Abstract
The objective of the development of this formulation was to develop a suitable drug delivery system that would bypass the hepatic metabolism and increase the bioavailability of Levodopa. Even though Levodopa exhibits low oral bioavailability it is the widely used drug for Parkinson’s disease treatment. By preparing the sublingual tablets that dissolve and disintegrate in the oral cavity, this method may overcome the limitations of low oral bioavailability and poor brain uptake of levodopa. Levodopa Sublingual tablets were prepared by direct compression method using different types of super disintegrants, Dissolution enhancers, and permeation studies ensuring the effective development of Levodopa tablets. Additionally, a new dissolution method was tailored for sublingual tablets to suit the requirements of the formulation to be studied. This study holds promise for potential advancement in Sublingual Levodopa formulations for effective Parkinson’s Disease management. The promising outcomes suggest a potential breakthrough in Parkinson’s management. A conclusion can be drawn that using an appropriate disintegrant, dissolution enhancer along with suitable media increased the bioavailability of Levodopa and prevented it from extensive first-pass metabolism.
Keywords
Levodopa, Sublingual delivery, Simulated Salivary fluid, Super Disintegrants, Bioavailability.
Introduction
Parkinson’s Disease is a neurodegenerative condition characterized by the degeneration of neurons in the region of substantia nigra pars compacta, leading to deficiency of dopamine. This deficiency results in motor disturbances such as dyskinesia, bradykinesia, resting tremors, and imbalance, impacting the quality of life. Levodopa being the most common choice of treatment for PD also induces Levodopa-induced parkinsonism due to its long-term use1. LD remains the best choice for Parkinson’s Disease due to its ability to replenish dopamine levels in the brain2. Levodopa faces challenges in its efficiency due to low oral bioavailability (30%)3, low brain uptake, and peripheral decarboxylation of enzymes by dopa decarboxylase in the gut, kidney, and liver reduces the amount of levodopa available for brain uptake4. The combination of Levodopa with a Dopa Decarboxylase Inhibitor (DCI), particularly Carbidopa or Benserazide, has been considered the gold standard in treating Parkinson's disease (PD). These DCIs work by inhibiting the peripheral conversion of levodopa into dopamine before it crosses the blood-brain barrier5. During the perioperative period, managing medications for conditions like Parkinson's disease becomes crucial. Sublingual administration of medications like levodopa can be particularly beneficial in such cases. The direct absorption of the drug into the bloodstream bypasses issues related to gastric emptying and potential interactions with anesthesia or other medications given orally. This method ensures a more reliable and rapid onset of action, crucial for patients who rely on continuous medication to manage their symptoms, especially in situations where oral intake might be compromised. It helps maintain therapeutic levels of the drug and prevents the worsening of Parkinson's symptoms during the perioperative period6. Sublingual administration provides a direct route for drug absorption into the bloodstream, bypassing the digestive system and the first-pass metabolism in the liver7. This can result in a faster onset of action, increased bioavailability, and potentially reduced side effects compared to oral intake, where the drug may be degraded by stomach acid or enzymes before reaching systemic circulation8. Sublingual administration is particularly advantageous for drugs that have poor oral bioavailability or are sensitive to degradation in the gastrointestinal tract7. Levodopa is currently available as a conventional tablet, orally disintegrating tablets9, controlled release tablets10, Extended release tablets11, etc. The plasma elimination half-life of levodopa in the presence of a decarboxylase inhibitor is ? 90 min. Therefore, Levodopa is always in combination with Carbidopa to prevent it from peripheral decarboxylation5. In this study, we have developed levodopa sublingual tablets using different super-disintegrants like sodium starch glycolate (SSG), cross-carmellose sodium, Cross-povidone to obtained good disintegration of tablets. For sublingual tablets, conventional dissolution methods might not be directly applicable due to the small volume of media present in the sublingual region and the properties of sublingual mucosa. To overcome this challenge new method was tailored to mimic sublingual conditions to study the disintegration, dissolution and permeation of sublingual tablets, considering the lack of specific guidelines in the official pharmacopoeias such as IP, BP, USP.
MATERIALS AND METHODS
Materials:
Levodopa was kindly purchased from RP Chemicals, Mumbai, India. Ludiflash (co-processed excipient) and Soluplus was provided as gift samples by BASF, India. Avicel PH 102 was a gift sample provided by Signet Excipients pvt ltd., India. Sodium starch glycolate (SSG), cross-carmellose sodium, Cross-povidone was purchased from Molychem, India.
Preformulation Study:
Preformulation studies are an important stage in pharmaceutical development that aims to study the physical and chemical characteristics of the therapeutic ingredient as well as possible excipients. These studies help in the selection of appropriate excipients for the formulation of a stable and effective dosage form. Preformulation study of following parameters were performed:
Melting point
To ensure the purity of the compound, the melting point of Levodopa was determined using the capillary tube method. A small amount of drug was placed in the capillary tube, which was then attached to the thermometer and partially immersed in Thiele's tube containing liquid paraffin. Thiele's tube was heated until the compound in the capillary melted. The temperature at which the drug melted completely.
Solubility study of drug in different Solvents
Solubility study was carried out as per the I.P.2018. In this maximum amount of solvent required to dissolve the solute was determined. The solubility study in SSF and Phosphate buffer pH 6.8 was also done to check how much drug solubilize in the given solvents. 4.3.3 Spectral characterization by UV spectroscopy.
Determination of maximum wavelength
The UV system consisted of a Shimadzu UV-visible spectrophotometer with 10 mm quartz cuvettes used for determination of ? max and construction of calibration curve. Levodopa was weighed accurately to get 50?g/ml solution (pH 6.8 Phosphate buffer). Further diluted with phosphate buffer solution (pH 6.8) to get 10?g/ml. The U.V spectrum was recorded in the range 200-400 nm using the same buffer as blank. The maximum wavelength of the drug was determined. Solubility study, Spectral characterization.
Drug- excipients compatibility
Compatibility studies were done by FTIR and DSC.
Method Of Preparation Of Levodopa Sublingual Tablets:
Direct compression method was used to prepare tablets. The Levodopa and Soluplus were mixed using mortar and pestle blended to get a uniform mixture and kept a side Super disintegrants such as Croscarmellose sodium, Sodium Starch Glycolate, Cross Povidone in varying ratio was taken. All the materials were passed through #60 mesh prior to mixing for uniformity in particle size. The additional ingredients were then weighed and blended in a geometrical order, and the tablets were compressed using a 7mm punch to get 150 mg total weight using ten station Rimek Minipress 1 Multistage tablet punching machine. Composition of different formulation were prepared by direct compression method which are given below.
The Preparation and Composition Of Simulated Salivary Fluid (SSF):
The ingredients required for the preparation of SSF were added individually in each test tube containing 5ml of distilled water. All the test tubes stirred until the added ingredients completely dissolves in the distilled water. Then the contents were added to beaker in the given order. The solution was filtered using Whatman filter paper if any particles are observed. And the obtained solution was used for the given studies
Table 1. Composition of saliva

