Abstract
The oral route is favored for drug administration. However, in the case of various drugs, physiological and biochemical reasons make it difficult to achieve an acceptable level of absorption. Other routes like parenteral administration also have limitations. The buccal mucosa is a promising site for medication delivery with advantages like high blood supply, rapid absorption, and bypassing hepatic processing.
Keywords
Buccal Drug Delivery, hepatic processing, like parenteral, medication delivery.
Introduction
One that medical professionals and patients generally favor. Many medications cannot be administered efficiently by the traditional oral route, according to our current understanding of the physiological and biochemical factors of absorption and metabolism. due to the considerable pre-systemic clearance that occurs in the liver upon delivery, which frequently results in a lack of meaningful association between membrane permeability, absorption, and bioavailability. challenges related to parenteral administration and inadequate oral availability It is thought that other absorptive mucosae could be used as medication administration sites. For a long time, medications have been given topically to the oral mucosa. But lately, there has been interest in using the mouth as a route for medication delivery to the bloodstream.1,2 The buccal has considerable appeal for both local and systemic drug bioavailability due to its capacity to maintain a delivery system at a specific place for an extended length of time. The buccal mucosa has a plentiful blood supply, which makes absorption effective here. The route also delivers drugs quickly to the systemic circulation, preventing gastrointestinal enzyme breakdown and bypassing hepatic processing first.3 One of the best transmucosal routes for administering controlled release dosage forms is the buccal mucosa because of its smooth muscular expanse, great accessibility, and generally immobile mucosa. In comparison to other non-oral transmucosal drug administration routes, buccal medication delivery also has a high patient acceptance rate. Due to its lower permeability compared to the sublingual location, buccal mucosa is a better option for long-term medication administration.4 The oral mucosa's permeability barrier is mostly caused by intercellular components that come from what are known as "membrane coating granules" (MCGs).1
The mucoadhesive delivery system can be classified based on their route of application as follows 3:
1. Buccal drug delivery system
2. Ocular drug delivery system
3. Nasal drug delivery system
4. Gastrointestinal drug delivery system
5. Rectal drug delivery system
Structure And Function of Oral Mucosa

Fig. no :1 Anatomical region of buccal cavity
The oral cavity is lined by a squamous epithelium that is stratified. It is possible to distinguish between three different types of oral mucosa: masticatory, lining, and specialized. The hard palate and gingiva are covered by the masticatory mucosa. Because it is made up of keratinized epithelium that is firmly linked to underlying tissues by collagenous connective tissue, it can endure the shearing and abrasion forces that occur during the masticatory process. With the exception of the tongue's dorsal surface, the lining mucosa is covered in a nonkeratinized epithelium that is more transparent. Because of its elastic deformation ability, this mucosa can expand to meet the needs of speech and chewing. Human epithelium varies in thickness depending on the area; for example, the hard palate has 310 ?m and the floor of the mouth has 190 ?m.5
Permeability
Comparing buccal mucosa permeability to skin permeability, the former is 4-4,000 times higher. The variations in the various oral mucosa's architecture and functions result in the mouth cavity's ability to pass via various areas. The thickness and level of tissue keratinization influence the permeability of the oral mucosa. This suggests that sublingual permeability is higher than buccal and buccal permeability is higher than palatal 6. Gaikwad SS, Kale YK, Gondkar SB, Darekar AB. Buccal tablet as a promising mucoadhesive drug delivery. Invent Rapid: Pharm Tech. 2012 May 1;3:1-8. 6
functions of oral cavity
The following are the key functions that are performed by the mouth cavity:
- To facilitate the chewing, mastication, and mixing of food ingredients. To serve as a conduit for the intake of food materials and fluids.
- creating a bolus and lubricating the meal substance.
- A tongue's taste buds are used to identify food that has been consumed.
- Start of the metabolism of fat and carbohydrates. Consumption of catabolized
- Items that are metabolized later on.
- To facilitate the process of speaking and breathing.
