Abstract
This review focuses on herbal drugs that can be used to treat anxiety disorders. The most common behavioural disorders are generalized anxiety disorder (GAD), obsessive-compulsive disorder (OCD), panic disorder, specific phobia, social phobia, and post-traumatic stress disorder (PTSD). Several medicinal plants have been proven to have anti-anxiety effects. These plants are passion flower (passiflora incarnata), Brahmi (Bacopa monnieri), Ashwagandha (Withania somnifera), Goti Kola or Mandookarparni (Centella asiatic), Chamomile (Matricaria recutita), Kava Kava (piper methysticum), Astragalus membranaceus. Depression is a chronic illness that can be fatal and affects people all over the world. The medications used to treat this illness have a variety of adverse effects and may interact with foods or other medications. Furthermore, only thirty percent of patients satisfactorily receive the current medications, and the remaining patients do not fully recover. Finding therapies that work well, have minimal side effects, and are less expensive appears to be essential. Reviewing double-blind clinical trials and animal studies on the antidepressant properties of medicinal herbs was the aim of this investigation. Clinical trials have demonstrated the beneficial effects of several herbs and their active compounds, including St. John's wort (Hypericum perforatum), curcumin, saffron (Crocus sativus), lavender (Lavandula angustifolia), Roseroot (Rhodiola rosea L.) and Borage (Echium amoenum) improving the symptoms of mild, moderate, or major depression. Compared to synthetic drugs, the aforementioned plants exhibit antidepressant properties and have fewer adverse effects. As such, they may be able to help patients who are depressed.Department of pharmacology, SNJB’s Shriman Sureshdada Jain College of Pharmacy, Chandwad Dist: Nashik, India
Keywords
Anxiety, Herbal Medicine, Anxiolytic, EPM-Elevated Plus Maze, Depression, Randomized clinical trials
Introduction
Anxiety is defined as feeling tense, having worried thoughts and experiencing physical changes such as higher blood pressure. Anxiety is a natural emotion that can lead to physical symptoms like shaking and sweating. Anxiety disorder can impact daily life but can get better with treatment. Anxiety, worry and fear are natural human feelings. When these feelings last a long time, they can harm mental and physical health, leading to clinical anxiety disorders. Various treatments including herbal remedies are used to address anxiety disorders. Anxiety is a common disorder in the community especially in secondary medical care. It often lasts for years causing personal distress, lower quality of life, higher disease rates, increased deaths and a heavy economic burden. In this article, various herbal remedies for anxiety disorders are discussed [85-86].
OCCURRENCE
In 2017 a study was conducted by Lancet found that nearly 197 million people in India had mental health disorders. Depression affected 45.9 million individuals, while anxiety impacted 44.9 million people (3.3% of the population). Females were more affected by anxiety than males. Anxiety was most common in Kerala followed by Manipur, west Bengal Himachal Pradesh and Andhra Pradesh. In 2017, the World Health Organization reported around 38 million people in India have anxiety disorders, making It the second most prevalent disorder after depression.
TYPES OF ANXIETY DISORDER
GENERALIZED ANXIETY DISORDER
Generalized anxiety disorder is characterized by Persistent anxiety and worry about various events or feelings that the patient considers excessive and inappropriate (DSM-IV-TR). People experience both psychological and physical symptoms that Cause substantial distress or disability. Additional Symptoms include exhaustion, agitation, disturbed sleep, difficulty focusing and tense muscles. A person diagnosed with generalized anxiety disorder (GAD) if they have excessive worry about a lot of events for longer than six months [82-84].
PANIC DISORDER
People with panic disorders can have panic attacks, which are sudden periods of intense symptoms lasting around 10 minutes and can happen with various anxiety disorders. Sweating, palpitations, trembling, chills, hot flashes, Chest pain and a feeling of choking are some of the symptoms that may be present. A diagnosis of this condition is done when an individual has recurrent, predictable panic attacks and at least four of the following symptoms appear suddenly and peak in less than 10 minutes [82-84]
OBSESSIVE-COMPULSIVE DISORDER
Repetitive ritualistic behaviors and thoughts as well as persistent and recurrent thoughts are characteristic of this kind of disease. In this disorder, there are repeated thoughts and actions known as compulsions, such as repeated handwashing, checking, ordering, repeated words, quietly preying and so forth are deliberate, purposeful and repetitive behaviours or mental acts that are typically carried out in response to an obsession [82-84].
POST-TRAUMATIC STRESS DISORDER
This disorder is caused by experiencing or witnessing stressful or terrible life experiences such as tragic accidents or severe crimes. This disorder's symptoms may include trouble sleeping or sleeplessness being overly sensitive to outside factors and forgetting what happened during a traumatic event [82-84].
Social phobia
Social anxiety disorder is also known as social phobia and it is characterized by intense fear of being rejected, negatively assessed or judged in a social or performance situations. A person suffering from social anxiety disorder may fear exhibiting visible signs of anxiety such as blushing, and stuttering. They may also worry about being recognised as unintelligent, socially inept, uninteresting or dull. people may avoid social events or performances because they feel anxious and distressed [82-84].
SPECIFIC PHOBIA
This phobia is when someone is really scared of a certain thing or situation like bugs, heights or public transportation [82-84].
Herbal medicine for Anxiety.