Evaluation of precompression powder blend
Powder blend characterization
The flow properties of the blend, including the Angle of Repose, Hausner’s ratio, and Carr’s Index, were within the expected range for tablet formulation. When dealing with tablet compression, the flow characteristics of both the active pharmaceutical ingredient (API) and the excipients plays a pivotal role in determining the appropriate compression technique12.
Evaluation of Post compression Parameters
Hardness
The hardness of a tablet is defined as the force required to break the tablet across its diameter. The tablet's hardness determines its resistance to chipping, abrasion, or fracture during storage and handling before use. The hardness of the tablet of each formulation was determined using the Monsanto Hardness tester13.
Tablet thickness & diameter
Tablet thickness is a significant factor in determining appearance. Tablet thickness and diameter can be determined with a simple approach. 5 tablets were taken and their thickness and diameter were measured using digital Vernier calliper13.
Weight variation
After compression, 20 tablets from each batch were selected and the average weight was calculated. The obtained results should not vary from the limit specified in the Indian Pharmacopoeia (IP) of 199613.
Friability test
Tablet friability was tested using a Roche friabilator. Twenty tablets were randomly selected from each formulation and initial weight of 20 tablets are calculated and then transferred into friabilator. The friabilator rotates at 25 rpm every four minutes (100 revolutions). The tablets were de dusted and weighed again (final weight). The percentage friability was calculated by the following equation,
F = ???????????????????????????? ????????????????????????????????????????????? ???????????????????????? / ???????????????????? ???????????????????????? × 100
Where, F= Percent Friability14
Wetting time (WT)
A piece of tissue paper (12 cm X 10.75 cm) folded twice was placed in a small petri dish (ID = 6.5 cm) containing 6 ml of SSF pH 6.8. A tablet was placed on the paper, and the time to complete wetting was measured. Each batch underwent three trials, and the standard deviation was determined. The time required for the water to diffuse from the wetted absorbent paper throughout the entire tablet was then recorded using a stopwatch14.
Water absorption ratio
A piece of tissue paper folded twice was placed in a small Petri dish Containing 6 ml of SSF. A tablet was placed on the tissue paper and allowed to completely wet. The wetted tablet was then weighted. The water absorption ratio, R, was found using the equation below,
R = ????????????????? / ???????? × 100
Where, R= Water absorption ratio
wa = Weight of tablet after water absorption
wb = Weight of tablet before water absorption15.
Drug content
For the determination of drug content 25 mg levodopa-containing samples were dispersed in 250 ml SSF pH 6.8. After 2 hours of stirring with a magnetic stirrer, the samples were vortexed, filtered, and the amount of levodopa was measured using a spectrophotometer (UV/VIS Spectrophotometer) at 280 nm. The content was calculated using the formula:
Drug content = Drug content experimental / Drug content theoretical × 100
Where, the “Drug content theoretical” is 25 mg levodopa, and the “Drug content experimental” was the spectrophotometrically measured effective amount of levodopa in the samples16.
Disintegration test
A relatively simple approach with stringent requirements was created. examined and recorded using a stopwatch. The visual inspection was enhanced by gently rotating the test tube at a 450 angle, without agitation, to distribute any tablet Each tablet was put into a 10-ml glass test tube (1.5 cm diameter) containing 2ml SSF, and the time it took for the tablet to completely dissolve was visually particles that might mask any remaining no disintegrated portion of the tablets. In the USP disintegration test for sublingual tablets, the disintegration apparatus for oral tablets is used without the covering plastic disks, and 2 minutes was specified as the acceptable limit for tablet disintegration17.