- Saline in the mouth cavity and on consumed material causes a slight antisepsis.2
The Mechanism of Mucoadhesion
In its simplest form, mucoadhesion is the occurrence where two materials—one of which may be an artificial substance, such as a polymer—adhere to each other over an extended length of time through the action of interfacial forces. The other material is the mucin layer that lines the mucosal tissue 7 Thus the mechanism of mucoadhesion is generally divided in two steps, the contact stage and the consolidation stage
Contact Stage
When the mucoadhesive material and the mucous membrane come into touch during this stage, a close relationship is formed between them.
Consolidation Stage
The term "consolidation stage" refers to the prolonged and deep intimate adherence that results from the attachment of mucoadhesive material to the mucous membrane by various physicochemical forces of attraction. 8
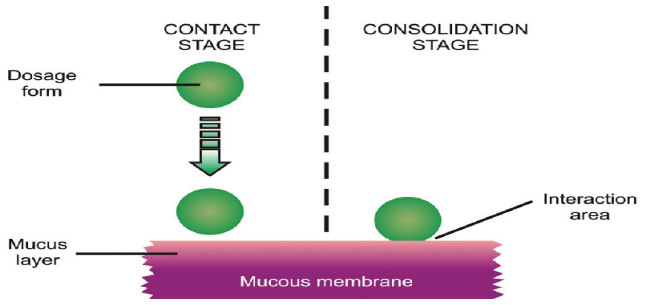
Fig.no 2 : Mechanism of Mucoadhesion
Theories of Bioadhesion or Mucoadhesion
Mucoadhesion is a complex process and various theories have been proposed to explain the mechanisms involved in mucoadhesion. These are as follows:
1.Wetting theory: Wetting theory studies the adhesive and contact affinity in terms of a liquid or a paste to spread over a biological system, and it is mostly applicable to liquid bioadhesive systems. Finding the contact angle will yield the answer to affinity. As a general rule, the affinity increases with decreasing contact angle. In order to achieve sufficient spreadability, the contact angle needs to be zero or almost zero.9
The spreadability coefficient (SAB) can be calculated by the equation:
SAB = ?B- ?A – ?AB
Where, ?B = Surface energy ?A =Interfacial energy. If interfacial energy is greater in relating to the individual surface energy, then adhesion work WA will be greater, i.e., greater the energy required to separate the two phases.
WA = ?A + ?B – ?ab 10

Fig no 3: Wetting theory
2.Diffusion theory
Diffusion theory explains how both polymer and mucin chains can interpenetrate to a depth that produces a semi-permanent adhesive bond. This idea states that a semipermanent adhesive bond was formed when the polymer chains and mucus combined thoroughly. The polymer chains' precise level of mucus penetration depends on a number of factors, including contact time, mobility, the type of mucoadhesive chains, and the diffusion coefficient's flexibility. The difference in molecular weight between cross links determines this diffusion coefficient. The diffusion coefficient dramatically drops as the cross connecting density does. In order to create an effective bioadhesive connection, the literature suggests that the depth of interpenetration should be between 0.2 and 0.5 ?m. This polymer's level of interpenetration and mucin chains can be determined by the following equation
l = (t Db)½
Where t = the contact time
Db = the diffusion coefficient of the mucoadhesive material in the mucus.
