Abstract
Cubosomes are lipid vesicles that are comparable to vesicular systems like liposomes. Cubosomes are created with certain amphiphilic lipids in the presence of a suitable stabiliser. Since its discovery and designation, self-assembled cubosomes as active drug delivery vehicles have drawn much attention and interest. Oral, ocular, transdermal, and chemotherapeutic are just a few of the drug delivery methods in which they are used. Cubosomes show tremendous potential in drug nanoformulations for cancer therapeutics because of their prospective advantages, which include high drug dispersal due to the structure of the cubic, large surface area, a relatively simple manufacturing process, biodegradability, ability to encapsulate hydrophobic, hydrophilic, and amphiphilic compounds, targeted and controlled release of bioactive agents, and biodegradability of lipids. The most typical technique of preparation is the simple emulsification of a monoglyceride with a polymer, followed by sonication and homogenisation. Top-down and bottom-up are two different sorts of preparation techniques. This review will critically analyse the composition, preparation techniques, drug encapsulation approaches, drug loading, release mechanism and applications relevant to cubosomes. Furthermore, the challenges faced in optimising various parameters to enhance the loading capacities and future potentialities are also addressed
Keywords
cubosomes, characterisation, nanomedicine, drug delivery systems
Introduction
The “cubosomes” term was firstly coined by Larsson, which is similar to liposomes. Cubosomes are the nanostructured particles and these are the discrete and submicron size particles of the bicontinuous cubic liquid crystalline phase. The bicontinuous cubic phases are having a specific benefit, that is, their ability to tune membrane curvature. Cubosomesare self-assembled liquid crystalline particles, which have rheology like a solid. Liquid crystals could be a quarter state of matter. These cubosomes are made up of lipids, polymers, and surfactants, which are usually amphiphilic. Here, the meaning of bicontinuous is that the enclosures of two different regions of water are divided by surfactant bilayers. Cubosomes are similar to liquid crystalline substance, viscous, optically isotropic as well as solid and having cubic crystallographic symmetry. Cubosomes are highly important in nanotechnology based drug delivery system
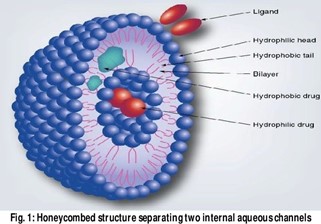
ADVANTAGES :-
- High drug payloads due to high internal surface area and cubic crystalline structures.
- Relatively simple method of preparation.
- Biodegradability of lipids.
- Capability of encapsulating hydrophilic, hydrophobic and amphiphilic substances.
- Targeted release and controlled release of bioactive agents.
- It has Skin permeation enhancement
- It is non-toxic and biocompatible
- It is economic
DISADVANTAGE :-
- Because cubosomes contain a large amount of water, water-soluble drugs are less likely to be trapped.
- Due to its high viscosity, large-scale cubosome manufacture is challenging.
- Without the use of a specific polymer, regulated drug delivery is not possible.
- They have the potential to leak during storage or in-vivo transmission.
- When particles are left alone for a long time, they may grow.
- If the external environment changes, cubosomes can produce a phase change (dynamics).
- Large scale production is sometimes difficult because of high viscosity.
APPLICATIONS :-
- In cancer therapy
- Oral drug delivery
- Intravenous drug delivery systems
- Drug delivery vehicle
- Controlled or sustained release behaviour
- Intreatment of viral disease
METHOD OF PREPARATION
Cubosomes formulation technique:-
- Bottom-up Approach
- Top-down Approach
- Solvant evaporation method
- Spray drying method ?
- TOP-DOWN METHOD :-
The top down-method is the most widely used technique for cubosomes preparation, it involves two main steps. Firstly, mixing the cubosomes forming lipid with a suitable stabilizer to form the bulk viscous cubic aggregates. Secondly, dispersion of the produced viscous cubic aggregates in aqueous media by the application of high energy as high-pressure homogenizer or sonication finally resulting in the formation of cubosomes. Fortunately, cubosomes prepared by the top-down method are found to be stable against aggregation up to a year. However, this method with drawbacks in large scale production as the formation of viscous cubic aggregates require high energy input to be dispersed into cubosomes, unfortunately, these may be a problem when incorporation of temperature-sensitive bioactive agents, especially peptides and protein are required.
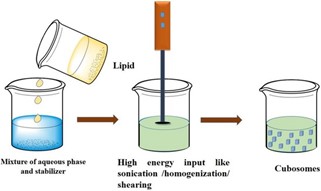
Preparation of cubosomes by top-down approach
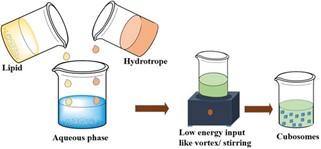
2.BOTTOM-UP METHOD :-
This approach is commonly referred to as solvent dilution method, it involves dispersion of mixture containing cubosomes forming lipid, the stabilizer and a hydrotrope in excess of water with the application of minimal energy input. Hydrotrope is the key factor in the bottom-up approach as it is added to dissolve water-insoluble lipids to form lipid precursors and prevent the formation of liquid crystals at high concentration . Hydrotrope is a molecule able to solubilize molecule that able to solubilize poorly soluble agents in aqueous media by hydrotropic solubilization which means enhancement of solubility of one solute by addition of another solute. Urea, sodium alginate and sodium benzoate are among the most commonly used hydrotropes. The solubilizing mechanism of hydrotrope involves complex formation between the hydrotrope and the hydrophobic agent.The bottom-up technique provides more advantages over the top-down approach as it requires less energy input thus it can be safely used for the preparation of cubosomes loaded with temperature-sensitive agents also the yielded cubosomes show long term stability due to the homogenous dispersion of stabilizers onto the surface of the produced nanovesicles .