In-vitro dissolution studies
Tablets from each batch were evaluated for drug dissolution using 50ml beaker containing 30ml SSF pH 6.8 at 37 ± 0.5 0C, 100rpm on magnetic stirrer. 2ml Samples were withdrawn and filtered through Whatman filter paper at specified time intervals (1, 5, 10, 15, 20, 25, and 30 minutes) and make up the volume up to 10ml. Replaced the aliquot by 2ml fresh medium. Samples were analyzed using UV visible Spectrophotometer at 280nm18.
In vitro permeation
In vitro permeation investigations were performed using the Franz diffusion cell (Singh Scientific Pvt. Ltd., India) and using Dialysis membrane M110 as a permeability barrier. Dialysis membrane was soaked for 24 hours in pH 7.4. In donor compartment, 2 mL of SSF (pH 6.8) at 37 ± 0.5 0C was taken as dissolution media which simulate the physiological conditions like pH and volume of saliva. In receptor compartment, phosphate buffer pH 7.4 at 37 ± 0.5 0C was taken as media which simulate the blood pH 7.4. A tablet from each batch was placed in donor compartment, which disintegrates in available media to release Levodopa. The released drug crossed the membrane barrier and entered the receptor compartment. Samples were withdrawn at specified time intervals (2, 5, 10, 20, 30, 45, 60, 90, 105, and 120 minutes) and analysed by UV-visible spectrophotometer at 280 nm19.
Ex-vivo permeation
For determination of the ex vivo permeability of the drug, the buccal mucosa of the goat had been selected. Permeation studies were performed by using Franz diffusion cell and using Sublingual mucosa obtained from Slaughterhouse as a permeability barrier. The buccal mucosa was excised from the buccal cavity and cleaned properly. In donor compartment, 2 mL of SSF (pH 6.8) at 37 ± 0.5 0C was taken as dissolution media which simulate the physiological conditions like pH and volume of saliva. In receptor compartment phosphate buffer pH 7.4 at 37 ± 0.5 0C was taken as media which simulate the blood pH. A tablet from each batch was placed in donor compartment, which disintegrates in available media to release Levodopa. Released drug was passed through the barrier and entered the receptor compartment. Samples were withdrawn at specified time intervals (2, 5, 10, 20, 30, 45, 60, 90, and 120 minutes) and analysed by UV-visible spectrophotometer at 280 nm20.
Stability Study
The purpose of stability testing was to provide evidence on how the quality of a drug substance or drug product varies with time under the influence of a variety of environmental factors such as temperature, humidity and light, enabling recommended storage conditions, re-test periods and shelf lives. Generally, the observation of the rate at which the product degrades under normal room temperature requires a long time. To avoid this undesirable delay, the principles of accelerated stability studies are adopted. ICH specifies the length of study and storage conditions. All the batches were subjected to stability studies for 3 months at room temperature and accelerated stability conditions (40±2°C and 75%±5% RH) and kept in a humidity chamber. The samples were evaluated for various tablet parameters such as Disintegration Time, Hardness, In vitro % cumulative drug release, Friability20.
RESULTS
Preformulation study:
Melting point
Melting point of the Levodopa was found to be 296-298 0C.
Solubility
As there is no Levodopa sublingual marketed formulation available in the market the solubility was performed in Simulated Saliva and Phosphate buffer pH 6.8. It was found that 1mg of levodopa is soluble in 10ml of SSF and Phosphate buffer pH 6.8.
Spectral characterization by UV method
UV Spectrum of the drug in SSF pH 6.8 and phosphate buffer pH 6.8.
The spectrum of levodopa in SSF pH 6.8 and phosphate buffer pH 6.8 is represented in Figure. After Studying the UV spectra of the drug, it showed maximum absorbance at 280 nm.