Both the bioadhesive and the mucus components involved must have strong mutual solubility, or similar chemical structures, for diffusion to occur. The strength of the mucoadhesive binding increases with increasing structural similarity.11

Fig.no 4: Diffusion theory
3.Electronic Theory:
Derjaguin and Smigla proposed the electronic theory of adhesion. This theory states that when an adhesive polymer and the mucus glycoprotein network come into contact, electron transfer takes place due to the differences in their electrical structures. As a result of this, there is the creation of an electrical double layer at the interface. Attractive forces acting across the double layer cause adhesion. According to this idea, adhesion between mucus and the mucoadhesive system happens through the transfer of electrons, which is caused by variations in their electron structures.12
4.Fracture Theory:
This theory states that an adhesive bond between systems is connected to the force needed to separate both surfaces from one another. This "fracture theory" connects the strength of the polymer's adhesive bond with the force needed to separate it from the mucus. The work fracture will be larger the longer the polymer network strands. Alternatively, work fracture will rise if the level of cross-linking in such a system is diminished. The following formula can be used to determine this:
r = (E x e / L) ½
where, r = fracture strength
e = fracture energy
E = Young?s modulus of elasticity
L = the critical crack length 11
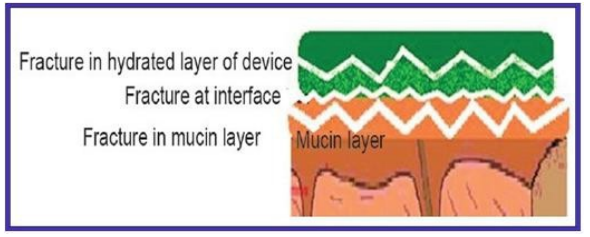
Fig.no 5: Fracture Theory
5.Adsorption Theory
This hypothesis states that surface forces operating between the atoms in the two surfaces cause the materials to adhere after an initial contact between them. The adsorption process involves two different kinds of chemical bonds: primary covalent (permanent) and secondary chemical connections, which include electrostatic forces, van der Waals forces, hydrogen bonds, and hydrophobic bonds.13

Fig.no 6: Adsorption Theory
Advantages Of Bucco-Adhesive Drug Delivery System 14
- Provides increased bioavailability by avoiding first pass metabolism.
- Permits long-term drug localization.
- Makes it easier to administer and stop therapy in an emergency.
- Easy to deliver to a patient who is unconscious.
- A considerable dosage reduction is achievable.
- It is possible to provide medications that are prone to enzymatic breakdown or that are likely to be unstable in an acidic or alkaline stomach and intestine.
- Passive diffusion is the method used to absorb drugs.
- Greater acceptance or compliance from patients.8
- Offers continuous medication delivery.
- Quick starting point for action.
Limitations Of Bucco-Adhesive Drug Delivery System15
1. It is not permitted to give medications that cause irritation to the oral mucosa, have an unpleasant odor, or taste harsh.
2. It is not possible to deliver medications that are unstable at buccal pH.
3. Medication can be administered at a low dosage required.
4. Drug swallowing may result from excessive salivation.
5. It is possible to deliver drugs that are absorbed through passive diffusion.
6. It could not be convenient to consume food and liquids.
7. Patients may inadvertently ingest the formulation.

Fig.no 7: Overview of the characteristics of the oral environment relevant to buccal drug delivery (black) and the factors that influence them (red) 16
Evaluation Of Buccal Tablets
1.Weight variation - An electronic balance was used to weigh the tablets (n = 20) in each batch, and the average weight was determined.17
2.Size and thickness -A micrometer or screw gauge with a minimum count of 0.01mm is used to measure the thickness of tablets. By sandwiching the tablets between two tiny slides at five distinct locations, the thickness may be determined. By measuring the thickness of samples with assembly using a micrometer or screw gauge and deducting the thickness of the two glass slides that were previously measured, the thickness of the film at various points was determined. Although the typical range of a suitable size for buccal tablets is 1 to 3 cm2, the greatest likely size is 15 mm. Tablets should not be thicker than a few millimeters. The two most comfortable shapes for patients to use are circular or ellipsoid.18
3.Hardness
The tablet's hardness determines how resistant it is to chipping, abrasion, or breaking when handled, transported, and stored before use. Ten buccal tablets from each batch were chosen at random, and the hardness of each was assessed using a Monsanto Hardness Tester (Secor Scientific Eng Corporation India) and expressed in kg/cm2. We computed and provided the mean and standard deviation values.19,20
4. Friability
Abrasion friabilator was used to hold ten tablets that had been previously weighed. It made 100 rotations in total while rotating at 25 rpm for 4 minutes. The tablets were weighed once more after being turned over on a #10 sieve to remove any remaining dust. Using the following formula, the % friability was determined
% Friability = Wi X Wf X 100Wi
Where Wi is the initial weight and Wf is the final weight of the tablet before and after the friability test. The percent friability must not be more than 0.8% for new formulations 21
5. % swelling study:
The percentage swelling investigation involved weighing each buccal tablet separately (W1), arranging them in separate 2% agar gel plates with the core facing the gel surface, and then incubating them at 37 ± 0.1°C. The tablets were taken out of the petridish, and any extra surface water was carefully wiped away with filter paper. After reweighing the swollen pill (W2), the swelling index was computed using the formula below.