Plants and their derivatives are not only important Sources of nutrition in our lives but they are also increasingly being used in the treatment of certain such as psychiatric disorders such as anxiety, sleep and mood disorders. Herbs are safer, more digestible, cost effective and have fewer side effects than pharmaceutical drugs. Herbs are more effective than pharmaceutical drugs for treating many health issues.
Passion flower (passiflora incarnata).
It acts as a partial agonist of the benzodiazepine receptor, resulting in GABA-mediated anxiolysis. Animal behavioural models demonstrate a non-sedative anxiolytic effect. passionflower anxiolytic effects on rodents have been thoroughly documented [1]. In a study Comparing passion flower extract to Oxazepam in 18 outpatients with a generalised anxiety disorder (GAD), the extract was found to be effective. Additionally, Job performance impartment was lower in the group treated with passion flower extract compared to the group treated with oxazepam. This study was a randomized double-blind study [2]. Another study found that preoperative oral passiflora incarnata reduced anxiety in ambulatory surgery patients in a double-blind placebo-controlled trial [3].

Brahmi (Bacopa monniera
Several herbs have been used as nerve tonics in traditional Indian medicine. The most widely used herb is brahmi, which is well known for enhancing memory. For nearly 3000 years, Ayurvedic physicians have utilised this herb. Research on both animals and humans supported the traditional Ayurvedic use of brahmi as an anti-anxiety treatment. In Indian traditional medicine, Brahmi is used to treat various brain disorders such as anxiety and poor memory [4]. In a recent clinical study, it was found that administering brahmi syrup to 35 individuals diagnosed with anxiety neurosis led to a significant decrease in anxiety symptoms and levels [5]. The Brahmi group showed a decrease in depression and anxiety scores as well as heart rate. In contrast, the placebo group experienced an increase in these measures [6].

Ashwagandha (Withania somnifera)
Ashwagandha has been a key herb used in Ayurvedic and indigenous medicine for over 3000 years. Research including clinical and preclinical studies Supports its effectiveness in treating anxiety, Parkinson’s disease, inflammation as well as cognitive and neurological disorders [7]. In preclinical studies, the extract of the Withania somnifera (WS) showed anxiolytic effects in various tests as elevated plus-maze, feeding latency in a new environment and social interaction [8]. A study was conducted on patients with anxiety disorders using an ethanolic extract of W. Somnifera for 6 weeks. The results showed that the extract had an anxiolytic effect compared to a placebo. Additionally, the extract was well-tolerated and did not result in more side effects than the placebo. This suggests that the ethanolic extraction of w. Somnifera could be beneficial for treating anxiety. The results show that ashwagandha has strong stress-relieving adaptogenic properties, supporting its traditional medicinal use in Ayurveda [9].

GOTU KOLA OR MANDOOKARPANI (CENTELLA ASIATICA)
Centella asiatica also known as Gotukola, is known for its positive impact on neurological disorders. This herb has been used for many years in Ayurvedic and traditional Chinese medicine to help lessen symptoms of anxiety and depression. Recent studies in rats have found that prolonged pretreatment with Gotu kola can reduce locomotor activity, improve performance in the elevated plus maze, and lessen the response to sudden noises [11]. In a clinical trial, 33 people were administered a 70% hydro-ethanolic extract of Centella Asiatica for two months. Hamilton's Brief Psychiatric Rating Scale (BPR3 / was used to screen them. The study found that Mandookarparni considerably reduced anxiety-related disorders [10]. This preliminary research indicates that Centella asiatica may have anxiolytic properties in humans, although its effectiveness in treating anxiety symptoms in large populations has to be seen.

Chamomile (Matricaria recutita)
One of the most widely used single ingredients in herbal teas or tisanes is chamomile. Dried flower heads are used to make chamomile tea, which had been traditionally used for gastrointestinal tract disorders and other medical conditions. Additional applications include attention deficit hyperactivity disorder (ADHD), Sleeplessness, Varicose ulcers, dysmenorrhea, restlessness, allergic rhinitis and mastitis. Flavonoids found in chamomile have properties similar to those of benzodiazepines [12]. A study compared the effectiveness of a standardised extract of Matricaria recutita (L) to a placebo for eight weeks in patients with mild to moderate GAD (DSM-IV). The extracted treated group had significantly lower HAMA (Hamilton Anxiety Rating Scale) ratings compared to the placebo group [13].

Kava Kava (Piper methysticum)
The principal effects of kava include weak GABA binding, which increases the Synergistic effect of [3H) muscimol binding of GABA-a- receptors and GABA channel modification lipid membrane shape and sodium channel functions. Additionally, it inhibits MAO-B and downregulates a-adrenergic activity. It prevents norepinephrine from being reabsorbed in the prefrontal cortex. On the Hamilton Anxiety Rating Scale (HAMA) kava is reported to have a strong anxiolytic action when compared to a placebo. One experiment showed that kava was useful in the short term for treating Anxiety [14-17]. In multiple controlled clinical trials, kava-kava was used to treat anxiety disorders. However, the individuals in this research Varied widely with diagnoses including agoraphobia, specific phobias, social phobias and adjustment disorder with anxiety [18-21]. Research on animals has shown that kava has anti-anxiety properties [22]. Several Randomized double-blind clinical investigations in GAD patients found that Kava-kava can reduce anxiety [23].

Astragalus membranaceus.
