chemicals like hypericin and chlorophyllin, as well as plant extracts, in the PDT process for bladder cancer.
Hypericin
Hypericum perforatum, also called the yellow flowering herb or St. John's wort, is the natural source of hypericin, an anthraquinone derivative. PDT combined with hypericin has been utilized to treat a variety of malignancies, such as glioblastoma, bladder, cervical, and skin cancers. The wavelength of light at which PS absorbs occurs between 514 and 593 nm. Studies reveal that photoactivation of hypericin can produce singlet oxygen and superoxide anion radicals with good quantum efficiency. Reactive oxygen species (ROS) generated by PDT have the ability to eradicate tumors. Studies have shown that light-induced hypericin activation can block several growth factors, including protein kinase C (PKC), leading to enhanced membrane lipid peroxidation. This method lowers cellular glutathione levels in the mitochondria by inducing superoxide dismutase activity [44–49]. Hypericin is found in the Golgi complex, the nuclear envelope, the endoplasmic reticulum (ER), and the mitochondria, according to the accumulation of cells in cancer cell lines. The use of hypericin in PDT therapy makes it abundantly evident that necrosis, autophagy, and apoptosis are the primary mechanisms contributing to cancer cell death. Cell death via caspase-dependent or autophagy-dependent apoptosis results from hypericin accumulation in the ER membrane, which causes a rapid depletion of Ca2+ reserves. An additional theory on PDT, including hypericin, highlights the significance of mitochondria, whose impairment could trigger the start of the intrinsic apoptotic pathway. The method includes the release of cytochrome c from the mitochondria, which leads to an increase in poly ADP-ribose polymerase cleavage (PARP) and procaspase-9/procaspase-3 activation. According to the data, hypericin is arguably one of the most effective drugs for PDT photosensitization. It is also inexpensive and easily accessible because it comes from natural sources [50, 51, 52]. For patients who had their bladder cancer removed, additional research has been done. An intravenous solution of hypericin was given to these individuals. Evidently, the outcomes show that the therapy is more sensitive [53]. The Kubin et al. team conducted research that was comparable but used hypericin. Results with hypericin procedures were better in those with suspected or recurring bladder cancer [54]. The polar methanol fraction of Hypericum perforatum L. extract was employed as a photosensitizer by the Stavropoulos et al. team for use in photodynamic diagnostics (PDD) and PDT. In the study, human bladder cancer cells T24 (high-grade metastatic cancer) and RT4 (low-grade primary papillary cancer) were used to examine the extract's effectiveness as a phototoxic agent against bladder cancer. In both cell lines, the photosensitizer at a concentration of 60 g/mL demonstrated considerable photocytotoxicity following irradiation (with laser light at a wavelength of 630 nm), resulting in cell loss from 80% to 86%. Neither of the cell lines died at the lower concentrations (20 g/mL). Comparing the obtained results with those in the same cell lines under the same conditions using the clinically approved photosensitizer, Photofrin enabled a comparison. Photofrin was stimulated with a 630 nm laser light and utilized at the highest clinically acceptable dose of 4 g/mL. Compared to the Hypericum perforatum L. extract, photofrin produced limited cell death (9%) in RT4 cells and slightly less photocytotoxicity (77% in T24 cells). In these two bladder cell lines, apoptosis is the primary cause of cell death resulting from the PDT activity of methanol extract [55]. Bhuvaneswari et al. studied the optimization of PDT methods for even higher efficacy. Researchers looked into the anti-tumor effects of hypericin PDT combined with Erbitux, an angiogenesis inhibitor that targets the EGFR receptor in human bladder cancer cells. Comparing the bladder tumor model to the other groups, the acquired data demonstrated that the combination of Erbitux and hypericin-PDT substantially reduced tumor growth. Increased apoptosis and the detected ErbB4 dephosphorylation at the 1284 tyrosine site both had an impact on tumor suppression [56]. The studies suggest that methanol extract can be a useful photosensitizer in PDT because of its high photocytotoxicity, selective localization, and low extraction costs.
