Abstract
Introduction:
The knowledge of severity of the error is necessary to prevent harm to the subject The severity of errors was classified based on NCC MERP classification that helps understand the potential impact and harm associated with medication errors, allowing for appropriate responses and preventive measures
Objective:
To classify the medication errors according to NCC MERP guidelines
Methodology: A prospective observational study of medication reconciliation was performed for 6 months in a Tertiary care hospital. The discrepancies were identified by reviewing medical records, all patients above 18years were included in the study.
Result:
Of the 225 patients (mean age, 57.82 years; 132 men, 123 women) included in the study a total of 633 discrepancies identified during the admission, 296 (46.76%) were categorized as unintentional discrepancies.412 discrepancies were identified of which 191 were unintentional during discharge. Majority of errors, accounting for 52.54%, were under Category C, indicating errors that reached the patient but did not cause harm.
Conclusion:
The study offers an advanced understanding of medication-related problems through the classification of differences into intentional and unintentional categories and the evaluation of error severity. In the end, this study highlights the importance medication reconciliation is to reducing adverse events, boosting patient outcomes, and raising the standard of healthcare delivery as an entire sector.
Keywords
Medication reconciliation, medication discreapancies,NCC MERP, severity of error
Introduction
Joint Commission on Accreditation of Health care Organization (JCAHO) defines Medication reconciliation is “the process of comparing a patient medication order to all of the medications that patient has been taking”1. Medication errors remain a significant patient safety concern and a financial burden in hospitalized patients. These organizations stated and proved that medication reconciliation reduces the incidence of medication discrepancies2. The knowledge of severity of the error is necessary to prevent harm to the subject3. The National Coordinating Council for Medication Error Reporting and Prevention (NCC MERP) classification system categorizes the severity of errors. It aids healthcare professionals and organizations in understanding the potential impact and harm of medication errors, facilitating appropriate responses and preventive measures.1. The NCC MERP, comprising 24 national healthcare organizations, standardizes medication error reporting and grading. Its index categorizes errors into nine severity-based categories, considering factors such as harm and extended hospital stays. This minimizes interpretation variability and enhances medication error recognition and prevention.4.
MATERIALS AND METHODS
STUDY DESIGN: A prospective, observational study was performed on medication reconciliation in inpatient of various department of the Hospital. The study was completed in the period of 6 months.
SAMPLE SIZE: 255 [5]
Inclusion criteria: Patient admitted in the hospital aged >18 years, of any sex and who is already having any comorbid condition and was using any drug prior to the admission.
Exclusion Criteria: Patients of vulnerable population, unconscious, who are not willing to participate, those who are not able to provide history within 24 hours are excluded from the study.
SOURCE OF DATA COLLECTION:
The Best Possible Medication History (BPMH) for each patient comprises all pre-admission medication details, including dosage, frequency, and any vitamins, herbal medications, non-prescription drugs, or food allergies.
STATISTICAL ANALYSIS:
Statistical analysis involves collecting and scrutinizing every data sample in a set of items from which samples can be drawn and a suitable statistical test was applied to analyze the,data. The collected data were analyzed using Microsoft Excel.
RESULT:
Patient demographics:
A total of 600 patients were included in the study of which 42.5% (225 patients) had discrepancies. majority of the patients were 60 & above (55.29%).Furthermore, 51.76% were male patients.
Table 1: Demographics of Subjects

Category of Discrepancies
In this study, medication discrepancies were classified as intentional and unintentional. Intentional discrepancies involved medication changes based on evolving clinical status, while unintentional discrepancies included issues like omissions or duplications. Out of the total 633 discrepancies identified during the admission process, a substantial 337 (53.23%) were classified as intentional discrepancies, while the remaining 296 (46.76%) were categorized as unintentional discrepancies. The breakdown of these categories of discrepancies during admission is presented in Table 4. This classification allows for a comprehensive understanding of the nature and origins of medication discrepancies in the study.
Table 2: Category of Discrepancies


Figure1: Category of Discrepancies During Admission
Category of Discrepancies During Discharge
Out of the 412 discrepancies identified during discharge, 53.64% (221 discrepancies) were intentional, and 46.35% (191 discrepancies) were unintentional. Table 2 provides a breakdown of these categories, offering insight into the nature and origins of medication discrepancies in the study
Table 3: Category of Discrepancies During Discharge


Figure2: Category of Discrepancies During Discharge
No. of Unintentional Discrepancies Per Prescription
In the case of prescriptions with unintentional discrepancies, 59 (23.13%) had 2 discrepancies, followed by 36 (14.11%) with 1 discrepancy, 17 (6.66%) with 3 discrepancies, 11 (4.31%) with 4 discrepancies, and 1 (0.39%) with more than 5 discrepancies. These unintentional discrepancies are detailed in Table 8, providing insight into the distribution of errors in the study. Table 8: No. of Unintentional Discrepancies Per Prescription
Table 4: No. of Unintentional Discrepancies Per Prescription

