The name Parkinson's disease (PD) honors James Parkinson, whose 1817 book "A Treatise on Concussive Palsy" provided the classic description of the treatment of the disease. [1] Parkinson's disease (PD) is a neurodegenerative disease caused by the loss of neurons specifically required for dopamine synthesis in the brain. [2,3] Parkinson's disease (PD) is the second most common disease, affecting 0.2% of the world's population, 1% of people over 60 years of age, and approximately 4% of people over 80 years of age. The rate of PD (PD) is increasing exponentially, especially in men. [4,5] The life expectancy of Parkinson's patients is up to 80%. [6] Although the etiology of Parkinson's disease is often unknown, age is still an important factor in Parkinson's disease. [7] Parkinson's disease is the most common genetic mutation that causes Parkinson's disease. It is an autosomal recessive mutation found in 50% of early-onset PD. [8] Parkinson's disease is a neurodegenerative disease that is seen significantly in all age groups. Bradykinesia or slowness of movement, stiffness, restlessness, and other symptoms that reduce the quality of life and ultimately cause serious damage due to lack of motor control are symptoms of Parkinson's disease (PD). [8,9] Characteristics of Parkinson's disease: Parkinson's disease is a movement disorder that mainly affects the elderly. Main symptoms include ? Inhibition of voluntary movement (hypokinesia) caused by muscle stiffness and motor dysfunction. Natural inertia makes it difficult to start and stop the engine. A resting tremor that often begins in the hands and goes away while engaging in intentional action. Muscular stiffness, can be felt as more resistance while moving the limbs passively. A level of mystery. People with Parkinson's disease have a noticeable wobble in their steps. Parkinson's disease can be caused by cerebral ischemia, viral encephalitis, or other damage, but usually has no obvious cause. Medications that reduce dopamine levels in the brain or block dopamine receptors (such as antidepressants such as chlorpromazine) are the main cause of symptoms. Rare familial cases of early-onset Parkinson's disease (PD) have been reported, and mutations have been identified in many genes, including those encoding parkin and synuclein. Studies on mutations in these genes provide some clues about the mechanisms behind neurodegenerative processes. Parkinson's disease affects the basal ganglia; Its neurochemical origins were discovered in the 1960s by Hornykiewicz, who showed that dopamine levels in the striatum and substantia nigra of the hindbrain of Parkinson's patients were abnormal, often less than 10% of what was always there. This is associated with degeneration of striatal nerve endings and loss of dopaminergic neurons in the substantia nigra. It affects more than other monoamines, such as dopamine, norepinephrine, and serotonin. Years of progressive dopamine depletion precede the onset of Parkinson's disease symptoms, which only appear when striatal dopamine levels drop to 20-40% of normal. Nigrostriatal damage or chemically triggered dopamine depletion in experimental animals can also cause symptoms of PD. [10]
Cardial Motor Symptoms in PD:
The most often recognized motor symptoms of PD include bradykinesia, rest tremor, stiffness, and loss of postural reflexes.
1.Bradykinesia:
Bradykinesia, a symptom of Parkinson's Disease (PD), is often characterized by slow movements, reduced amplitude, and motor control problems due to reduced neuronal density in the SN. People with bradykinesia cannot provide enough energy for their muscles to work, which prevents them from moving quickly. Time slowing down and difficulty juggling various activities are the first symptoms, but other symptoms include difficulty swallowing, loss of movement and decreased blinking. [11] Patients with Parkinson's disease (PD) may experience bradykinesia and need additional external support to initiate motor activities. Bradykinesia and dopamine deficiency are often closely related. [12]
2.Tremor:
Since tremors are the most prominent symptom of Parkinson's disease, the disease is called tremor paralysis. This condition occurs in 75% of patients at some stage during the course of the disease. Usually this is a tremor that occurs when the patient is resting and sitting. Severe tremors can affect the body and movement. The shaking frequency is between 3.5 and 7 Hz. 13 Many patients have smelled something from the inside before; others will feel that they are shaking even if they are not. In one sample of patients, 44% reported an internal feeling. [13] For most patients, tremors eventually become clearly visible to outside observers. Patients become anxious while reading newspapers or using the phone. Tremors are a clear indication of a nervous system problem while the patient is sitting still. Any limb can cause the first tremor. It usually starts in one arm, usually in one finger or hand, and then spreads to the other arm. Tremors may occur in the same leg or the opposite leg. In general, the limb most affected at the onset of the disease is also the limb that suffers the most. Tremors do not usually affect the head but can affect the