Chlorophyllin
Chlorophyllin is a chlorophyll derivative found in cyanobacteria, algae, and plants' chloroplasts. Studies on its use have revealed that chlorophyllin accumulates in lysosomes and mitochondria. Its localization position suggests that autophagy and apoptosis are the primary mechanisms underlying chlorophyllin PDT in cancer cells. Researchers also highlighted its good optical characteristics, which range from 600 to 670 nm in wavelength. Chlorophyllin is easily soluble in aqueous solutions, making extraction a simple and cost-effective alternative to synthesized PS. It is low in toxicity and quickly eliminated from the body [40, 41]. The authors of the study on chlorophyllin in the application of PDT clearly discovered the anticancer impact. They discovered that chlorophyllin causes apoptosis and autophagy in cells. The findings imply that chlorophyllin is a novel, effective PS that, when paired with autophagy inhibitors, could be a promising therapy method for non-invasive human bladder cancer in PDT [57]. Patients with non-invasive bladder cancer (NMIBC) frequently recur following surgery because of insufficient resection and chemotherapy resistance. The team of Zhuo et al. sought to assess the anti-tumor efficacy of PDT utilizing chlorophyllin and bladder cancer human tumor models (T24 and 5637). The data clearly demonstrated that chlorophyllin-PDT causes considerable cytotoxicity in the cells tested. Cell movement and consequent invasion capacity were dramatically reduced, and cells displayed the characteristic morphological alterations seen after apoptosis. In cells treated with chlorophyllin-PDT, the quantity of intracellular reactive oxygen species (ROS) increased dramatically, whereas superoxide dismutase (SOD) activity fell significantly [58]. Chlorophyllin has a photo-cytotoxic action, is an effective PS, and has the potential for use in bladder cancer treatment.
LIGHT SOURCES IN APPLICATIONS FOR PDD AND PDT OF BLADDER CANCER
Lasers and other light sources transform input energy into light. The laser contains an amplifying medium, a resonant cavity, and an energy source. Diode lasers are currently the most commonly used light source for clinical PDT therapy. Lasers can work in both pulsed and continuous modes. The following are the most frequent types of lasers used in PDT: The interaction of electrons and holes in a diode laser produces light. A diode laser is a semiconductor device that includes an amplifying medium and a resonant cavity. The source of energy is electricity. Diode lasers are compact, lightweight, portable, and affordable. In turn, dye lasers use an organic dye molecule to generate light in a specific spectral region. This makes it easy to match the absorption wavelength to the appropriate photosensitizer. The dye takes the shape of a fluid that circulates constantly. The PDT also employs fluorescent, incandescent, metal halide, xenon, and sodium lights. The lamp's light spectrum is vast, which is why optical filters are used in laboratories to match the wavelength to the photosensitizer's absorption band [59]. In recent years, a lot of research has gone into establishing novel measurement techniques for each of the parameters utilized in PDT. Several optical approaches are in use or in development for measuring parameters in PDT. The light dose is primarily assessed using optical fibers, which can capture light at large, solid angles. Positron emission tomography (PET) can be used to evaluate photosensitizer concentration in tissue by utilizing labeled photosensitizers as detectable tracers. Fluorescence is an alternative to PET. This technique is based on monitoring the phosphorescence released by singlet oxygen in the tissue when it returns to its ground state following stimulation with the light employed in PDT [60]. PDT enables quantifiable light dosimetry in the treatment of superficial bladder cancer with targeted or total bladder wall irradiation. PDT is the local or systemic delivery of a photosensitizing agent that, when exposed to light and oxygen, produces tissue damage, including tumor elimination. The clinical efficacy of PDT and PDD in the treatment of bladder cancer is dependent on the irradiation source's parameters, such as bandwidth, maximum intensity, and half-width. The irradiation source's characteristics must be employed to optimize the main factors, such as the low intensity and low cost of the PDT or PDD. As a result, a low light level is employed before surgery to optimize the position of the light source. Parameters such as bladder volume, laser energy output, and desired light dose are important for adjusting the photon irradiation period to compensate for changes in laser light generation, energy losses during transmission, and changes in light intensity due to bladder action in terms of photoirradiation luminous flux. Hypericin is a hydroxylated phenanthroperylenquinone that is commonly utilized in bladder cancer research and treatment [61]. Hypericin (30 mM) in the bladder was treated with 595 nm laser light. This study found that light intensities of 12-48 J/cm2 caused selective PDT-induced urothelial tumor destruction without affecting the detrusor muscle [62]. Fluorescence microscopy identified hypericin (30 mM) in human bladder cancers and normal bladders. In situ, hypericin measurement revealed that there was much more hypericin fluorescence in the tumor than in the normal bladder, with the tumor-to-normal bladder ratio reaching 12:1 after 4 hours of hypericin treatment [63]. The whole bladder wall PDT was done using approximately 630 nm of light generated by an isotropic light source centered in the bladder cavity. The integrating sphere effect refers to the phenomenon of a higher fluence rate in this spherical geometry due to light scattering. The optical properties of cancerous human bladder tissue, including absorption coefficient, scattering coefficient, anisotropy factor, and refractive index, were measured in vitro in the wavelength range of 450–880 nm [64]. The bladder was detected by fluorescence endoscopy with a hematoporphyrin derivative and an argon-ion laser utilizing a video monitoring system coupled with an image intensifier; however, this was challenging due to the excitation light reflecting. Three patients with multicentric CIS of the bladder underwent whole bladder wall photo radiation therapy with argon dye laser light (630 × 1.6 nm) at 5 to 25 J/cm2 [65]. Marynissen and colleagues used an isotropic light detector (0.8 mm-diameter probe on 200 microns of fiber) connected to an amplifier displaying the light dose rate (in mW/cm2) and integrated light dose (in J/cm2) for whole bladder wall PDT with in vivo monitoring and dose control. In this work, a dosage rate uniformity of _20% was reached in vivo in the dog bladder using red light (wavelength 630 nm). Uniform irradiation of green light (wavelength 514.5 nm) proved problematic, most likely due to a substantially lower percentage of scattered light [66]. PDT on 46 patients with superficial bladder cancers was performed using a hematoporphyrin derivative, red light (630 nm), and an argon-dye laser. Hematoporphyrin derivative (2-4 mg/kg) was administered intravenously 48 to 72 hours before PDT. The light power was 200 mW/cm2 for 5–10 minutes or more, with a total light energy of 100 J/cm2 or more in tumors up to 2 cm in size [67]. Bladder tissue is more translucent than other human tissues; hence, PDT has a lot of potential for treating bladder cancer [68].