A Korean herb called astragalus membranaceus has been therapeutically recommended to treat illnesses related to stress. In persistently stressed mice, AM dramatically improves memory and learning deficiencies. Compared to the control group, AM therapy results in a considerable increase in the amount of time spent with open arms. Additionally, in stressed rats, it increased the expression of Choline acetyltransferase (ChAT) [24]. There are no clinical studies on its anxiolytic effects. However, a clinical investigation showed that astragalus had a protective effect on the State of oxidative stress in hemodialysis [25].
Ginkgo (Ginkgo biloba)
The ginkgo tree is indigenous to Asia, and its standardised leaf extract, which contains terpenes, has been the subject of much research in Europe on its potential benefits for dementia and cognition and flavonoids as the components thought to be active. In rats, chronic oral therapy with Ginkgo biloba EGb 761 (100 mg/kg per day) resulted in dose-dependent increases in frontocortical dopamine levels after 14 days [26]. Additionally, in vivo studies have indicated that chronic administration inhibits noradrenaline reuptake [27]. A study using the EPM in mice evaluated the anxiolytic effects of ginkgo extract and its four terpenoid components (Bilobalide, ginkgolide-A, ginkgolide-B and ginkgolide-C,) [28]. The single human study found was a 4-week RCT comparing Ginkgo biloba EGb 761 extract (480 or 240 mg daily) to placebo in 107 individuals diagnosed with GAD, or adjustment disorder with anxious mood. Both active treatment groups showed significant dose-dependent reductions in HAMA compared to placebo [29].

Skullcap (Scutellaria Lateriflora)
Skullcap plants are used in traditional medicine in Europe and North America for their calming effects on the nervous system. They contain many active compounds, with flavones being the main ones responsible for reducing anxiety. One specific compound, baicalein, has been found to have similar effects to certain medications in reducing anxiety and promoting relaxation. Skullcap aerial portions have been employed in European and North American traditional medicine for relaxing, nervous system and anticonvulsant actions [30-32]. Skullcap contains diterpenoids, amino acids (GABA and glutamine), essential oil, and phenolic compounds [32-33]. Flavones, such as wogonin, baicalein, chrysin and baicalin wogonin, have been linked to anxiolytic properties [31,33]. Baicalein, a weak benzodiazepine receptor ligand, has been shown in in-vitro to have anxiolytic and sedative effects. In a study of 12 participants, 5 (42%) were rated as ‘much' or ‘very much improved' by clinicians, 6 (50%) as ‘minimally improved', and 1 (8%) as 'no change.[35]

DEPRESSION
Depression is a mental health condition that negatively impacts a person's emotions, thoughts, and behaviours in addition to their physical well-being. Depression's primary signs and symptoms include anhedonia, melancholy, slow motion and a sad attitude [36]. The interplay of genetic and environmental variables is the primary cause of depression; the precise aetiology and pathogenic processes of depression are yet unknown [37-38].
There are currently many theories being discussed regarding depression. The following is a list of the generally acknowledged potential mechanisms: First, the monoamine Hypothesis: Patients with depression have demonstrated insufficient activation of monoamine neurotransmitters in both their central and peripheral neural systems. Certain antidepressants work by inhibiting serotonin reuptake, norepinephrine reuptake, and serotonin and norepinephrine reuptake, among other mechanisms, to raise the concentration of monoamine neurotransmitters in the synaptic cleft of neurons and control mood [39-41] The HPA axis (hypothalamic, pituitary, adrenal): It was discovered that patients with depression have abnormal HPA axis stimulation. The HPA's sustained activity axis by emotional stress causes a persistent rise in brain glucocorticoid levels, which in turn causes depression [42-43]. A HPA axis imbalance is linked to a decline in glucocorticoid receptor (GR) activity, which in turn causes dysfunction of the central nervous system [44-45]. It has been demonstrated that antidepressants enhance the biological activities and expression of GRs in cellular and animal models as well as in depressed individuals [43-46].
The third theory, known as the neuroinflammation hypothesis, holds that stress can cause the release of pro-inflammatory cytokines and neurotransmitters, such as interleukin (IL)1?, IL6, and tumour necrosis factor-?, all of which are directly linked to the onset of depression [47-48]. The neurotrophic theory: Patients with depression had lower serum and postmortem brain tissue levels of brain-derived neurotrophic factor (BDNF), and antidepressant effectiveness is linked to higher BDNF levels in the central nervous system [49-50].
TYPES OF DEPRESSION
Major Depression
Major depression is a common and complicated mental condition that presents ongoing difficulties for both patients and doctors. It's a common disorder [55]. The most common kind of depression is this one. Patients of this kind go through repeated experiences throughout their lives [56]. Antidepressants are typically used in its treatment [57].
Chronic depression or dysthmia
It is described as having a protracted, depressed mood [51,54]. Compared to medicine, talk therapy is more effective in treating dysthymia. Certain studies also propose their combination [57].
Seasonal affective disorder
The cause of this kind of depression is a lack of sunlight [52,57]. SAD is a common type of depression that has a seasonal pattern, starting in the autumn and lasting through the winter. The main symptoms were melancholy and low energy. Younger women who live far from the equator and have a family history of depression, SAD, or bipolar disorder are more common. Antidepressants, vitamin D, light therapy, and counselling are commonly used in treatment [53,58].
Atypical Depression
The patient may feel heaviness in their limbs, be irritable, and have relationship issues [6]. Several studies have indicated that talk therapy is an excellent treatment for this kind of depression [54,57].