Figure 1: UV spectra of the drug in phosphate buffer pH 6.8

Figure 2: UV spectra of the drug in SSF pH 6.8.
Calibration curve of drug in SSF pH 6.8
The curve suggested that levodopa obeying Beer’s law was in the linearity range of 10-80?g/ml and the obtained experimental data (Y = 0.0113x + 0.0184) with a correlation coefficient of 0.9901) were used to estimate the levodopa in in-vitro dissolution study.

Figure 3: Levodopa Calibration curve in SSF pH 6.8 at 280nm using a UV spectrophotometer.
Calibration curve of the drug in Phosphate buffer 7.4
The curve suggested that levodopa obeying Beer’s law was in the linearity range of 10-50?g/ml and the obtained experimental data (Y = 0.0077x + 0.0032) with a correlation coefficient of 0. 9938) were used to estimate the levodopa in In-vitro and Ex-vivo permeation study.
Figure 4: Levodopa Calibration curve in Phosphate buffer pH7.4 at 280nm using a UV spectrophotometer.
Drug- excipients compatibility studies
Fourier Transform infrared (FTIR) spectroscopy studies
The result of FTIR showed that there were no major changes in peak value obtained for pure drug and physical mixture of drug and excipients which indicates that there was no interaction between drug and excipients used. The FTIR spectrum of pure L-dopa showed characteristic bands at the below-mentioned wavelengths.

Figure 5: FTIR Spectra of Levodopa
Table 2: Result of FTIR.