Palladium Bacteriochlorophyll Derivatives
WST11 (Tookadc solubleH), the most often characterized drug in this group, is a laser-activated vaso-occlusive agent that selectively remains in the bloodstream and is quickly eliminated by the liver and kidneys. Photoactivation of WST11 causes the generation of reactive oxygen species, which activates reactions that culminate in vascular damage and clogging, followed by tumor cell necrosis 48 hours after treatment. To achieve this selectivity, the optical fiber must be placed inside the tumor. This process differs from the traditional PDT impact at the cellular level. This type of PDT is known in the literature as "vascular targeted photodynamic treatment" (VTP). WST11-VTP has even shown success in phase II clinical studies for the treatment of localized prostate cancer. This type of PDT stimulates the growth of dendritic cells and macrophages. Mac2- and CD3+-stained cells revealed the ability to penetrate the tumor, resulting in increased lymphoid cell activation. This type of PDT appears to generate a long-term immunological response, as indicated by higher T cell counts at all foci, as well as a particular increase in CD8+ and active CD4+ T cells long after treatment [69].
Chlorins
Chlorins (dihydroporphyrins) are effective porphyrin-derived PSs that respond to near-infrared light. The most commonly described PS in this group are m-tetra-hydroxyphenyl chlorin (temoporfin or Fo scan), benzoporphyrin (verteporfin), and rad-a chlorin (a combination of sodium salts of chlorin e6, chlorin p6, and purpurin) [70].
Tetrahydroporphyrin Tetratosylate (THPTS)
THPTS is a hydrophilic cationic PS that is believed to accumulate in lysosomes. Pinocytosis is assumed to be the mechanism for uptake into cells. Cells accumulate independently of their nature or metabolic activity, but only tumor cells accumulate in lysosomes. The toxic effect on the tumor cell occurs through the release of lysosomal enzymes or the release of PS from lysosomes following light exposure and damage to the other cell components. Caspases 3 and 9 showed increased activity. Photoactivation of certain genes causes growth arrest and apoptosis. Upregulation of HSP105 and increased concentrations of mRNA for GADD45? activate MEKK4 and p38, resulting in apoptotic activity. The maximum absorption occurs in the near-infrared band (760 nm), enabling tissue penetration of up to 15 mm [71].
CLINICAL TRIALS OF PDT FOR UROTHELIAL CARCINOMA
Clinical evidence for PDT in urothelial carcinoma is currently restricted to several series of patients with NMIBC [72, 73], with the majority of these series employing this therapy for carcinoma in situ (Cis), while some use it for multiple and recurrent papillary Ta/T1. Table 1 highlights the various series of PDTs of the urinary bladder. When considering laser type, light delivery mode, light dosimetry (energy density), PS utilized and dosage, kind of non-muscle invasive bladder cancer (NMIBC) (papillary vs. cis, size), and follow-up, reported recurrence rates differ dramatically between series Overall, PDT appears to be a promising therapy option for patients with recurrent NMIBC who would otherwise require radical cystectomy. Combining it with mitomycin C in intravenous chemotherapy can enhance tumoricidal effects. In rare circumstances where the tumor is located in a bladder diverticulum, focused PDT can be employed to eliminate the disease without causing perforation [74]. PDT can also be repeated in individuals who have previously undergone therapy or are now undergoing radiotherapy or chemotherapy [75] without contraindication. An RCT compared the efficacy of BCG instillations (induction and maintenance) to a single session of PDT with Photofrin in the treatment of patients with intermediate and high risks of non-muscle-invasive bladder cancer. A total of 124 participants participated in the trial. After the intention-to-treat and as-treated analyses, the estimated median recurrence-free survival was 24.9 (BCG) versus 16.6 months (PDT) and 25.8 (BCG) versus 14.7 (PDT) months, respectively. In this patient population, the authors found that a single session of photoprint-based PDT was not superior to BCG maintenance therapy. However, the findings of this study did not rule out the advantages of BCG. Other publications have noted that both side effects and economic factors continue to favor BCG [76]. Concerns about bladder PDT's complexity and potential side effects continue to limit its practical application. Indeed, systemic (mostly skin photosensitivity) and local toxicity (bladder wall fibrosis/contracted bladder, vesicoureteral reflux, storage symptoms) are significant issues to address in treatment [77, 78].