tongue, lips, jaw, and neck. The affected areas have the consistency of tremors. [14] Stress may cause the patient to shiver. Tremors can be caused by further stress, whether from an emotional state or physical stress. When the tremor disappears during sleep or anesthesia and reappears during sleep, the patient wakes up. Asking the patient to use the contralateral body to perform gross motor activities, such as making and releasing a fist, or power-intensive tasks, such as counting numbers, may result in the presence or absence of tremors in the clinical setting. Because of the disabling effect on intentional movement, tremors are often the worst cause of Parkinson's symptoms, but they are one of the most noticeable. The thalamus is believed to be the source of tremors. Both peripheral and spinal reflexes affect tremors. [15]
3.Rigidity:
The second most common symptom of Parkinson's disease (PD) is rigidity, defined as stiffness of the neck, trunk, and extremities. While bradykinesia reduces movement speed, rigidity reduces muscle movement and the inability to relax. Shoulder pain, sometimes confused with arthritis, is a symptom of Parkinson's disease (PD), which is caused by stiffness. Studies have shown that dopaminergic agonists are effective in reducing skin inflammation. [16] Although the degree of stiffness varies, it is present almost from the moment the disease begins. Patients may report fatigue, weakness, or muscle aches. Not being able to swing your arm freely may be the first sign of bradykinesia, or stiffness affecting the arm. Similar to tremors, rigors often begin in a single way. It can then spread outside. It takes years for symptoms to become asymmetrical. [17]
4.Postural Instability:
PD often occurs with post-traumatic stress disorder. In the early stages, patients showing signs of weakness or a history of falls should be evaluated for the consequences of Parkinson's disease, such as multiple atrophy or supranuclear palsy. The most unpleasant symptoms of Parkinson's disease (PD) and those least likely to be treated are unpleasant. The tension in PD causes the person to be pushed forward and downward due to abnormal body movement resulting from the neck and body bending forward and the arm bending at the elbow. This change in the body makes it susceptible to losses or decline. Since the patient's motor skills have already changed, loss of postural reflexes increases the risk of falling. Depending on how the force is used, the patient will fall forward (impulse) or backward (retraction). If the patient loses his balance, he will start to stand up again by taking a few steps. Due to poor posture, they will fall more often and lose control of themselves. Patients with stability problems "use their toes and forefoot for support; at the same time they are irresistibly forced to move faster, make their steps shorter, and therefore experience anxiety, which they admit will not refuse to run quickly." 18] The main symptom of impaired postural reflexes is postural instability, which often occurs in the late stages of the disease. This is the first symptom in most patients with PD, leading to falls and subsequent fractures. [19]
5.Gait Disorder:
PD often occurs with post-traumatic stress disorder. In the early stages, patients showing signs of weakness or a history of falls should be evaluated for the consequences of Parkinson's disease, such as multiple atrophy or supranuclear palsy. The most unpleasant symptoms of Parkinson's disease (PD) and those least likely to be treated are unpleasant. The tension in PD causes the person to be pushed forward and downward due to abnormal body movement resulting from the neck and body bending forward and the arm bending at the elbow. This change in the body makes it susceptible to losses or decline. Since the patient's motor skills have already changed, loss of postural reflexes increases the risk of falling. Depending on how the force is used, the patient will fall forward (impulse) or backward (retraction). If the patient loses his balance, he will start to stand up again by taking a few steps. Due to poor posture, they will fall more often and lose control of themselves. Patients with stability problems "use their toes and forefoot for support; at the same time they are irresistibly forced to move faster, make their steps shorter, and therefore experience anxiety, which they admit will not refuse to run quickly." 18] The main symptom of impaired postural reflexes is postural instability, which often occurs in the late stages of the disease. This is the first symptom in most patients with PD, leading to falls and subsequent fractures. [19]
Secondary Manifestations of Parkinson's Disease:
These are nonetheless crucial in the clinical progression of the illness while being categorized as secondary. Instead, some can be important prodromal symptoms of the disease that emerge before any of the main motor characteristics, in particular paresthesias, cramps, focal foot dystonia, and sadness. Furthermore, compared to the core symptoms, secondary symptoms including dementia and orthostatic hypotension might be more incapacitating. The next section discusses the most prevalent and clinically significant of them.