Final weight (W2) – Initial weight (W1) % Swelling index = ×100 Initial weight (W1)22
6. Content uniformity
Each batch of twenty tablets was precisely weighed, and the powdered form, equivalent to 6.2 mg of carvedilol, was shaken with 50 ml of methanol in a 50 ml volumetric flask. Four milliliters of this standard solution were pipetted out, and the remaining milliliter was diluted with 100 ml of phosphate buffer (pH 6.8). The resulting solution was filtered, and the amount of carvedilol was estimated by measuring the filtrate's absorbance at 242 nm using a spectrophotometer.23
7. Moisture absorption study
The investigations on moisture uptake provide insight into the relative moisture absorption capacities of polymers and provide information about whether the formulations retain their integrity during moisture absorption. 5 percent w/v agar was dissolved in hot water, then put into petriplates and left to solidify. Before the investigation, six tablets from each formulation series were laminated on one side with a water-impermeable backing membrane and kept in a vacuum oven for the whole night to remove any possible moisture. After an hour of incubation at 370 C, they were taken out and weighed again. Using the formula, the percentage of moisture absorption was determined.24
% moisture absorption = (Final weight – Initial weight) x100 Initial weight
8. Surface pH
A combination glass electrode is used to measure the buccal tablets' surface pH. The pill is left to swell for two hours at room temperature with 1 milliliter of distilled water (pH 6.8 ± 0.05). After allowing the electrode to acclimate to the tablet surface for one minute, the pH is determined.25
9. In vitro drug release study
The matrix tablets' in vitro drug release investigations were carried out in a USP type II dissolving equipment that was calibrated at 37 ± 0.5°C and 100 rpm. For 12 hours, the dissolving investigations were conducted in triplicate in 900 cc of pH 1.2 stomach juice. Every hour, the dissolution samples were taken and replaced with an equivalent volume of stomach fluid to keep the volume constant. A UV spectrophotometer (Shimadzu, Kyoto, Japan) was used to analyze the sample solution at 272 nm, as specified in USP and BP. The sample solution had been adequately diluted. The necessary calibration curves, which were created using the medication's reference standard, were used to compute the amount of drug present in the samples. Plotting of drug dissolved at particular times was done as a percent release versus time (hours) curve.26
10. Mucoadhesion Strength
A modified balance method was used to check the strength of the mucoadhesion. The device consists of two panbalances that have been changed by swapping out one pan with a Teflon assembly that is used to attach the tablet and lower it onto another Teflon assembly that is covered with a buccal mucosa tie. The model membrane employed in this study was porcine buccal mucosa. Before use, the mucosa was kept at room temperature in phosphate buffer (pH 7.4) By eliminating the underlying connective and adipose tissue, the mucosal membrane was removed. After that, it was allowed to acclimate for 30 minutes at 37±1°C in 0.2 molar phosphate buffer (pH 6.8).Using cyanoacrylate adhesive, the tablet was adhered to the Teflon arm and then dropped onto the mucosa for a five-minute contact period while maintaining a constant weight of five grams. To measure the mucoadhesion strength, the weight (g) needed to separate the pill from the membrane was used.27
11. In vitro drug diffusion study