Bipolar Depression
Manic-depressed disease, another name for bipolar disorder, is characterised by periodic manic and depressive episodes in the patient [56]. Psychosocial therapies combined with antidepressant medication can improve depression's acute stabilisation and long-term maintenance. Drugs that interfere with sleep and circadian rhythm have been shown to have an impact on mood and could be useful in treatment plan selection for some patients [59].
Psychotic depression
Patients with psychotic depression experience delusions and hallucinations, which cause them to become catatonic [56]. A combination of antidepressants and antipsychotics was found to be more successful in treating this type of depression, according to many reviews that have already been published [57].
Post-partum Depression
Every year, it affects about 10–15% of moms. This kind of sadness typically strikes six months to a year after birth [60]. Postpartum depression was noted in a study, however, four years following delivery [61]. Postpartum depression can be caused by physiological, situational, or complex causes [62]. Among the triggers are a history of postpartum depression [63], as well as being a single parent [64]. Typically, a combination of talk therapy and medication is provided [57].
St. John’s wort (Hypericum Perforatum)
It is known by its scientific name Hypericum perforatum L., common Saint John's wort is a herbaceous perennial plant that is indigenous to North Africa, Asia, and Western Europe. It belongs to the Hypericaceae family. This plant is extensively distributed throughout Iran, growing in the western regions of the country, Chalus, Mazandaran, and the hillsides of the Alborz Mountains [65]. In recent times, this plant has gained substantial attention as a herbal cure for depression. Numerous studies have been conducted on its antidepressant benefits in animal models and human subjects [66]. Hyperforin and hypericin are the herb's primary active components; research has indicated that hyperforin has a higher antidepressant impact than hypericin [67]. The amount of 3-methoxy-4-hydroxyphenylglycol increased dramatically after taking the hypericin extract, according to research done on depressed women aged 55 to 65. Methoxy-4-hydroxyphenylglycol is a metabolite that is generated during the metabolism of norepinephrine and is indicative of the antidepressant response [68].

Saffron (Crocus sativus)
Saffron, whose scientific name is Crocus sativus L. and belongs to the Iridaceae family, is one of the costliest spices on the planet. Apart from its conventional use as a food additive, it possesses several therapeutic properties. Generally speaking, traditional medicine has utilised saffron, its extracts, and its tinctures as a sweat enhancer, anti-inflammatory, expectorant, sedative, carminative, stimulant, analgesic, gastric strengthener, antispasmodic, enhancer of sexual desires, and as a means of promoting early menstruation [69-70] Animal models have been used to show that the aqueous and hydroalcoholic extracts of saffron have antidepressant properties [69-70]. Forty patients with mild to moderate depression were treated with either fluoxetine (20 mg/d) or a hydroalcoholic extract of saffron (30 mg/d) in a randomised double-blind clinical trial. Saffron produced a significant improvement after six weeks of treatment, comparable to fluoxetine. Furthermore, no discernible variations were observed in the adverse effects between the two cohorts. Saffron has been associated with a higher risk of bleeding, however, this study found that saffron did not result in abnormal bleeding [71]. It is hypothesised that the antidepressant activity of saffron in the inhibition of serotonin, dopamine and norepinephrine reuptake is by two of its active constituents, safranal and crocin [69].

Roseroot (Rhodiola rosea)
Rhodiola rosea L., a member of the family Crassulaceae, grows in North America, Asia, and Europe naturally. Traditional medicine in these regions has long employed this herb to treat a variety of illnesses, such as depression and anxiety. As of right now, R. rosea is recognised as an adaptogen plant that promotes physical vitality and strengthens stress resistance [72]. Research has demonstrated that the combination of R. rosea and tricyclic antidepressants, when given to patients suffering from depression, produces a more potent antidepressant effect than either medication used alone [73]. Darbinyan et al. investigated the antidepressant effects of R. rosea in patients with mild to chronic depression in a randomised double-blind clinical trial. Ninety patients were split into two groups: one received a placebo and the other received an extract at doses of 340 and 640 mg/d. While the placebo had no discernible effects, the extract, at both doses, significantly reduced overall depression as well as emotional instability and sleeplessness. Not a single group voiced concerns regarding the adverse effects of the medication [74]. By raising norepinephrine, serotonin and dopamine levels in various brain regions, this plant's extract demonstrates antidepressant action [72,75].

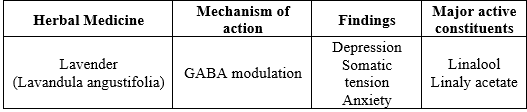
Lavender (Lavandula angustifolia)
The Lamiaceae family includes the lavender plant, scientifically known as Lavandula angustifolia, which has long been used in traditional medicine. Traditional medicine has employed lavender as an anti-spasmodic, analgesic and sedative. Lavender essential oil aromatherapy has been demonstrated in clinical trials to alleviate pain, depression, anxiety and stress [76]. 45 patients with mild to moderate depression participated in a second double-blind clinical trial. They were split into three groups: one that received imipramine tablet (100 mg) + placebo drop (60 drops), another that received lavender drop (60 drops) + placebo tablet, and a third group that received imipramine tablet (100 mg) + lavender drop (60 drops). After four weeks, the antidepressant effect of lavender by itself was less than that of imipramine. Lavender and imipramine together had much stronger antidepressant effects than imipramine by itself. While the lavender group complained of headaches, the imipramine group complained of anticholinergic side effects like urinary retention and dry mouth [77].
