Solid lipid nanoparticles are at the forefront of the rapidly developing field of nanotechnology with several potential applications in drug delivery, clinical medicine and research, as well as in other varied sciences. Due to their unique size-dependent properties, lipid nanoparticles offer the possibility to develop new therapeutics. The ability to incorporate drugs into nanocarriers offers a new prototype in drug delivery that could be used for secondary and tertiary levels of drug targeting. Hence, solid lipid nanoparticles hold great promise for reaching the goal of controlled and site specific drug delivery and hence have attracted wide attention of researchers. This review presents a broad treatment of solid lipid nanoparticles discussing their advantages, limitations and their possible remedies. The different types of nanocarriers which were based on solid lipid like solid lipid nanoparticles, nanostructured lipid carriers, lipid drug conjugates are discussed with their structural differences. Different production methods which are suitable for large scale production and applications of solid lipid nanoparticles are described. Appropriate analytical techniques for characterization of solid lipid nanoparticles like photon correlation spectroscopy, scanning electron microscopy, differential scanning calorimetry are highlighted. Aspects of solid lipid nanoparticles route of administration and their biodistribution are also incorporated. If appropriately investigated, solid lipid nanoparticles may open new vistas in therapy of complex diseases.
Solid lipid nanoparticles (SLN), colloidal drug carriers, homogenization, TEM, PCS, biodistribution, targeting.
Solid lipid nanoparticles (SLNs), formerly known as lipospheres, are among the most promising pharmaceutical nanocarriers for regulated drug delivery. SLNs often contain lipidic substances that are safe and biodegradable. The capacity of SLNs to transfer a variety of therapeutics, including vaccine antigens, large biomolecules (polysaccharides, etc.), genetic material (DNA/siRNA), and small pharmacological molecules1, is what makes them special. Nanoparticles vary in size from 1 to 100 nanometers and can be composed of metal, carbon, metal oxides, or organic materials. The structure of nanoparticles (NPs) is intricate. There are two or three levels to them: (i) a surface layer functionalized with various metal ions, small molecules, surfactants, or polymers The central area of the core material, which can be purposefully added, is composed of nanoparticles and differs chemically from the outermost layer2. SLNs combine the advantages of polymeric nanoparticles, emulsions, and liposomes. They offer both the robustness of a solid matrix and the biological compatibility of lipid carriers, avoiding the drawbacks of those drug delivery technologies. Nanomaterials with exceptional biodegradability and biocompatibility are considered the best carriers for drug delivery systems in biomedical applications. The subsequent characteristics are essential for an ideal nanoparticulate drug delivery system to possess: (1) Maximum bioavailability of the medication. (2) Targeting tissues. (3) Kinetics of release under control. (4) Very little immunological reaction. (5) The capacity to provide medications that have historically proven challenging, such as biomolecules, lipophiles, and amphiphiles. (6) Adequate ability for medication loading. (7) Satisfactory patient adherence. Drug delivery has undergone a radical transformation thanks to solid lipid nanoparticles, which combine the best aspects of liposomes, polymeric nanoparticles, and microemulsion. All of the characteristics of lipid nanoparticles are improved through surface modification, increased pharmacokinetic acceptability, inclusion complex formation, improved stability pattern, and integration of chemotherapeutic drugs3. SLNs were first presented in the 1990s as a large-scale industrially produced substitute for traditional colloidal carriers including liposomes and polymeric nanoparticles. Biodegradable lipids having high melting points, such as triglycerides, partial glycerides, fatty acids, fatty alcohols, and waxes, are frequently employed in the creation of SLNs. Surfactants work by lowering the surface tension between lipid and aqueous media, stabilizing the structure of SLNs. It was said that smaller SLN particle sizes were obtained with increased surfactant concentrations. Nonetheless, toxicity could result from a high surfactant concentration. As a result, it's necessary to optimize the surfactant concentration employed in SLN development. One of the most significant factors influencing the effectiveness of medication delivery is the particle size of SLNs. According to certain research, SLNs' skin permeability rises as their particle size lowers. The sub-100 nm size range is best for skin delivery since it allows the particles to