A diffusion research was conducted utilizing a Franz diffusion cell to assess the drug's permeability across the goat buccal mucosal membrane. Within two hours of the goats' slaughter, the buccal mucosa was used. It was purchased at a nearby butcher. After being collected, the tissue was kept in an ice-cold water solution. For permeation investigations, the epithelium was clamped in between the donor and recipient chambers of the diffusion cells after being cut using surgical scissors from the underlying connective tissues. Three milliliters of simulated pH 6.8 phosphate buffer saliva were placed in the donor compartment, whereas twenty milliliters of pH 6.8 phosphate buffer saliva were housed in the receptor compartment. The tablet was positioned on the mucosal surface of the donor compartment. Fresh 2 ml medium was added each time, and 2 ml aliquots were taken out of the receptor compartment at appropriate intervals while the solution was being constantly stirred with a magnetic stirrer. Using a UV visible spectrophotometer, the absorbance was measured at 238 nm.28
List of some of the marketed buccal products 29
|
Product Name Drugs
|
Drug
|
Dosage Form
|
Manufacturer
|
|
Buccastem
|
Prochlorperazine
|
Tablet
|
Reckitt Benckiser
|
|
Suscard
|
Glyceryl trinitrate
|
Tablet
|
Forest laboratories
|
|
Aphtach Tablet
|
Triamcinolone acetonide
|
Tablet
|
Teijin Ltd
|
|
Straint SR
|
Testosterone
|
Tablet
|
Ardana Bioscience Ltd
|
|
Subutex
|
Buprenorphine HCl
|
Tablet
|
Reckitt Benckiser
|
|
Nitrostat Tablet
|
Nitroglycerine
|
Tablet, Spray
|
Pfizer Pharmaceuticals
|
|
Suboxone
|
Buprenorphine hydrochloride-naloxone HCl
|
Tablet
|
Reckitt Benckiser
|
CONCLUSION:
The buccal route has proven to be a viable alternative for medication delivery, providing effective absorption and rapid delivery to systemic circulation. This is an advantage that makes the route an attractive option for drugs that face challenges with traditional oral administration. Further research and development of buccal delivery systems may improve treatment outcomes and compliance in patients.
REFERENCES
- Reddy PC, Chaitanya KS, Rao YM. A review on bioadhesive buccal drug delivery systems: current status of formulation and evaluation methods. DARU Journal of Pharmaceutical Sciences. 2011;19(6):385.
- Dhotre, Bhagyashree. (2021). Formulation And Evaluation Of Buccal Tablets: A Review.
- Singh J, Deep P. A review article on mucoadhesive buccal drug delivery system. International journal of pharmaceutical sciences and research. 2013 Mar 1;4(3):916.
- Koirala S, Nepal P, Ghimire G, Basnet R, Rawat I, Dahal A, Pandey J, Parajuli-Baral K. Formulation and evaluation of mucoadhesive buccal tablets of aceclofenac. Heliyon. 2021 Mar 1;7(3).
- Squier CA, NW Johnson and RM Hopps. Human oral mucosa: development, structure and function. Oxford: Blackwell Scientific, 1976
- Gaikwad SS, Kale YK, Gondkar SB, Darekar AB. Buccal tablet as a promising mucoadhesive drug delivery. Invent Rapid: Pharm Tech. 2012 May 1;3:1-8.
- Gupta S, Das S, Singh A, Ghosh S. A Brief Review on Bucco-adhesive Drug Delivery System. Journal of Drug Delivery and Therapeutics. 2021 Aug 15;11(4-S):231-5.
- Patil AV, Mehetre GD, Akotkar AM. A review on mucoadhesive buccal drug delivery system. World Journal of Pharmacy and Pharmaceutical Sciences. 2020;9(5):237-60.
- Bhatt M, Bhatt G, Kothiyal P, Chaudhary S. A review on buccal mucosal route of drug administration. World J Pharm Res. 2016 Apr 13;5:868-90.
- Kaur N, Nirmala SL, Kumar H. A review on study of buccal patches: current status of formulation and evaluation methods. Journal of Drug Delivery & Therapeutics. 2014;4(3):69-79.
- Roychowdhury S, Gupta R, Saha S. A review on buccal mucoadhesive drug delivery systems. Indo-Global Journal of Pharmaceutical Sciences. 2011;1(3):223-33.