Borage (Echium amoenum)
Iranian echium, scientifically known as Echium amoenum, is a member of the Boraginaceae family and is only found in Iran's Alborz Mountain Range. This plant is traditionally used in Iran to improve mood and treat anxiety [78]. 35 patients with mild to moderate depression received either a placebo or E. amoenum (375 mg/d) for six weeks as part of a double-blind clinical trial. After four weeks, the group receiving E. amoenum treatment had a significant decrease in depression. There was no discernible difference in the side effects of E. amoenum and the placebo [78].

Curcumin
Turmeric (Curcuma longa Linn) naturally contains a chemical compound called curcumin. Curcumin has demonstrated notable antidepressant properties in numerous animal models of depression. However, because of its poor absorption through the digestive system, its efficacy in clinical trials is lower [80]. Lopresti et al. investigated the impact of daily curcumin intake on patients with major depressive disorder in a double-blind clinical trial. A random assignment was made to place 56 patients in the placebo or curcumin (500 mg twice daily) groups. Following four weeks of therapy, there was no discernible difference in the two groups' rates of remission of depressive symptoms. However, after eight weeks, the curcumin group's depression symptoms considerably improved. Additionally, the subgroup of atypical major depression showed improved effects from curcumin [81].
60 patients with major depression were split into three groups and given fluoxetine (20 mg), curcumin (1000 mg), and both in a double-blind clinical trial. When compared to fluoxetine (64.7%) and curcumin (62.4%) alone, the combination of fluoxetine and curcumin led to a greater reduction of symptoms (77.8%) after 6 weeks of treatment. Compared to fluoxetine, curcumin produced fewer side effects and was well tolerated by the patients in this study [79].
Research indicates that curcumin alleviates depression symptoms by affecting the biological mechanisms that underlie depression, such as the nitrative and oxidative pathways, monoaminergic activity, inflammatory process, and HPA axis activity. In mice subjected to depression, curcumin also promotes neurogenesis in the hippocampus and frontal cortex [80].
CONCLUSION
Herbs are a beneficial alternative to pharmaceutical drugs for treating various health conditions. When herbs are combined with a raw vegan diet and regular exercise, they can improve overall health more effectively than pharmaceutical drugs. Some herbs such as kava-kava and gingko have shown promising results in clinical studies, demonstrating significant effectiveness when compared to benzodiazepines, buspirone, and antidepressants. However, despite the increasing evidence supporting the use of herbs in treating neuropsychiatric disorders, there are challenges in translating these findings into effective patient treatment. One major obstacle is the limited understanding of the chemical composition of herbal products, as well as the lack of standardized preparations and a scarcity of well-controlled research studies. Initial findings indicate that herbal medicines could be beneficial in treating anxiety disorders and should be further investigated. However, it is important to note that many of these remedies have not been approved for clinical use and herbal treatments should not be considered as a replacement for traditional clinical therapies. Additionally, certain herbal remedies may have adverse interactions with other medications, possibly resulting in serious side effects or even death. Therefore, it is recommended to use herbal medications under the close supervision of qualified Ayurvedic physicians with regular check-ins. In our review study, we examined the anxiolytic effects of various plant extracts on mice and rats at different doses. The results showed significant levels of anxiolytic activity using the Elevated Plus Maze (EPM) and other parameters. However, the effectiveness of using particular medicinal plants to treat specific types of anxiety disorders still requires further evidence. When medicinal herbs are used to treat patients with major depression as well as mild to moderate depression, certain medicinal herbs have demonstrated antidepressant effects comparable to those of conventional antidepressants. Patients who use medicinal plants report no significant adverse effects, and those side effects are not substantially different from those of placebos. Nevertheless, more research with a bigger sample size is required to validate their toxicity in various people, side effects, and antidepressant properties.
REFERENCES
- Dhawan, K., Kumar, S. and Sharma, A. (2001). Anti- anxiety studies on extracts of Passiflora incarnata Linneaus. J. Ethnopharmacol., 78: 165–170
- Akhondzadeh S, Naghavi HR, Vazirian M, Shayeganpour A, Rashidi H & Khani M. (2001). Passionflower in the treatment of generalized anxiety: a pilot double-blind randomized controlled trial with oxazepam. Journal of Clinical Pharmacy & Therapeutics 26: 363-367
- Movafegh A, Alizadeh R, Hajimohamadi F, Esfehani F & Nejatfar M. (2008). Preoperative oral Passiflora incarnata reduces anxiety in ambulatory surgery patients: a double-blind, placebo-controlled study. Anesthesia & Analgesia 106: 1728-1732