SUMMARY AND CONCLUSIONS
Finally, in this study we present the most current data on the use of PDT in the treatment of bladder cancer. Photodynamic therapy (PDT) is an alternative cancer treatment that induces cell death through various mechanisms. This includes apoptosis and closure of tumor vasculature, while also triggering autophagy as a cellular defense process. However, excessive light exposure can lead to photodamage and prevent necrosis. PDT has the potential to reduce the death rate in cancer patients and is being researched for its effectiveness against brain tumors. Natural photosensitizers derived from plants and other biological sources are being explored as green alternatives in PDT due to their selective anti-cancer activity and minimal damage to normal cells. Funding for research on plant-based photosensitizers and their efficacy in PDT should be increased. PDT is also showing promise in urology, particularly for bladder cancer, with photodynamic diagnostic techniques being developed to improve diagnosis. Combining PDT with cystoscopy has shown effectiveness in reducing cancer cell numbers. Overall, PDT has the potential to become a first-line treatment for various cancers, with further research and investment needed to fully explore and optimize its applications.
REFERENCE
- Sung, H., Ferlay, J., Siegel, R. L., Laversanne, M., Soerjomataram, I., Jemal, A., & Bray, F. (2021). Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA: a cancer journal for clinicians, 71(3), 209-249.
- Andersson, K. E., & Arner, A. (2004). Urinary bladder contraction and relaxation: physiology and pathophysiology. Physiological reviews, 84(3), 935-986.
- Wong, M. C., Fung, F. D., Leung, C., Cheung, W. W., Goggins, W. B., & Ng, C. F. (2018). The global epidemiology of bladder cancer: a joinpoint regression analysis of its incidence and mortality trends and projection. Scientific reports, 8(1), 1129.
- Edwards, T. J., Dickinson, A. J., Natale, S., Gosling, J., & McGrath, J. S. (2006). A prospective analysis of the diagnostic yield resulting from the attendance of 4020 patients at a protocol?driven haematuria clinic. BJU international, 97(2), 301-305.
- Leal, J., Luengo-Fernandez, R., Sullivan, R., & Witjes, J. A. (2016). Economic burden of bladder cancer across the European Union. European urology, 69(3), 438-447.
- TAPPEINER, V. (1904). Uber die wirkung der photodynamischen (fluorescierenden) stoffe auf protozoen und enzyme. Dtsch. Arch. Klin. Med., 80, 427-487.
- Blum, H. F. (1943). Photodynamic action and diseases caused by light.
- Moan, J., & Berg, K. (1991). The photodegradation of porphyrins in cells can be used to estimate the lifetime of singlet oxygen. Photochemistry and photobiology, 53(4), 549-553.
- Henderson, B. W., & Dougherty, T. J. (1992). How does photodynamic therapy work?. Photochemistry and photobiology, 55(1), 145-157.
- Henderson, B. W., Waldow, S. M., Mang, T. S., Potter, W. R., Malone, P. B., & Dougherty, T. J. (1985). Tumor destruction and kinetics of tumor cell death in two experimental mouse tumors following photodynamic therapy. Cancer research, 45(2), 572-576.
- Korbelik, M., & Krosl, G. (1994). Cellular levels of photosensitisers in tumours: the role of proximity to the blood supply. British journal of cancer, 70(4), 604-610.
- Tromberg, B. J., Orenstein, A., Kimel, S., Barker, S. J., Hyatt, J., Nelson, J. S., & Berns, M. W. (1990). In vivo tumor oxygen tension measurements for the evaluation of the efficiency of photodynamic therapy. Photochemistry and photobiology, 52(2), 375-385.
- Zilberstein, J., Bromberg, A., Frantz, A., Rosenbach?Belkin, V., Kritzmann, A., Pfefermann, R., ... & Scherz, A. (1997). Light?dependent oxygen consumption in bacteriochlorophyll?serine?treated melanoma tumors: on?line determination using a tissue?inserted oxygen microsensor. Photochemistry and photobiology, 65(6), 1012-1019.
- Sitnik, T. M., & Henderson, B. W. (1997, May). Effects of fluence rate on cytoxicity during photodynamic therapy. In Optical Methods for Tumor Treatment and Detection: Mechanisms and Techniques in Photodynamic Therapy VI (Vol. 2972, pp. 95-102). SPIE.