- Rao NR, Shravani B, Reddy MS. Overview on buccal drug delivery systems. Journal of pharmaceutical sciences and research. 2013 Apr 1;5(4):80.
- Krishnarajan D, Jithin TG, Nikhil V, Nair AM, Sherin A, Thomas S, Purushothaman M. Recent Trend And Approaches Of Buccal Drug Delivery System: A Review. Pharmacophore. 2016 Sep 1;7(5).
- Patel AR, Patel DA, Chaudhry SV. Mucoadhesive buccal drug delivery system. International Journal Of Pharmacy & Life Sciences. 2011 Jun 1;2(6).
- Qidra R.K. In-depth recent advances in buccal mucoadhesive drug delivery system. European Journal of Pharmaceutical and Medical Research, 2018; 5(3):81-103
- Shipp L, Liu F, Kerai-Varsani L, Okwuosa TC. Buccal films: A review of therapeutic opportunities, formulations & relevant evaluation approaches. Journal of Controlled Release. 2022 Dec 1;352:1071-92.
- Koirala S, Nepal P, Ghimire G, Basnet R, Rawat I, Dahal A, Pandey J, Parajuli-Baral K. Formulation and evaluation of mucoadhesive buccal tablets of aceclofenac. Heliyon. 2021 Mar 1;7(3).
- Shaikh S, Pawar J, Raykar M. A Review On Bucoadhesive Drug Delivery System.
- Pranshu T, et al. mucoadhesive drug delivery, mechanism and methods of evaluation. International journal of Pharma and bio sciences, 2011; 2(1): 458.
- Khurana SH, Madhav NS, Tangri PR. Mucoadhesive drug delivery: mechanism and methods of evaluation. Int J Pharm Biosci. 2011;2(1):458-67.
- Li KL, Castillo AL. Formulation and evaluation of a mucoadhesive buccal tablet of mefenamic acid. Brazilian Journal of Pharmaceutical Sciences. 2020 Jun 21;56:e18575.
- Roy AK, Kumar V, Basha S, Haque R, Karki R. Formulation and evaluation of mucoadhesive buccal tablets of valsartan. Int J Drug Dev Res. 2013;5(4):145-55.
- Kadam SS, Yeole D, Unjavani HK, Ganure AG, Kabra RP. Formulation and Evaluation of Mucoadhesive Buccal Tablets Containing Carvedilol. Int J Curr Pharm Res. 2014;1:67-80.
- Raju KN, Velmurugan S, Deepika B, Vinushitha S. Formulation and in-vitro evaluation of buccal tablets of metoprolol tartrate. International Journal of Pharmacy and Pharmaceutical Sciences. 2011;3(2):239-46.
- Shirsand SB, Wadageri GV, Raju SA, Kolli G, Reddy V. Design and evaluation of mucoadhesive bilayer buccal tablets of nebivolol. RGUHS J Pharm Sci. 2013;3(1):40-7.
- Deshmukh VN, Jadhav JK, Sakarkar DM. Formulation and in vitro evaluation of theophylline anhydrous bioadhesive tablets. Asian Journal of Pharmaceutics (AJP). 2009;3(1).
- Shidhaye SS, Thakkar PV, Dand NM, Kadam VJ. Buccal drug delivery of pravastatin sodium. Aaps Pharmscitech. 2010 Mar;11(1):416-24.
- Padsala KR, Desai K, Swamy SM. Formulation, Evaluation And Optimization Of Mucoadhesive Buccal Tablet Of Simvastatin. Pharma Science Monitor. 2014 Apr 2;5
- Garg A, Garg S, Kumar M, Kumar S, Shukla AK, Kaushik SP. Applications of natural polymers in mucoadhesive drug delivery: An overview. Adv. Pharm. J. 2018;3(2):38-42.


 Rushikesh Anandache*
Rushikesh Anandache*
 Priya Patil
Priya Patil







 10.5281/zenodo.14868696
10.5281/zenodo.14868696