- Singh HK & Dhawan BN. (1997). Neuropsychopharmacological effects of the Ayurvedic nootropic Bacopa Monniera Linn. (Brahmi). Indian Journal of Pharmacology 29: 359–365
- Asthana OP, Srivastava JS, Ghatak A, Gaur SPS & Dhawan BN. (1996). Safety and tolerability of bacosides A and B in healthy human volunteers. Indian Journal of Pharmacology 28: 37
- Calabrese C, Gregory WL, Leo M, Kraemer D, Bone K & Oken B. (2008). Effects of a standardized Bacopa monnieri extract on cognitive performance, anxiety, and depression in the elderly: a randomized, double-blind, placebo-controlled trial. The Journal of Alternative & Complementary Medicine 14: 707-713
- Mishra LC, Singh BB & Dagenais S. (2000). Scientific basis for the therapeutic use of Withania somnifera (ashwagandha): a review. Alternative Medicine Review 5: 334-346
- Bhattacharya SK, Bhattacharya A, Sairam K & Ghosal S. (2000). Anxiolytic-antidepressant activity of Withania somnifera glycowithanolides: an experimental study. Phytomedicine 7: 463-469
- Bhattacharya SK & Muruganandam AV. (2003). Adaptogenic activity of Withania somnifera: an experimental study using a rat model of chronic stress. Pharmacology Biochemistry & Behavior 75: 547-555
- Jana U, Sur TK, Maity LN, Debnath PK & Bhattacharyya D. (2010). A clinical study on the management of generalized anxiety disorder with Centella asiatica. Nepal Medical College Journal 12: 8-11
- Chen SW, Wang WJ, Li WJ, Wang R, Li YL, Huang YN & Liang X. (2006). Anxiolytic-like effect of asiaticoside in mice. Pharmacology Biochemistry & Behavior 85: 339-344
- Avallone R, Zanoli P, Puia G, Kleinschnitz M, Schreier P & Baraldi M. (2000). Pharmacological profile of apigenin, a flavonoid isolated from Matricaria chamomilla. Biochemical Pharmacology 59: 1387-1394
- Amsterdam JD, Li Y, Soeller I, Rockwell K, Mao JJ & Shults J. (2009). A randomized, double-blind, placebo-controlled trial of oral Matricaria recutita (chamomile) extract therapy for generalized anxiety disorder. Journal of Clinical Psychopharmacology 29: 378-382
- Boonen, G. and Haberlein, H. (1998). Influence of genuine kavapyrone enantiomers on the GABA-A binding site. Planta Med., 64: 504–506.
- Davies, L.P., Drew, C.A., Duffield, P., Johnston, G.A. and Jamieson, D.D. (1992). Kava pyrones and resin: Studies on GABAA, GABAB and benzodiazepine binding sites in rodent brain. Pharmacol. Toxicol., 71: 120–126.
- Jussofie, A., Schmiz, A. and Hiemke, C. (1994). Kavapyrone enriched extract from Piper methysticum as modulator of the GABA binding site in different regions of rat brain. Psychopharmacol. (Berl), 116: 469–474.
- Magura, E.I., Kopanitsa, M.V., Gleitz, J., Peters, T. and Krishtal, O.A. (1997). Kava extract ingredients, (+)-methysticin and (+/”)- kavain inhibit voltage-operated Na(+)- channels in rat CA1 hippocampal neurons. Neurosci., 81: 345–351.
- Volz HP & Kieser M. (1997). Kava-kava extract WS 1490 versus placebo in anxiety disorders--a randomized placebo-controlled 25-week outpatient trial. Pharmacopsychiatry 30: 1-5
- Malsch U & Kieser M. (2001). Efficacy of kava-kava in the treatment of non-psychotic anxiety, following pretreatment with benzodiazepines. Psychopharmacology (Berl)157: 277-283
- Gastpar M & Klimm HD. (2003). Treatment of anxiety, tension and restlessness states with Kava special extract WS 1490 in general practice: a randomized placebo-controlled double-blind multicenter trial. Phytomedicine 10: 631-639
- Lehrl S. (2004). Clinical efficacy of kava extract WS 1490 in sleep disturbances associated with anxiety disorders. Results of a multicenter, randomized, placebo-controlled, double-blind clinical trial. The Journal of Affective Disorders 78: 101-110
- Bruner NR & Anderson KG. (2009). Discriminative-stimulus and time-course effects of kava-kava (Piper methysticum) in rats. Pharmacology Biochemistry & Behavior 92: 297-303
- Watkins LL, Connor KM & Davidson JR. (2001). Effect of kava extract on vagal cardiac control in generalized anxiety disorder: preliminary findings. Journal of Psychopharmacology 15: 283-286
- Park HJ, Kim HY, Yoon KH, Kim KS & Shim I. (2009). The Effects of Astragalus Membranaceus on Repeated Restraint Stress-induced Biochemical and Behavioral Responses. Korean Journal of Physiology and Pharmacology 13: 315-319
- Qu XL, Dai Q, Qi YH, Tang YH, Xu DH, Wu ZH & Wang XX. (2008). Effects of Astragalous Injection on oxidative stress status in maintenance hemodialysis patients. Journal of Chinese Integrative Medicine 6: 468-472
- Yoshitake T, Yoshitake S, Kehr J. The Ginkgo biloba extract EGb 761(R) and its main constituent flavonoids and ginkgolides increase extracellular dopamine levels in the rat prefrontal cortex. Br J Pharmacol. 2010;159(3):659–68.
- Fehske CJ, Leuner K, Muller WE. Ginkgo biloba extract (EGb761) influences monoaminergic neurotransmission via inhibition of NE uptake, but not MAO activity after chronic treatment. Pharmacol Res. 2009;60(1):68–73.
- Kuribara H, Weintraub ST, Yoshihama T, et al. An anxiolytic-like effect of Ginkgo biloba extract and its constituent, ginkgolide-A, in mice. J Nat Prod. 2003;66(10):1333–7.
- Woelk H, Arnoldt KH, Kieser M, et al. Ginkgo biloba special extract EGb 761((R)) in generalized anxiety disorder and adjustment disorder with anxious mood: a randomized, double-blind, placebo-controlled trial. J Psychiatr Res. 2007;41(6):472–80.