- Foster, T. H., Murant, R. S., Bryant, R. G., Knox, R. S., Gibson, S. L., & Hilf, R. (1991). Oxygen consumption and diffusion effects in photodynamic therapy. Radiation research, 126(3), 296-303.
- Gibson, S. L., VanDerMeid, K. R., Murant, R. S., Raubertas, R. F., & Hilf, R. (1990). Effects of various photoradiation regimens on the antitumor efficacy of photodynamic therapy for R3230AC mammary carcinomas. Cancer research, 50(22), 7236-7241.
- Henderson, B. W., & Fingar, V. H. (1989). Oxygen limitation of direct tumor cell kill during photodynamic treatment of a murine tumor model. Photochemistry and photobiology, 49(3), 299-304.
- Fingar, V. H., & Henderson, B. W. (1987). Drug and light dose dependence of photodynamic therapy: a study of tumor and normal tissue response. Photochemistry and photobiology, 46(5), 837-847.
- Sitnik, T. M., Hampton, J. A., & Henderson, B. W. (1998). Reduction of tumour oxygenation during and after photodynamic therapy in vivo: effects of fluence rate. British journal of cancer, 77(9), 1386-1394.
- Reed, M. W., Wieman, T. J., Schuschke, D. A., Tseng, M. T., & Miller, F. N. (1989). A comparison of the effects of photodynamic therapy on normal and tumor blood vessels in the rat microcirculation. Radiation research, 119(3), 542-552.
- Allison, R. R., & Moghissi, K. (2013). Photodynamic therapy (PDT): PDT mechanisms. Clinical endoscopy, 46(1), 24.
- Bachor, R., Reich, E., Rück, A., & Hautmann, R. (1996). Aminolevulinic acid for photodynamic therapy of bladder carcinoma cells. Urological research, 24, 285-289.
- Waidelich, R., Beyer, W., Knchel, R., Stepp, H., Baumgartner, R., Schrder, J., ... & Kriegmair, M. (2003). Whole bladder photodynamic therapy with 5-aminolevulinic acid using a white light source. Urology, 61(2), 332-337.
- Filonenko, E. V., Kaprin, A. D., Alekseev, B. Y. A., Apolikhin, O. I., Slovokhodov, E. K., Ivanova-Radkevich, V. I., & Urlova, A. N. (2016). 5-Aminolevulinic acid in intraoperative photodynamic therapy of bladder cancer (results of multicenter trial). Photodiagnosis and Photodynamic Therapy, 16, 106-109.
- Inoue, K. (2017). 5?Aminolevulinic acid?mediated photodynamic therapy for bladder cancer. International Journal of Urology, 24(2), 97-101.
- Witjes, J. A., & Douglass, J. (2007). The role of hexaminolevulinate fluorescence cystoscopy in bladder cancer. Nature clinical practice Urology, 4(10), 542-549.
- Vaucher, L., Jichlinski, P., Lange, N., Ritter?Schenk, C., van den Bergh, H., & Kucera, P. (2007). Hexyl?aminolevulinate?mediated photodynamic therapy: How to spare normal urothelium. An in vitro approach. Lasers in Surgery and Medicine: The Official Journal of the American Society for Laser Medicine and Surgery, 39(1), 67-75.
- Bader, M. J., Stepp, H., Beyer, W., Pongratz, T., Sroka, R., Kriegmair, M., ... & Waidelich, R. (2013, October). Photodynamic therapy of bladder cancer–a phase I study using hexaminolevulinate (HAL). In Urologic Oncology: Seminars and Original Investigations (Vol. 31, No. 7, pp. 1178-1183). Elsevier.
- Kamuhabwa, A. R., Agostinis, P., D'Hallewin, M. A., Kasran, A., & de Witte, P. A. (2000). Photodynamic activity of hypericin in human urinary bladder carcinoma cells. Anticancer research, 20(4), 2579-2584.
- Li, B., Wu, Z., Li, W., Jia, G., Lu, J., Fang, J., & Chen, G. (2012). Chlorophyllin e4 is a novel photosensitizer against human bladder cancer cells. Oncology reports, 27(5), 1455-1460.
- Gomaa, I., Ali, S. E., El-Tayeb, T. A., & Abdel-kader, M. H. (2012). Chlorophyll derivative mediated PDT versus methotrexate: An in vitro study using MCF-7 cells. Photodiagnosis and Photodynamic Therapy, 9(4), 362-368.
- Dhaneshwar, S., Patil, K., Bulbule, M., Kinjawadekar, V., Joshi, D., & Joshi, V. (2014). Photodynamic therapy for cancer. Int. J. Pharm. Sci. Rev. Res, 27(2), 125-141.