DSC
A DSC thermogram of pure Levodopa showed a sharp endothermic peak at 296.02°C. Similar endothermic peaks were obtained at 289.1°C for the best formulation F1. The presence of all peaks signifies that all of the components were compatible with Levodopa, and there was no incompatibility between the compounds.

Figure 6: Thermograms of pure Levodopa

Figure 7: Thermograms of formulation F1
Evaluation Parameters Of Sublingual Tablets:
Pre-compression parameters of sublingual tablets
Table no 3 Pre formulation study results

The different ratio powders were subjected to pre-compression parameters, and F1 formulation values were found that the Angle of repose >25, Bulk density was 0.53, Tapped density was 0.6, Hausner’s ratio 1.17, and Carr’s Index was 15.2 indicating good compressibility.
Post-compression parameters of sublingual tablets:
For formulating a Sublingual tablet of the desired characteristic trial batches were prepared by employing varied concentrations of Super disintegrants were used such as SSG, Croscarmellose Sodium, and Cross-povidone. From all these SSG was the most desired as it provided good hardness and disintegration.
The results of all the tablets have passed the Weight variation test, as all the tablets were within the acceptable limits
The Hardness of all the tablets of Sodium Starch Glycolate formulations was found to be 3- 4 kg/cm2, which was within the acceptable limits
Friability test results showed that the friability of all the formulations ranged from 0.1-0.4.0%. All the values of formulation were less than 1% which indicated tablets have good mechanical resistance.
The Water Absorption Ratio of all the formulations was in the range of 20 to 40 %. The results showed that as the concentration of super disintegrants increased, the water absorption ratio also increased. From the results, the water absorption of super disintegrants was in the order,
Cross Povidone > Croscarmellose sodium > SSG
The Drug content of all the formulations was in the range of 91 – 100? showed the highest, drug content compared to others.
Table 4: Results of post-compression parameters.
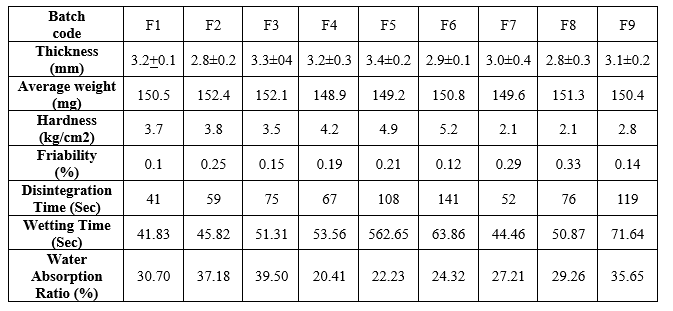
Comparative study of in-vitro disintegration time in sublingual tablets:
The in-vitro disintegration time of all the formulations was in the range of 41 – 141sec. From the table, it was observed that formulations F1 & F7 contain SSG and Cross Povidone respectively disintegrated rapidly in a short time of (41 –52 sec), from the result, the In-vitro disintegration time of super disintegrants was in order,
SSG > Cross Povidone > Croscarmellose sodium.

Figure 7: In-vitro disintegration of tablets.
Wetting time
The Wetting time of the formulation was in the range of 40-70 seconds it was indicated that as the concentration of super disintegrants increased the wetting time decreased. From the results, the wetting time of super disintegrants was in the order,
SSG > Croscarmellose sodium > Cross Povidone.
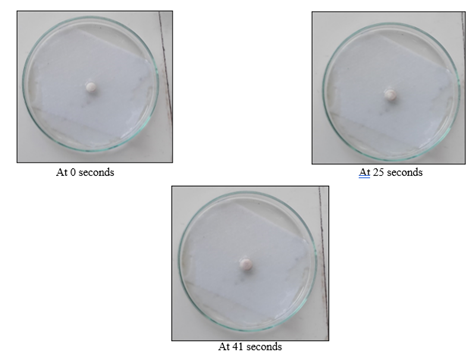
Figure 8: Wetting time of F1 formulation