- Wolfson P, Hoffmann D. An investigation into the efficacy ofScutellaria lateriflora in healthy volunteers. Altern Ther Health Med. 2003;9(2):74.
- Li J, Wang Y-H, Smillie TJ, et al. Identification of phenolic compounds from Scutellaria lateriflora by liquid chromatography with ultraviolet photodiode array and electrospray ionization tandem mass spectrometry. J Pharml Biomed Anal. 2012;63:120–7.
- Zhang Z, Lian X-y, Li S, et al. Characterization of chemical ingredients and anticonvulsant activity of American skullcap (Scutellaria lateriflora). Phytomedicine. 2009;16(5):485–93.
- Kuroda M, Iwabuchi K, Mimaki Y. Chemical constituents of the aerial parts of Scutellaria lateriflora and their alpha-glucosidase inhibitory activities. Nat Prod Commun. 2012;7(4):471.
- Yaghmai MS. Volatile constituents of Scutellaria lateriflora L. Flavour Fragr J. 1988;3(1):27–31.
- Kobak KA, Taylor LV, Bystritsky A, et al. St John’s wort versus placebo in obsessive-compulsive disorder: results from a double-blind study. Int Clin Psychopharmacol. 2005;20(6):299–304.
- Association AP. Diagnostic and Statistical Manual of Mental Disorders. American Psychiatric Association; 2013.
- Belmaker R, Agam G. Major depressive disorder. N Engl J Med.2008;358(1):55?68.
- Krishnan V, Nestler EJ. The molecular neurobiology of depression. Nature. 2008;455(7215):894?902.
- Hirschfeld RM. History and evolution of the monoamine hypothesis of depression. J Clin Psychiatry. 2000;61(suppl_6):4?6.
- Hjorth S, Bengtsson H, Kullberg A, et al. Serotonin auto receptor function and antidepressant drug action. J Psychopharmacol.2000;14(2):177?185.
- Marchand WR, Dilda V, Jensen C, et al. Neurobiology of mood disorders. Hosp Physician. 2005;41(9):17?26.
- Tsigos C, Chrousos GP. Hypothalamic–pituitary–adrenal axis, neuroendocrine factors and stress. J Psychosom Res. 2002;53(4):865?871.
- Pariante CM, Lightman SL. The HPA axis in major depression: classical theories and new developments. Trends Neurosci.2008;31(9):464?468.
- Ozbolt LB, Nemeroff CB. HPA axis modulation in the treatment of mood disorders. In: Schoepf D ed., Psychiatric Disorders-NewFrontiers in Affective Disorders. IntechOpen; 2013:21-40.
- Anacker C, Zunszain PA, Carvalho LA, et al. The glucocorticoid receptor: pivot of depression and of antidepressant treatment? Psychoneuroendocrinology. 2011;36(3):415?425.
- Xu J, Wang R, Liu Y, et al. Short-and long-term alterations of FKBP5-GR and specific microRNAs in the prefrontal cortex and hippocampus of male rats induced by adolescent stress contribute to depression susceptibility. Psychoneuroendocrinology. 2019;101:204?215.
- Makhija K, Karunakaran S. The role of inflammatory cytokines on the aetiopathogenesis of depression. Aust N Z J Psychiatry. 2013;47(9):828?839.
- Woelfer M, Kasties V, Kahlfuss S, et al. The role of depressive subtypes within the neuroinflammation hypothesis of major depressive disorder. Neuroscience. 2019; 403:93?110.
- Duman RS. Role of neurotrophic factors in the etiology and treatment of mood disorders. NeuroMol Med. 2004;5(1):11?25.
- Park H, Poo M-M. Neurotrophin regulation of neural circuit development and function. Nat Rev Neurosci. 2013;14(1):7?23
- Parekh, R. (2017). American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders (DSM-5), Fifth edition. 2013.
- Legg, T. J. (2016). Depression Overview, health-line newsletter.
- Villanueva, R. (2013). Neurobiology of major depressive disorder. Neural plasticity, 2013.
- Halverson, J., Beevers, C., & Kamhoiz, B. (2016). Diagnostic and Statistical Manual of Mental Disorders (DSM-5) Clinical Practice Review for Major Depressive Disorder. Anxiety and Depression association of America.
- Culpepper, L., Muskin, P. R., & Stahl, S. M. (2015). Major depressive disorder: understanding the significance of residual symptoms and balancing efficacy with tolerability. The American journal of medicine, 128(9), S1-S15.
- Cagliostro, D. (2018). Depression; Persistent Sadness & Loss of Interest in Life.
- Illiades, C., & Keegan K. (2018). 9 Different Types of Depression, Everyday Health.
- [58] Melrose, S. (2015). Seasonal Affective Disorder: An Overview of Assessment and Treatment Approaches; Depress Res Treat. 178564.
- [59] Geddes, J. R., & Miklowitz, D. J. (2013). Treatment of bipolar disorder. The lancet, 381(9878), 1672-1682.
- nokye, R., Acheampong, E., Budu-Ainooson, A., Obeng, E. I., & Akwasi, A. G. (2018). Prevalence of postpartum depression and interventions utilized for its management. Annals of general psychiatry, 17(1), 18.
- Mauthner, N. S. (1998). Re-assessing the importance and role of the marital relationship in postnatal depression: Methodological and theoretical implications. Journal of Reproductive and Infant Psychology, 16(2-3), 157-175.