Classification of Severity of Medication Errors
Assessing medication error severity is crucial for patient safety, as per NCC-MERP guidelines. Categorizing errors using NCC MERP showed that 52.54?ll under Category C, reaching the patient but causing no harm. Category D, requiring monitoring to confirm no harm, constituted 36.07% of errors observed.
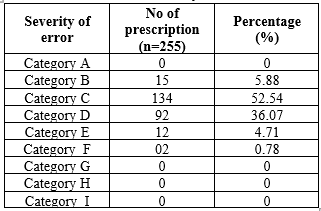
Table 5: Classification of Severity of Medication Errors

Fig no 3: severity of medication errors
DISCUSSION
The current study of 600 patients showed a slightly higher percentage of men and a majority aged over 60, highlighting the need to address older individuals' healthcare. In contrast, Meda V.S. et al.'s study of 106 participants had 42% men and 58% women, with 63% over 60. Reddy P.S. et al. found most patients were above 61, followed by the 46-60 age group. These findings underscore the importance of reconciling healthcare for older patients and ensuring precise medication administration to enhance patient care and safety.6 In the current study, out of 633 discrepancies identified during admission, the majority were intentional, while others were unintentional, indicating deviations from the original plan. A similar pattern was observed during discharge, with most of the 412 discrepancies being intentional. Alanazi A.S. et al. found 26.6% of 237
medication discrepancies were unintended, while 73.4% were intentional changes made as part of the new discharge plan. Their research focused on how discharge plan changes affected these discrepancies, contributing to improving patient safety during healthcare transitions.7. In the current study, medication discrepancies were classified by severity using the NCC MERP classification. The majority fell into Category C, indicating errors that reached the patient but caused no harm. Category D errors, reaching the patient but requiring monitoring, were also common. In contrast, Meda V.S. et al. found a majority of errors in Category E, suggesting temporary harm. Mohammed Aseeri et al. noted that Category C errors in NCC MERP refer to situations where an error affected the patient but did not result in harm.8.
CONCLUSION
This study highlights the urgency of medication reconciliation in hospitals, particularly for elderly patients, given the significant prevalence of discrepancies. Categorizing discrepancies and assessing error severity offer valuable insights into patient safety. Overall, medication reconciliation is essential for preventing adverse events and improving healthcare quality.
ACKNOWLEDGEMENT
Authors would like to extend deepest gratitude to Srinivas College of Pharmacy and would like to extend our thanks and appreciation to the study participants for smooth completion.
REFERENCES
- Meda VS, Babu KL, Reddy VP, Mounika M, Reddy SS, Reddy YM. Significance of medication reconciliation in intercepting admission medication errors in the General Medicine Department. Indian Journal of Pharmaceutical Sciences. 2021;83(5):925–30.
- Sheikh D, Mateti UV, Kabekkodu S, Sanal T. Assessment of medication errors and adherence to WHO prescription writing guidelines in a tertiary care hospital. Future Journal of Pharmaceutical Sciences. 2017 Jun 1;3(1):60-4.
- Sacks GS, Rough S, Kudsk KA. Frequency and severity of harm of medication errors related to the parenteral nutrition process in a large university teaching hospital. Pharmacotherapy: The Journal of Human Pharmacology and Drug Therapy. 2009 Aug;29(8):966-74.
- Forrey RA, Pedersen CA, Schneider PJ. Interrater agreement with a standard scheme for classifying medication errors. American journal of health-system pharmacy. 2007 Jan 15;64(2):175-81.
- Al-Hashar A, Al-Zakwani I, Eriksson T, Sarakbi A, Al-Zadjali B, Al Mubaihsi S, et al. Impact of medication reconciliation and review and counselling, on Adverse DrugEvents and Healthcare Resource Use. International Journal of Clinical Pharmacy. 2018;40(5):1154–64.
- Reddy PS, Biju V, Bhavana I. Identifying Medication Errors in a Tertiary Care Teaching Hospital: A Prospective Observational Study. Journal of Drug Delivery and Therapeutics. 2019 Dec 15;9(6-s):103-
- Alanazi AS, Awwad S, Khan TM, Asdaq SM, Mohzari Y, Alanazi F, et al. Medication reconciliation on discharge in a Tertiary Care Riyadh Hospital: An observational study. PLOS ONE. 2022;17(3).
- Aseeri M, Banasser G, Baduhduh O, Baksh S, Ghalibi N. Evaluation of medication error incident reports at a tertiary care hospital. Pharmacy. 2020 Apr 19;8(2):69.


 Sandra Thattiott*
Sandra Thattiott*







 10.5281/zenodo.13712642
10.5281/zenodo.13712642