Figure 9: In-vitro dissolution of Sublingual levodopa tablet containing 30ml dissolution media.
In-Vitro Dissolution Studies:
The In-vitro dissolution study of Levodopa in 30ml of SSF pH 6.8 was performed to mimic the oral sublingual region and the results are shown in figure 5.15. From the results, it was obtained that formulation F1 showed higher drug release compared to other formulations suitable for sublingual delivery. The release of the drug was prompted by employing SSG as a super disintegrant and Soluplus as a dissolution enhancer.
Table 5: Cumulative drug release of tablet
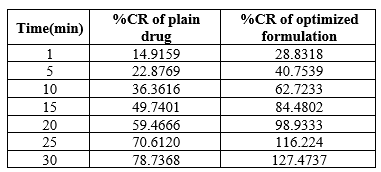
Soluplus was an important ingredient for improving the solubility of levodopa and dissolute in SSF.
In vitro permeation studies:
In the In-vitro permeation study using Dialysis membrane on Franz diffusion cell, the results are shown in (Fig 5.17). It was found that when Soluplus and Drug were used in a 1:1 ratio, the permeability was improved significantly as compared to drugs without Soluplus. Thus, the use of Soluplus helped increase the permeability of the drug. More than 90% permeability was observed within 90 min.

Figure 10: Comparison of In-vitro dissolution of plain drug with optimized formulation.
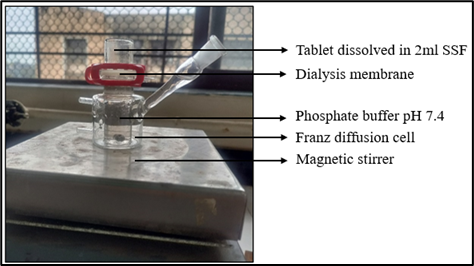
Figure 11: In-vitro permeation on Franz-diffusion cell

Figure 12: Comparative study of % Cumulative release of dru EX-vivo permeation study:
EX-vivo permeation study:
The Ex-vivo permeation study showed good permeation. The permeability enhancer had a prominent role in the permeability of the drug and they enhanced the percentage permeability of the drug upon increasing their concentrations in a 1:1 ratio. More than 90% permeability was observed in 90 min.