- Szliszka, E., Kawczyk-Krupka, A., Czuba, Z. P., Sieron, A., & Krol, W. (2011). Effect of ALA–mediated photodynamic therapy in combination with tumor necrosis factor–related apoptosis–inducing ligand (TRAIL) on bladder cancer cells. Central European Journal of Urology, 64(3), 175.
- Szygula, M., Wojciechowski, B., Adamek, M., Pietrusa, A., Kawczyk-Krupka, A., Cebula, W., ... & Siero?, A. (2004). Fluorescent diagnosis of urinary bladder cancer—a comparison of two diagnostic modalities. Photodiagnosis and Photodynamic therapy, 1(1), 23-26.
- Kawczyk-Krupka, A., Bugaj, A. M., Latos, W., Wawrzyniec, K., Ole?, P., Mertas, A., ... & Siero?, A. (2015). ALA-mediated photodynamic effect on apoptosis induction and secretion of macrophage migration inhibitory factor (MIF) and of monocyte chemotactic protein (MCP-1) by colon cancer cells in normoxia and in hypoxia-like conditions in vitro. Photodiagnosis and photodynamic therapy, 12(1), 27-35.
- Nakatani, S., Ida, M., Wang, X., Naito, Y., & Kawaguchi, M. (2021). Oral 5-aminolevulinic acid administration prior to transurethral resection of bladder tumor causes intraoperative hypotension: Propensity score analysis. Photodiagnosis and Photodynamic Therapy, 34, 102342.
- Rytlewski, J. D., Scalora, N., Garcia, K., Tanas, M., Toor, F., Miller, B., ... & Monga, V. (2021). Photodynamic therapy using Hippo pathway inhibitor verteporfin: a potential dual mechanistic approach in treatment of soft tissue sarcomas. Cancers, 13(4), 675.
- Kiesslich, T., Berlanda, J., Plaetzer, K., Krammer, B., & Berr, F. (2007). Comparative characterization of the efficiency and cellular pharmacokinetics of Foscan®-and Foslip®-based photodynamic treatment in human biliary tract cancer cell lines. Photochemical & Photobiological Sciences, 6, 619-627.
- Josefsen, L. B., & Boyle, R. W. (2008). Photodynamic therapy and the development of metal-based photosensitisers. Metal-based drugs, 2008.
- Lee, J. Y., Diaz, R. R., Cho, K. S., Lim, M. S., Chung, J. S., Kim, W. T., ... & Choi, Y. D. (2013). Efficacy and safety of photodynamic therapy for recurrent, high grade nonmuscle invasive bladder cancer refractory or intolerant to bacille Calmette-Guerin immunotherapy. The Journal of urology, 190(4), 1192-1199.
- Gándara, L., Sandes, E., Di Venosa, G., Mc Cormick, B. P., Rodriguez, L., Mamone, L., ... & Casas, A. (2014). The natural flavonoid silybin improves the response to Photodynamic Therapy of bladder cancer cells. Journal of Photochemistry and Photobiology B: Biology, 133, 55-64.
- Kriegmair, M., Baumgartner, R., Knuchel, R., Stepp, H., Hofstadter, F., & Hofstetter, A. (1996). Detection of early bladder cancer by 5-aminolevulinic acid induced porphyrin fluorescence. The Journal of urology, 155(1), 105-110.
- Waidelich, R., STEPP, H., BAUMGARTNER, R., WENINGER, E., HOFSTETTER, A., & KRIEGMAIR, M. (2001). Clinical experience with 5-aminolevulinic acid and photodynamic therapy for refractory superficial bladder cancer. The Journal of urology, 165(6), 1904-1907.
- Schempp, C. M., Winghofer, B., Langheinrich, M., Schöpf, E., & Simon, J. C. (1999). Hypericin levels in human serum and interstitial skin blister fluid after oral single-dose and steady-state administration of Hypericum perforatum extract (St. John’s wort). Skin Pharmacology and Physiology, 12(5), 299-304.
- Vantieghem, A., Xu, Y., Declercq, W., Vandenabeele, P., Denecker, G., Vandenheede, J. R., ... & Agostinis, P. (2001). Different pathways mediate cytochrome c release after photodynamic therapy with hypericin. Photochemistry and photobiology, 74(2), 133-142.
- Vantieghem, A., Assefa, Z., Vandenabeele, P., Declercq, W., Courtois, S., Vandenheede, J. R., ... & Agostinis, P. (1998). Hypericin-induced photosensitization of HeLa cells leads to apoptosis or necrosis: Involvement of cytochrome c and procaspase-3 activation in the mechanism of apoptosis. FEBS letters, 440(1-2), 19-24.