enter deeper skin layers through hair follicles. Moreover, because of their occlusive characteristics, SLNs may improve the penetration of medications into the stratum corneum. Three models—the homogeneous matrix, the drug-enriched shell, and the drug-enriched core—can be used to explain how pharmaceuticals are incorporated into split leaf nanostructures. • Homogeneous matrix model: no surfactants or solubilizers are needed to distribute the medication throughout the lipid matrix. Typically, this model is created using the cold homogenization method. The drug-enriched shell model involves heating a lipid and drug mixture over the lipid's melting point. At the core, lipid precipitates upon fast cooling. In contrast, the medication is concentrated at the melting lipid's outer layer. At normal temperature, the melted mixture solidifies into the drug-enriched shell. Solid lipid nanoparticles (SLNs) have been employed as a substitute for colloidal drug delivery systems, such as polymeric nanoparticles, oil-in-water emulsions, liposomes, and microparticles. They are made up ofspherical lipid particles that range in size from nanometers. SLNs are utilized to incorporate lipophilic and hydrophilic medicines as well as to distribute pharmaceuticals in a regulated and targeted manner. Water, emulsifier, and/or co-emulsifier, along with solid lipids, comprise SLNs. At temperatures higher than body temperature (37°C), a common solid lipid utilized in these delivery devices dissolves. Triglycerides, fatty acids, waxes, steroids, acylglycerols, and their mixtures are a few lipids that have been studied. To stabilize the lipid dispersion, emulsifiers of all kinds have been used, either singly or in combination. Lecithin, bile salts like sodium taurocholate, non-ionic emulsifiers such ethylene oxide/propylene oxide copolymers, sorbitan esters, fatty acid ethoxylates, and their mixtures are a few examples of the emulsifiers that have been studied. The dispersion medium is deionized water. A novel medication formulation or delivery method is solid lipid nanoparticles (SLN). They are composed of solid lipids that don't change form at normal temperature. The use of physiological lipids, the avoidance of chemical solvents, a potentially broad application range (intravenous, topical, and peroral), and high pressure homogenization as a proven production method are all benefits of stem cell nanotechnology (SLN). Additionally, it was believed that adding poorly water soluble pharmaceuticals to the solid lipid matrix would increase bioavailability, shield sensitive drug molecules from the outside world (water, light), and even provide controlled release features. Since physiological lipids are used to prepare SLNs, they do not exhibit biotoxicity. First off, the pace of chemical degradation processes may be slowed down because reactive chemicals have less mobility in solids than in liquids. Secondly, it is possible to regulate the micro phase separations between the carrier lipid and active components within individual liquid particles. This prevents the build-up of active chemicals at the surface of lipid particles, which is a common site for chemical degradation reactions. Thirdly, it has been demonstrated that incorporating poorly absorbed bioactive chemicals into solid lipid nanoparticles increases their absorption. Numerous studies have also demonstrated that using a solid matrix rather than a liquid matrix might slow down lipid digestion, enabling a longer-lasting release of the encapsulated chemical. An additional important component of SLNs are aqueous type surfactants. Their selection primarily depends on the route of administration. They serve as stabilizers for SLNs dispersion and emulsifiers to form o/w type emulsion. Usually, they consist of a solid hydrophobic core with the medicine dissolved or distributed throughout.
PLANT PROFILE:
CURCUMIN
Biological source: Curcuma longa Curcumin, a bright yellow chemical produced by the turmeric plant, is approved as a food additive by the World Health Organization, European Parliament, and United States Food and Drug Administration. Curcuma longa is a flowering plant in the ginger family Zingiberaceae.
Chemical Structure:
Figure 1: Diferuloylmethane
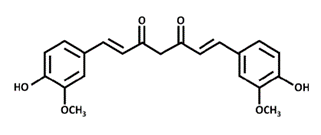
Scientific classification
Kingdom: Plantae
Phylum: Tracheophyta
Class: Liliopsida
Order: Zingiberales
Family: Zingiberaceae
Genus: Curcuma
Species: C. longa
Phytochemicals:
Phytochemical components of turmeric include diarylheptanoids, a class including numerous curcuminoids, such as curcumin, demethoxycurcumin, and bisdemethoxycurcumin. Essential oils are present in turmeric, among which turmerone, germacrone, atlantone, and zingiberene are major constituents. Turmeric powder consists of 60–70?rbohydrates, 6–13% water, 6– 8% protein, 5–10?t, 3–7% dietary minerals, 3–7% essential oils, 2–7% dietary fiber, and 1– 6% curcuminoids.