Figure 13: Ex-Vivo Permeation On Franz-Diffusion Cell

Figure 14: Ex-vivo permeation of optimized formulation.
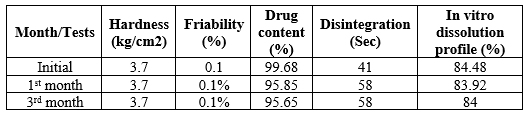
Stability study
Table no. 6: Stability study of optimized formulation
DISCUSSION
Levodopa is the gold standard for treating Parkinson's disease. The available conventional formulations available in market are in combination with Carbidopa. Levodopa gets eliminated by peripheral decarboxylase enzymes present in the body. This enzyme is usually present in high concentrations in Gut, liver, and kidney. Thus, the amount of drug reaching brain is less and dose requirement is more. Thus, the need of an alternative drug delivery system was needed which will bypass the metabolism. Sublingual drug delivery system provides promising route to bypass hepatic metabolism. Levodopa was procured and various pre formulation study was performed. Pre-compression parameters were evaluated for good flow properties. Post compression study showed that the formulation was developed successfully. Different super-disintegrants like SSG, Croscarmellose sodium, Cross povidone was used to get the disintegration of sublingual tablets within 2 minutes as per IP. Use of SSG more than 8?used sticking of the tablets to the die cavity of tablet punching machine. Thus, SSG should be used up to 5-8% of the total weight of tablet. Ludiflash improved the flow properties. Use of binder was avoided as it was making tablets very hard. And because of the extreme hardness of tablets the disintegration was hampered. Soluplus was used as a dissolution enhancer which improved the permeability of drug as tablets without Soluplus were showing less permeability. There is no specific dissolution method given in IP, USP, BP for the dissolution study of Sublingual tablets. Therefore, new method was prepared for the Sublingual tablet’s dissolution study. Dissolution was done in a beaker containing 30ml SSF on magnetic stirrer for 30 mins, the dissolution study results showed more than 80% within 15mins. Further the permeation studies were done in Franz diffusion cell containing Phosphate buffer pH 7.4 using Dialysis membrane. As the drug was permeating across membrane the buffer changed its colour from transparent to light brown. The colour changed during permeation study in pH 7.4 occurred because Levodopa containing compounds in the presence of Magnesium compounds or alkaline conditions gets converted to melanin thus solution turned dark in colour. Ex-vivo permeation study was performed on Goat mucosa. Tablets were kept for stability studies for 3 months. The optimized batch showed good dissolution and permeation therefore it can be summarized that the prepared formulation bypassed the GI and the bioavailability can be increased as the drug will directly go into the system circulation by avoiding peripheral degradation of Levodopa. The drug dose was reduced as Levodopa was formulated alone instead of in combination with Carbidopa.
CONCLUSION
- The ? max of Levodopa was found to be 245nm in phosphate buffer and SSF of pH 6.8.
- FTIR investigations revealed no interaction between drugs and excipients. Sublingual tablets were effectively manufactured using direct compression method.
- The pre-compression parameters and post-compression parameters were within the required limit. F1 batch was selected as an optimized formulation based on pre-compression, post-compression, and in-vitro drug release.
- There was no change from its initial nature till the period of 3 months.
- From the research, it can be concluded that the formulation prepared by both super disintegrants and dissolution enhancers showed good results in terms of pre-compression and post-compression parameters and stability studies.
- Thus, from the performed research work we can suggest that the formulation of Sublingual tablets can be promising route for the delivery of Levodopa.
FUTURE SCOPE
Further long-term stability studies could be done regarding the shelf life of the drug, using different types of dissolution enhancers to increase the dissolution of the drug in SSF and also introduce an In-vivo study. The blister packaging can be the reliable packaging for the proposed formulation.
ACKNOWLEDGEMENT