- Weller, M., Trepel, M., Grimmel, C., Schabet, M., Bremen, D., Krajewski, S., & Reed, J. (1997). Hypericin-induced apoptosis of human malignant glioma cells is light-dependent, independent of bcl-2 expression, and does not require wild-type p53. Neurological research, 19(5), 456-470.
- Zupko, I., Kamuhabwa, A. R., D'Hallewin, M. A., Baert, L., & De Witte, P. A. (2001). In vivo photodynamic activity of hypericin in transitional cell carcinoma bladder tumors. International journal of oncology, 18(5), 1099-1105.
- Couldwell, W. T., Hinton, D. R., He, S., Chen, T. C., Sebat, I., Weiss, M. H., & Law, R. E. (1994). Protein kinase C inhibitors induce apoptosis in human malignant glioma cell lines. FEBS letters, 345(1), 43-46.
- Krammer, B., & Verwanger, T. (2012). Molecular response to hypericin-induced photodamage. Current medicinal chemistry, 19(6), 793-798.
- Davids, L. M., Kleemann, B., Cooper, S., & Kidson, S. H. (2009). Melanomas display increased cytoprotection to hypericin-mediated cytotoxicity through the induction of autophagy. Cell biology international, 33(10), 1065-1072.
- Agostinis, P., Vantieghem, A., Merlevede, W., & de Witte, P. A. (2002). Hypericin in cancer treatment: more light on the way. The international journal of biochemistry & cell biology, 34(3), 221-241.
- Sim, H. G., Lau, W. K., Olivo, M., Tan, P. H., & Cheng, C. W. (2005). Is photodynamic diagnosis using hypericin better than white?light cystoscopy for detecting superficial bladder carcinoma?. BJU international, 95(9), 1215-1218.
- Kubin, A., Meissner, P., Wierrani, F., Burner, U., Bodenteich, A., Pytel, A., & Schmeller, N. (2008). Fluorescence diagnosis of bladder cancer with new water soluble hypericin bound to polyvinylpyrrolidone: PVP?hypericin. Photochemistry and photobiology, 84(6), 1560-1563.
- Stavropoulos, N. E., Kim, A., Nseyo, U. U., Tsimaris, I., Chung, T. D., Miller, T. A., ... & Skalkos, D. (2006). Hypericum perforatum L. extract–Novel photosensitizer against human bladder cancer cells. Journal of Photochemistry and Photobiology B: Biology, 84(1), 64-69.
- Bhuvaneswari, R., Gan, Y. Y., Soo, K. C., & Olivo, M. (2009). Targeting EGFR with photodynamic therapy in combination with Erbitux enhances in vivo bladder tumor response. Molecular cancer, 8, 1-11.
- Lihuan, D., Jingcun, Z., Ning, J., Guozeng, W., Yiwei, C., Wei, L., ... & Gang, C. (2014). Photodynamic therapy with the novel photosensitizer chlorophyllin f induces apoptosis and autophagy in human bladder cancer cells. Lasers in surgery and medicine, 46(4), 319-334.
- Zhuo, Z., Song, Z., Ma, Z., Zhang, Y., Xu, G., & Chen, G. (2019). Chlorophyllin e6 mediated photodynamic therapy inhibits proliferation and induces apoptosis in human bladder cancer cells. Oncology reports, 41(4), 2181-2193.
- Kim, M. M., & Darafsheh, A. (2020). Light sources and dosimetry techniques for photodynamic therapy. Photochemistry and photobiology, 96(2), 280-294.
- Beigzadeh, A. M., Rashidian Vaziri, M. R., Ziaie, F., & Sharif, S. (2020). A new optical method for online monitoring of the light dose and dose profile in photodynamic therapy. Lasers in surgery and medicine, 52(7), 659-670.
- Kamuhabwa, A., Agostinis, P., Ahmed, B., Landuyt, W., Van Cleynenbreugel, B., Van Poppel, H., & de Witte, P. (2004). Hypericin as a potential phototherapeutic agent in superficial transitional cell carcinoma of the bladder. Photochemical & Photobiological Sciences, 3, 772-780.
- Kamuhabwa, A. A., Roskams, T., D'Hallewin, M. A., Baert, L., Van Poppel, H., & de Witte, P. A. (2003). Whole bladder wall photodynamic therapy of transitional cell carcinoma rat bladder tumors using intravesically administered hypericin. International journal of cancer, 107(3), 460-467.
- Kamuhabwa, A. A., Cosserat?Gerardin, I., Didelon, J., Notter, D., Guillemin, F., Roskams, T., ... & de Witte, P. A. (2002). Biodistribution of hypericin in orthotopic transitional cell carcinoma bladder tumors: implication for whole bladder wall photodynamic therapy. International journal of cancer, 97(2), 253-260.