Properties
Chemical formula: C21H20O6
Molar mass: 368.385 g·mol?1
Appearance: Bright yellow-orange powder
Melting point: 183 °C (361 °F; 456 K)
Category: Anti-bacterial, anti-microbial
The chemical structure of Cur is diferuloylmethane, which belongs to the polyphenol class. Its chemical name is (1E,6E)-1,7-bis(4-hydroxy-3-methoxyphenyl)-1,6 heptadiene-3,5-dione. Solubility: is insoluble in water and ether, soluble in ethanol and propylene glycol, and easily soluble in glacial acetic acid and alkaline solutions. Curcumin can be made water soluble by using special techniques such as microencapsulation or nanotechnology.
Uses:
It helps in the management of oxidative and inflammatory conditions, metabolic syndrome, arthritis, anxiety and hyperlipidemia. It can also help in the management of inflammation caused by exercise and muscle pain, thereby improving recovery and performance in working people. Use in Traditional Indian Medicine It helps in Oral Health, Cancer Prevention, Brain Health, Managing Diabetes, Vision, Treats Allergies and Asthma, Treats Liver Conditions, Anti-Aging and Skin Health, Pain Management, Respiratory Health, Mood and Stress.
EXCIPIENT PROFILE:
Stearic acid:
Chemical structure:
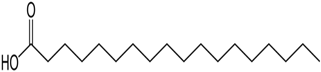
Fig no 2 Stearic Acid
Description:
Stearic acid is a saturated fatty acid with an 18-carbon chain. The IUPAC name is octadecanoic acid. It is a soft waxy solid with the formula CH3(CH2)16CO2H. The triglyceride derived from three molecules of stearic acid is called stearin. Stearic acid is a prevalent fatty-acid in nature, found in many animal and vegetable fats, but is usually higher in animal fat than vegetable fat. It has a melting point of 69.4 °C and a pKa of 4.50. Synonyms and Trade Names: Stearic Acid; Cetylacetic Acid; 1-Heptadecanecarboxylic Acid; Pearl Steric; E570; Pristerene®; Tego-Stearic®; Crodacid®; Cristal® G; Emersol® Chemical formula: C18H36O2 Molecular Weight: 284.484 g/mol Density: 0.9408 g/cm3 (20 °C) 0.847 g/cm3 (70 °C) Boiling point: 361 °C (682 °F; 634 K) Melting point: 69.3 °C (156.7 °F; 342.4 K)
Solubility:
Stearic acid is not very soluble in water. Only a fraction of a milligram of stearic acid can be dissolved in a hundred grams of water. However, this compound is soluble in many organic solvents. stearic acid is moderately soluble in alcohols, phenyls and alkyl acetates. The solubility of stearic acid in ethyl acetate was found to be the highest followed by ethanol, acetone and methanol. Uses: Tablet and Capsule Lubricant; Emulsifying Agent; and Solubilising Agent.
Tween 80
Chemical structure:

Fig no 3 Polysorbate 80
Polysorbate 80
Description:
Polysorbate 80 is a nonionic surfactant and emulsifier often used in pharmaceuticals, foods, and cosmetics. This synthetic compound is a viscous, water- soluble yellow liquid.Polysorbate80 is derived from polyethoxylated sorbitan and oleic acid. The hydrophilicgroupsin this compound are polyethers also known as polyoxyethylene groups, which are polymersof ethylene oxide. In the nomenclature of polysorbates, the numeric designation following polysorbate refers to the lipophilic group, in this case, the oleic acid.
Synonyms:
Polyoxyethylene (80) sorbitan monooleate, (x)-sorbitan mono-9-octadecenoate
poly(oxy-1,2-ethanediyl).
Category:
Non-ionic surfactant
Brand names:
Alkest TW 80, Scattics, Canarcel, Poegasorb 80, Montanox 80, Kotilen-80.