I wish to express my sincere thanks, with a deep sense of gratitude to my research guide, Mrs. Rachana D. Sarawade. I express my deep sense of gratitude to RP Chemicals, Mumbai for providing me with a drug without their support this work was impossible. My sincere thanks to Bharati Vidyapith College of Pharmacy, Navi Mumbai, for analyzing my DSC sample. Mrs. Avinash Phadatare, for providing chemicals from BASF. I want to express my gratitude to our Principal, Dr. Paraag Gide, Dr. L H Hiranandani College of Pharmacy, Ulhasnagar-03 for his generous consideration and facilities and of course for his constant support and encouragement to keep my morale high in times of difficulties.
REFERENCES:
- Johnston, T. H.; Fox, S. H.; Brotchie, J. M. Advances in the Delivery of Treatments for Parkinson’s Disease. Expert Opin. Drug Deliv. 2005, 2 (6), 1059–1073. https://doi.org/10.1517/17425247.2.6.1059.
- Goole, J.; Amighi, K. Levodopa Delivery Systems for the Treatment of Parkinson’s Disease: An Overview. Int. J. Pharm. 2009, 380 (1–2), 1–15. https://doi.org/10.1016/j.ijpharm.2009.07.026.
- Deleu, D.; Northway, M. G.; Hanssens, Y. Clinical Pharmacokinetic and Pharmacodynamic Properties of Drugs Used in the Treatment of Parkinson’s Disease. Clin. Pharmacokinet. 2002, 41 (4), 261–309. https://doi.org/10.2165/00003088-200241040-00003.
- Thanvi, B. R.; Lo, T. C. N. Long Term Motor Complications of Levodopa: Clinical Features, Mechanisms, and Management Strategies. Postgrad. Med. J. 2004, 80 (946), 452–458. https://doi.org/10.1136/pgmj.2003.013912.
- Salat, D.; Tolosa, E. Levodopa in the Treatment of Parkinson’s Disease: Current Status and New Developments. J. Park. Dis. 2013, 3 (3), 255–269. https://doi.org/10.3233/JPD-130186.
- Bhirud, P. H.; Kate, J. A. Perioperative Sublingual Levodopa in Parkisnon’s Disease: A Useful Alternative! Indian J. Anaesth. 2017, 61 (5), 432–434. https://doi.org/10.4103/ija.IJA_178_17.
- Pawar, P. P.; Ghorpade, H. S.; Kokane, B. A. Sublingual Route for Systemic Drug Delivery. J. Drug Deliv. Ther. 2018, 8 (6-s), 340–343. https://doi.org/10.22270/jddt.v8i6-s.2097.
- Nibha, K. An Overview on: Sublingual Route for Systemic Drug Delivery; 2012.
- Kumaran, K. S. G.; Sreekanth, J.; Sivanandy, Dr. P. Formulation Development and Evaluation of Levodopa-Carbidopa Orally Disintegration Tablets. 2011, 3, 169–175.
- Gauthier, S.; Amyot, D. Sustained Release Antiparkinson Agents: Controlled Release Levodopa. Can. J. Neurol. Sci. J. Can. Sci. Neurol. 1992, 19 (1 Suppl), 153–155.
- Freitas, M. E.; Ruiz-Lopez, M.; Fox, S. H. Novel Levodopa Formulations for Parkinson’s Disease. CNS Drugs 2016, 30 (11), 1079–1095. https://doi.org/10.1007/s40263-016-0386-8.
- Wagh, M.; Yewale, C.; Zate, S.; Kothawade, P.; Mahale, G. Formulation and Evaluation of Fast Dispersible Tablets of Aceclofenac Using Different Superdisintegrant. Int. J. Pharm. Pharm. Sci. 2010, 2, 154–157.
- FR, S.; D., G.; S, R.; J, J. Formulation and Evaluation of Nifedipine Sublingual Tablets. Asian J. Pharm. Clin. Res. 2009, 2, 44–48.
- Bushra, R.; Shoaib, M. H.; Aslam, N.; Hashmat, D.; Ur-Rehman, M. Formulation Development and Optimization of Ibuprofen Tablets by Direct Compression Method. Pak. J. Pharm. Sci. 2008, 21 (2), 113–120.
- Aghera, N.; Shah, S.; Vadalia, K. Formulation and Evaluation of Sublingual Tablets of Losartan Potassium. Asian Pac. J. Trop. Dis. 2012, 2, S130–S135. https://doi.org/10.1016/S2222-1808(12)60138-8.
- Bartos, C.; Pallagi, E.; Szabó-Révész, P.; Ambrus, R.; Katona, G.; Kiss, T.; Rahimi, M.; Csóka, I. Formulation of Levodopa Containing Dry Powder for Nasal Delivery Applying the Quality-by-Design Approach. Eur. J. Pharm. Sci. Off. J. Eur. Fed. Pharm. Sci. 2018, 123, 475–483. https://doi.org/10.1016/j.ejps.2018.07.061.
- Orally Disintegrating Tablets: A Review | Tropical Journal of Pharmaceutical Research. https://www.ajol.info/index.php/tjpr/article/view/44525 (accessed 2023-09-21).
- Phadke, A.; Amin, P. Orally Disintegrating Film of High-Dose BCS II Drug by Hot Melt Extrusion through Design of Experiment. J. Pharm. Innov. 2022, 18. https://doi.org/10.1007/s12247-022-09631-3.
- Verma, H.; Verma, S.; Singh, H.; Prasad, Dr. S. Sublingual Delivery of Frovatriptan: An Indication of Potential Alternative Route. Int. Sch. Res. Not. 2014, 2014, 1–9.
- Bonthagarala, B.; Kumar, P. Formulation and Evaluation of Sublingual Tablets of Rizatriptan,. Int. J. Pharm. Pract. Drug Res. ISSN 2249-7633 Vol 31 45-50 2013 2013


 Amruta Pol*
Amruta Pol*
 Rachana Sarawade 2
Rachana Sarawade 2




















 10.5281/zenodo.11109715
10.5281/zenodo.11109715