- Fishel, A. H. (2004). Mental health disorders and substance abuse. Maternity & women’s health care, 960-82.
- Leopold, K. A., & Zoschnick, L. B. (1997). Women's Primary Health Grand Rounds at the University of Michigan: Postpartum Depression. Female Patient-Total Health Care For Women, 22, 12-30.
- Andrews-Fike, C. (1999). A review of postpartum depression. Primary care companion to the Journal of clinical psychiatry, 1(1), 9.
- Harrer G, Schmidt U, Kuhn U, Biller A. Comparison of equivalence between the St. John’s wort extract LoHyp-57and fluoxetine. Arzneimittelforschung. 1999;49(4):289-96
- Rabiei Z, Rabie S. A review on antidepressant effect of medicinal plants. Bangladesh J Pharmacol. 2017;12(1):1-11.
- Naghdi Badi H, Amin G, Makizadeh Tafti M, Ziai SA. St. John’s wort (Hypericum perforatum L.): a review. Journal ofMedicinal Plants. 2005;4(16):1-14. [Persian].
- Muldner VH, Zoller M. Antidepressive Wirkung einesauf den Wirkstoffkomplex hypericin standardisierten hypericum-extraktes. Arzneim-Forsch. 1984;34:918-20
- Hosseinzadeh H, Karimi G, Niapoor M. Antidepressanteffect of Crocus sativus L. stigma extracts and theirconstituents, crocin and safranal, in mice. Acta Hortic. 2004;650:435-45.
- Karimi G, Hosseinzadeh H, Khaleghpanah P. Study ofantidepressant effect of aqueous and ethanolic extract of Crocus sativus in mice. Iranian Journal of Basic Medical Sciences. 2001;4(3):153-86. [Persian].
- NoorbalaAA, AkhondzadehS, Tahmacebi-PourN, JamshidiAH. Hydro-alcoholic extract of Crocus sativus L. versus fluoxetine in the treatment of mild to moderate depression: double-blind, randomized pilot trial. J Ethnopharmacol. 2005;97(2):281-4
- van Diermen D, Marston A, Bravo J, Reist M, Carrupt PA, HostettmannK. Monoamine oxidase inhibition by Rhodiola rosea L. roots. J Ethnopharmacol. 2009;122(2):397-401
- Brichenko VS, Kupriyanova IE, Skorokhodova TF. The use of herbal adaptogens together with tricyclic antidepressants in patients with psychogenic depressions. ModernProblems of Pharmacology and Search for New Medicines. 1986;2:58-60.
- Darbinyan V, Aslanyan G, Amroyan E, Gabrielyan E, Malmström C, Panossian A. Clinical trial of Rhodiola rosea L. extract SHR-5 in the treatment of mild to moderatedepression. Nord J Psychiatry. 2007;61(5):343-8.
- Kelly GS. Rhodiola rosea: a possible plant adaptogen. Altern Med Rev. 2001;6(3):293-302.
- Louis M, Kowalski SD. Use of aromatherapy with hospicepatients to decrease pain, anxiety, and depression and to promote an increased sense ofwell-being. Am J Hosp Palliat Care. 2002;19(6):381-6.
- Akhondzadeh S, Kashani L, FotouhiA, Jarvandi S, Mobaseri M, Moin M, et al. Comparison of Lavandula angustifolia Mill. tincture and imipramine in the treatment of mild to moderate depression: a double-blind, randomizedtrial. Prog Neuropsychopharmacol Biol Psychiatry. 2003;27(1):123-7.
- Sayyah M, Boostani H, Pakseresht S, Malaieri A. Efficacy of aqueous extract of Echium amoenum in treatment of obsessive-compulsive disorder. Prog Neuropsychopharmacol Biol Psychiatry. 2009;33(8):1513-6.
- SanmukhaniJ, Satodia V, Trivedi J, Patel T, Tiwari D, Panchal B, et al. Efficacy and safety of curcumin in major depressive disorder: a randomized controlled trial. Phytother Res. 2014;28(4):579-85.
- Kulkarni S, Dhir A, Akula KK. Potentials of curcumin as an antidepressant. Scientific World Journal. 2009; 9:1233-41.
- Lopresti AL, Maes M, Maker GL, Hood SD, Drummond PD. Curcumin for the treatment of major depression: a randomised, double-blind, placebo-controlled study. JAffect Disord. 2014; 167:368-75.
- Narrow WE, Rae DS, Robins LN and Regier DA. Revised prevalence estimates of mental disorders in the United States using a clinical significance criterion to reconcile 2 surveys’ estimates. Archives of General Psychiatry, 2002; 59(2): 115-123.
- Goodwin RD. Asthma and anxiety disorders. Advances in psychosomatic Medicine, 2003;24: 51-71.
- Ballenger JC, Davidson JR, Lecrubier Y et al. Consensus statement on social anxiety disorder from the international consensus group on depression and anxiety. The Journal of Clinical Psychiatry., 1998; 59(17): 54-60
- Gross C & Hen R. (2004). The developmental origins of anxiety. Nature Reviews Neuroscience 5: 545-552
- Weinberger DR. (2001). Anxiety at the frontier of molecular medicine. New England Journal of Medicine 344: 1247-1249


 Aman Upaganlawar*
Aman Upaganlawar*













 10.5281/zenodo.11353415
10.5281/zenodo.11353415