Properties:
Chemical formula: C64H124O26
Molar mass:
1310 g/mol
Appearance:
Amber colored oil
Density:
1.102 g/mL
Boiling point:
> 100°C
Solubility:
Easily soluble in cold water, hot water. Soluble in water, ethanol, methanol, ethylacetate and toluene. Insoluble in mineral oil and petroleum ether.
Applications:
Used as emulsifiers and dispersing agents in medicinal products, defoamers and emulsifiers infoods, and surfactants for pharmaceuticals.Because of their hydrophilic and lyophilic characteristics, these nonionic surfactants are veryuseful as emulsifying agents in pharmaceuticals, cosmetics, and other types of products. Polysorbate 80 is an ingredient in coal tar ointment and solution.
MECHANISM OF ACTION:
Polysorbate 80 is one of the primary components of protein formulations. This drug inhibitsinterfacial damage of the protein molecule that undergoes mechanical stress during shippingand handling. Polysorbate 80 also affects the formulation photostability. Exposure to light ofpolysorbate 80 aqueous solution results in peroxide generation, which in turn may lead tooxidation of the susceptible amino acid residues in the protein molecule.
EXTRACTION OF CURCUMIN
Sample Preparation: In this step, turmeric rhizomes were collected and appropriately washed.Then drying and grinding were done. Drying and size reductionare essential for processing, as size plays an important role(smaller the size, higher the diffusion of bioactive compounds from source to solvent) in the extraction process.
Extraction and Purification:
The ground samples are thensubject to an extraction procedure. The most commonly usedextraction process for curcumin is the traditional method due toits low cost of operation and simple handling. Among the different extraction processes, the soxhlation method of powdered turmeric withethanol for 420 minutes at 70?C was done and the extract is collected.
FORMULATION:
Cur-loaded SLNs were prepared via sonication using a modified O/W emulsion method. Briefly, the oil phase was prepared by dissolving Cur in melted lipid (SA) at 10 °C above thelipid melting point. The molten oil phase was poured into a hot aqueous solution containingsurfactants (TW 20, or TW 80) and homogenized at 1000 rpm, which resulted in an O/Wemulsion. SLN were then fabricated by sonicator. The effects of different compositions of Cur-loaded SLN were evaluated.
1. Photostability studies:
The photostability of Cur in SLNs was determined according to the modified method describedcompared with that of Cur in a 0.1% MeOH solution. The photostability of Cur wasmonitored by recording its absorption spectrum at 425 nm. Briefly, 20 mL of Cur- or Cur-loaded SLNs in a 0.1% MeOH solution (4 ppm) was irradiated (2 J/cm2) with LED for differenttime intervals (0, 10, 20, 30, and 40 min). Cur was then extracted from the formulations by layercontaining the extracted Cur was filtered through 0.22 ?m filters and analyzed using the UV?vis spectrophotometer as described in the Development of Analytical Method for Cur section.
2. Determination of Drug-Encapsulation Efficiency (EE):
SLN specimens were diluted 10 times to a final volume of 1 mL and then gently vortexed. Thesuspension was then centrifuged at suitable rpm for 1 h at 4 °C. The concentration of the freenon-encapsulated drug in the supernatant was analyzed using a UV?vis spectrophotometer, asin the Development of Analytical Method for Cur section. EE was calculated using the formula.
EE (%) = Total drug amount - Free nonencapsulated drug amount × 100
Total drug amount
3. Drug content:
1ml of the prepared curcumin loaded solid lipid nanoparticle suspension was made to 10ml with methanol and was homogenously dispersed. The suitable dilutions were made with phosphate buffer saline of pH 7.4 and the concentration of the drug was analyzed using UV-visible spectrophotometer at 430nm.
4. Dialysis tubing:
Dialysis tubing may be used to achieve in vitro drug release. The pre-washed dialysis tubingcan be hermetically sealed with the solid lipid nanoparticle dispersion. The dialysis sac is thendialyzed at room temperature against a suitable dissolution medium, with samples withdrawn from the dissolution medium at appropriate intervals, centrifuged, and drug content determined using a suitable analytical process.