- van Staveren, H. J., Keijzer, M., Keesmaat, T., Jansen, H., Kirkel, W. J., Beek, J. F., & Star, W. M. (1996). Integrating sphere effect in whole-bladder-wall photodynamic therapy: III. Fluence multiplication, optical penetration and light distribution with an eccentric source for human bladder optical properties. Physics in Medicine & Biology, 41(4), 579.
- Stewart, F. A., Oussoren, Y., Te Poele, J. A. M., Horenblas, S., & Mooi, W. J. (1992). Functional and histological damage in the mouse bladder after photodynamic therapy. British journal of cancer, 65(6), 884-89
- Marynissen, J. P. A., Jansen, H., & Star, W. M. (1989). Treatment system for whole bladder wall photodynamic therapy with in vivo monitoring and control of light dose rate and dose. The Journal of urology, 142(5), 1351-1355.
- Misaki, T., Hisazumi, H., Hirata, A., Kunimi, K., Yamamoto, H., Amano, T., ... & Nakazima, K. (1986). Photodynamic therapy of superficial bladder tumors. Hinyokika kiyo. Acta Urologica Japonica, 32(12), 1941-1948.
- Hisazumi, H., Naito, K., Uchibayashi, T., Hirata, A., & Komatsu, K. (1991). Integral photodynamic therapy of superficial bladder tumors with special reference to carcinoma in situ. Scandinavian Journal of Urology and Nephrology, 25(sup138), 161-165.
- Mühleisen, L., Alev, M., Unterweger, H., Subatzus, D., Pöttler, M., Friedrich, R. P., ... & Janko, C. (2017). Analysis of hypericin-mediated effects and implications for targeted photodynamic therapy. International journal of molecular sciences, 18(7), 1388.
- Madar-Balakirski, N., Tempel-Brami, C., Kalchenko, V., Brenner, O., Varon, D., Scherz, A., & Salomon, Y. (2010). Permanent occlusion of feeding arteries and draining veins in solid mouse tumors by vascular targeted photodynamic therapy (VTP) with Tookad. PloS one, 5(4), e10282.
- Laranjo, M., Aguiar, M. C., Pereira, N. A., Brites, G., Nascimento, B. F., Brito, A. F., ... & e Melo, T. M. P. (2020). Platinum (II) ring-fused chlorins as efficient theranostic agents: Dyes for tumor-imaging and photodynamic therapy of cancer. European Journal of Medicinal Chemistry, 200, 112468.
- Alvim, R. G., Georgala, P., Nogueira, L., Somma, A. J., Nagar, K., Thomas, J., ... & Coleman, J. A. (2021). Combined OX40 agonist and PD-1 inhibitor immunotherapy improves the efficacy of vascular targeted photodynamic therapy in a urothelial tumor model. Molecules, 26(12), 3744.
- Jocham, D., Beer, M., Baumgartner, R., Staehler, G., & Unsöld, E. (2007, September). Long?term experience with integral photodynamic therapy of TIS bladder carcinoma. In Ciba Foundation Symposium 146?Photosensitizing Compounds: Their Chemistry, Biology and Clinical Use: Photosensitizing Compounds: Their Chemistry, Biology and Clinical Use: Ciba Foundation Symposium 146 (pp. 198-208). Chichester, UK: John Wiley & Sons, Ltd.
- Waidelich, R., Hofstetter, A., Stepp, H., Baumgartner, R., Weninger, E., & Kriegmair, M. (1998). Early clinical experience with 5-aminolevulinic acid for the photodynamic therapy of upper tract urothelial tumors. The Journal of urology, 159(2), 401-404.
- Waidelich, R., STEPP, H., BAUMGARTNER, R., WENINGER, E., HOFSTETTER, A., & KRIEGMAIR, M. (2001). Clinical experience with 5-aminolevulinic acid and photodynamic therapy for refractory superficial bladder cancer. The Journal of urology, 165(6), 1904-1907.
- Nseyo, U. O. (1996). Photodynamic therapy in the management of bladder cancer. Journal of Clinical Laser Medicine & Surgery, 14(5), 271-280.
- Alvim, R. G., Georgala, P., Nogueira, L., Somma, A. J., Nagar, K., Thomas, J., ... & Coleman, J. A. (2021). Combined OX40 agonist and PD-1 inhibitor immunotherapy improves the efficacy of vascular targeted photodynamic therapy in a urothelial tumor model. Molecules, 26(12), 3744.
- Railkar, R., & Agarwal, P. K. (2018). Photodynamic therapy in the treatment of bladder cancer: past challenges and current innovations. European urology focus, 4(4), 509-511.


 Pranali V. Ghate* 1
Pranali V. Ghate* 1
 Akshaykumar A. Kedar 1
Akshaykumar A. Kedar 1
 Jaya P. Ambhore 1
Jaya P. Ambhore 1

 10.5281/zenodo.11228124
10.5281/zenodo.11228124