GREEN SYNTHESIS IN CUBOSOME
Green synthesis of nanoparticles is done to reduce the waste generated and implement sustainable process. In this process mild reaction conditions and nontoxic precursors are used. It does not require expensive and harmful chemicals; we can synthesize various metallic nanoparticles by this method. It can be done by one step using various biological organisms such as bacteria, algae, yeast, molds and plants or their products. We can also use molecules from plants and microorganisms like phenolic compounds, amines, proteins and alkaloids. It is free from toxic compounds, different parts of the plant can be used for synthesis of nanoparticles. Green synthesis of cubosomes by using plant extracts has drawn a considerable attention. We can also extract reducing and stabilising agents from plants to synthetize metallic nanoparticles.
Preparation of cubosomes by the bottom-up approach
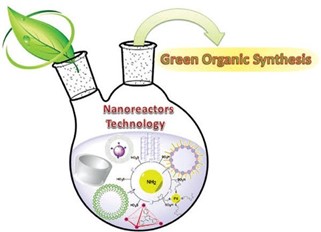
Green synthesis in Cubosome
Various components can be extracted from plants and used in the synthesis of cubosomes such as curcuma longa, capsicum, neem plant, tulasi herb, red onion, moringa oleifera, lippia citriodora, alium sepa, eucalyptus, tridax procumbens etc.
CONCLUSION
This information gives an understanding of study of Cubosomes. As cubosomes are bicontinuous lipid cubic phases, they have capability to incorporate many hydrophilic and lipophilic drugs that get delivered to the targeted tissues such as central nervous system and brain efficiently. Stability can be imparted to potent and highly sensitive moieties by these cubic systems which contain selective and stable lipids. Two reproducible methods such as top down and bottom up approaches could be easily employed to produce cubosomes either by high pressure homogenization or ultrasonication technique. Cubosomes are applicable to wide range of drug candidates, proteins, immunogenic substances and also to cosmetics.Due to the potential site specificity, the cubosomal preparations may be widely employed as targeted drug delivery systems for ophthalmic, diabetic and also for anticancer therapy. The cubosome technology is relatively new with high through output and would have wide scope of research in developing formulations with commercial and industrial viability.
REFERENCE
- Karami, Z. and M. Hamidi, Cubosomes: remarkable drug delivery potential. Drug Discovery Today, 2016. 21(5): p. 789-801.
- Esposito, E., et al., Cubosome dispersions as delivery systems for percutaneous administration of indomethacin. Pharmaceutical research, 2005. 22(12): p. 2163-2173. Rao, S.V., B.N. Sravya, and K. Padmalatha, A review on cubosome: The novel drug delivery system. GSC Biological and Pharmaceutical Sciences, 2018. 5(1): p. 076-081. Limbachiya, M.I., et al., Solubility enhancement techniques for poorly soluble drugs: A review. IJPRD, 2011. 4(4): p. 71-86.
- Croy, S. and G. Kwon, Polymeric micelles for drug delivery. Current pharmaceutical design, 2006. 12(36): p. 4669-4684.
- Thadanki, M., P.S. Kumari, and K.S. Prabha, Overview of cubosomes: a nano particle.Int J Res Pharm Chem, 2011. 1(3): p. 535-541.
- Bhosale, R.R., et al., Cubosomes: the inimitable nanoparticulate drug carriers. Scholars Academic Journal of Pharmacy, 2013. 2(6): p. 481-486.
- Tajmal, M. and D.N. Reddy, preparation and characterization of cubosomes, as advanced drug delivery system for low solubility drugs. 2018.
- Anbarasan, B., X.F. Grace, and S. Shanmuganathan, An overview of cubosomes– smart drug delivery system. Sri Ramachandra J. Med., 2015. 8(1):p. 1-4.
- Gan, L., et al., Self-assembled liquid crystalline nanoparticles as a novel ophthalmic delivery system for dexamethasone: improving preocular retention and ocular bioavailability. International journal of pharmaceutics, 2010. 396(1-2): p.179-187.
- Kulkarni, C.V., et al., Monoolein: a magic lipid? Physical Chemistry Chemical Physics, 2011. 13(8): p. 3004-3021.
- Luzzati, V. and F. Husson, The structure of the liquid-crystalline phases of lipidwater systems. The Journal of cell biology, 1962. 12(2): p. 207-219.
- Clogston, J. and M. Caffrey, Controlling release from the lipidic cubic phase. Amino acids, peptides, proteins and nucleic acids. Journal of controlled release, 2005. 107(1): p. 97-111.
- Aleandri, S., et al., Biotinylated cubosomes: a versatile tool for active targeting and codelivery of paclitaxel and a fluorescein-based lipid dye Langmuir, 2015. 31(46): p. 12770-12776.
- Chong, J.Y.T., et al., Chapter Five - Steric Stabilizers for Cubic Phase Lyotropic Liquid Crystal Nanodispersions (Cubosomes), in Advances in Planar Lipid Bilayers and Liposomes, A. Igli?, C.V. Kulkarni, and M. Rappolt, Editors 2015, Academic Press. p. 131-187.
- Boge, L., et al., Cubosomes for topical delivery of the antimicrobial peptide LL-37.
- European Journal of Pharmaceutics and Biopharmaceutics, 2019. 134: p. 60-67.


 Rutuja Suresh Jagtap*
Rutuja Suresh Jagtap*
 Prajkta Sadanand Ingale
Prajkta Sadanand Ingale




 10.5281/zenodo.11784008
10.5281/zenodo.11784008