5. Stability of Cur-SLNs against pH and Ionic Strength:
The stability of Cur-SLN was evaluated by adjusting the pH of the suspension from 2 to 7 using0.1 M NaOH or HCl at ambient temperature. The ionic stability of Cur-SLNs was assessed byadding NaCl (0–200 mM) to the dispersions at ambient temperature. The z-average and zetapotential of samples were measured 2 h after equilibrium
6. Chemical Stability of Cur in SLNs
The chemical stability of Cur after storage at 4 °C for 4 weeks was evaluated to investigate the protective effect of Cur-SLNs. Briefly, the Cur-SLNs dispersion (100 ?L) was diluted 100 times using ethanol and sonicated for 30 min to release Cur. The residual amount of Cur was quantified using the above UV-Vis spectrophotometer (425 nm) and calculated as the percentage of Cur with reference to that on day 0. The control was the free Cur dissolved in ethanol.
RESULTS AND DISCUSSION:
- Photostability Studies:
The photostability of Cur is important for the storage of Cur-loaded SLNs before administration. A moderate dosage should be maintained due to the side effects of thedegradation products and the decrease in drug effects. All formulations improved the photostability of Cur (73.82 to 94.39% for 40 min) compared with that of the pure Cur solution(58.26% for 40 min). The Cur photostability of the formulations decreased in the followingorder after 40 min: F4 > F5 > F3 > F2 > F1 > Cur. This is because SLN can structurally protectencapsulated drugs from the external environment. Thus, the photodegradation of Cur wasinhibited. Concerning the effects of lipids and surfactants, formulations using lipids with longcarbon chains and surfactants with high hydrophilicity tended to have high photostability. Thissuggeststhat the encapsulated drug inhibitslight exposure, as mentioned above in the EE study.
- Determination of Drug-Encapsulation Efficiency (EE):
EE is an important parameter in particle formulations because it affects drug stability against the external environment, avoids side effects in the human body that result from exposure to the drug, and enables the sustained release of Cur from the formulations.The EE study for Cur-loaded SLN was carried out using the centrifugation method, followed by concentration estimation using the UV?vis method. The EE values ranged from 97.24 to 97.74% (Figure 3).The EE of Cur in the SLNs proportionally increased as the number of lipid carbon chainsincreased. This was because the log p value of Cur was 3.2, and the lipophilicity of Cur mighthave a high affinity for lipids with longer carbon chains. The higher affinity between the drug and the lipid matrix induces stable encapsulation of the drug in the particle core and shell. For formulations using different surfactants, the results showed that an increase in the HLB value of the surfactant increased EE.
- Drug content:
The drug content of all the prepared SLN formulations by sonication is shown in
Table 3: Drug content of curcumin loaded SLNs

Chemical Stability of Cur in SLNs
During storage, the transition to more ordered lipid crystals could reduce the number of amorphous lipids and imperfect crystals, which lead to the expulsion of Cur initially trapped in the voids of lipid bases. In the previous studies, surfactant type, carrier oil type, droplet size and pH significantly influence the chemical stability of encapsulated Cur. Therefore, the Curretention in SLNs was measured to assess its chemical stability. No direct relationship was observed between the chemical stability of Cur and the size of SLNs. This might be because he low storage temperature could efficiently keep the compound from oxidation and degradation.
The development of curcumin-loaded solid lipid nanoparticles presents a promising avenue for enhancing the bioavailability and therapeutic efficacy of curcumin. Through our project, wehave demonstrated the successful formulation and characterization of these nanoparticles highlighting their stability, enhanced cellular uptake compared to free curcumin. Moreover, our findings suggest that curcumin-loaded SLNs exhibit improved pharmacokinetic properties,potentially leading to enhanced therapeutic outcomes in various diseases such as cancer,inflammation. However, further studies are warranted to explore the long-term safety, efficacy,and clinical applicability of these nanoparticles. Overall, the successful development of curcumin-loaded SLNs opens up exciting opportunities for the targeted delivery of curcumin and holds great promise for future therapeutic interventions.


 Ch. Bhavani*
Ch. Bhavani*





 10.5281/zenodo.11261094
10.5281/zenodo.11261094