1.Sleep Disorder:
While troubled sleep is a typical complaint among the elderly, 74% to 98% of Parkinson's disease patients report having trouble sleeping.[20] Initiation of sleep is impacted in 67% and maintenance of sleep in 88%. Sleeping might be impeded by motor symptoms like stiffness or trembling. Tremor can impede the onset of sleep and continue during the first few hours of light sleep. REM sleep causes the tremor to go away, however, it returns during arousal.[21] For some patients, this may be a sign of depression. Restlessness may result from restless legs, which occurs in some people with Parkinson's disease. The most common treatment for this condition is benzodiazepines, nocturnal levodopa, or dopamine agonists. Parkinson's disease is associated with rapid eye movement behavior problems. In some people, onset occurs before the diagnosis or onset of Parkinson's disease. [22, 23] In older people, it occurs more frequently. In certain patients, day-night reversal may result from irregularities in their circadian cycle. To increase the likelihood of sleeping through the night, an effort should be made to avoid taking naps during the day. Cognitively impaired Parkinson's disease individuals are more prone to experience this issue.[24]
2.Constipation:
Constipation is one of the most common symptoms of Parkinson's disease (PD), affecting more than 60% of patients. [25] It can have many causes, including decreased appetite, impotence, poor nervous system function, and side effects of medications (trihexyphenidyl), but has also been shown to be associated with the accumulation of ?-synuclein. Although persistent constipation can also cause urinary incontinence, it is often considered a risk factor for Parkinson's disease (PD). [26] The classic symptom of Parkinson's disease is gastrointestinal problems. Gastrointestinal disorders may also be affected by bradykinesia, which affects motor movements. Constipation is caused by slow digestion. More than half of PD patients report constipation. [27]
3.Dysphagia:
One of the most common complaints of PD patients is dysphagia. The majority of PD patients (more than 50%) report dysphagia. Thirty percent of patients complain of cough and cough. [28] Patients often complain of feeling like something is stuck in their throat or having to swallow while eating. Patients who have difficulty swallowing tablets on their own will find it easier to swallow tablets coated with pudding or applesauce, which thickens the medicine and food. Packing your mouth and food for long periods can extend meal time. Oropharyngeal dyskinesia has been widely documented. Due to the lack of motor control of the tongue, food is slow and often difficult to bolus and push down into the throat. [29]
4.Urinary dysfunction:
Difficulty urinating can affect 58% to 71% of people with Parkinson's disease (PD). [30] Usually, night is the first and most important sign. As the pain worsens, patients will urinate more frequently and urgently during the day, and the situation will worsen due to the inability to go to the toilet in time. In general, urinary dysfunction is mild and less severe than in patients with multiple atrophy. Comparison of urodynamic findings in patients with multiple sclerosis and striatonigral degeneration with those in Parkinson's disease. All study participants experienced urinary symptoms. In Parkinson's disease (PD), detrusor hyperreflexia with elevation of the urethral bodies is the most common finding. In contrast, most patients with striatonigral degeneration exhibit detrusor hyporeflexia (atonic bladder) or a mixed pattern of hyporeflexia and hyperreflexia with decreased urethral pressure. [31] In the early stages, it may be difficult to differentiate between multiple organ atrophy and Parkinson's disease (PD) based on a single clinical area, and urodynamic testing is even recommended as a diagnostic method in different diagnoses. [32]
5.Dermatologic Findings:
PH and seborrheic dermatitis are often associated. Seborrhea is a skin condition that causes areas of red skin on the face and scalp. Creams and ointments can be used to treat this type of seborrhea; If you have dandruff on your scalp, shampoo can help you control it. Recurrence of seborrhea is common and can be embarrassing.
6.Visual Disturbance:
Visual complaints are another prevalent PD issue. Eye strain, double vision, and blurry vision are common complaints from patients. An ophthalmologist should examine them before linking their visual symptoms to Parkinson's disease. Numerous research have examined visual issues in individuals with Parkinsonian disease. There have been reports of visual evoked potentials with extended PlOO wave latencies.[33] Certain findings may be explained by a lack of dopamine in the retina and the visual pathway, which includes the visual cortex.[34, 35]
7.Olfactory Dysfunction:
Ansari and Johnson (1975) showed that reduced sensation is associated with Parkinson's disease. [36] Since then, it has been generally accepted that olfactory dysfunction is the main symptom of PD. [37] Approximately 80% of people with Parkinson's disease experience hyposmia, indicating that it is a symptom of the disease. A possible disadvantage of olfactory receptor neurons in terms of neurodegeneration is that they produce odorants and use long unmyelinated axons to transmit electrical signals to the brain when they smell odors. According to Braak staging, anosmia may not only be a prodromal symptom of Parkinson's disease (PD) but also an early sign of neurodegenerative disease that occurs in the body years before the onset of symptoms. In Stage 1, alpha-synuclein debris and associated Lewy body pathology begin in the olfactory bulb. [39] This may be important in the diagnosis of Parkinson's disease (PD) because loss of smell may indicate the need for neuroprotective therapy to prevent or delay disease progression. Loss of smell has many advantages over other prodromal symptoms; One of these is the use of perfume products without the help of a nurse or psychiatrist. [40]
8.Depression:
Depression refers to changes in a patient's mood and its impact on behavior. Depression is often associated with a deficiency of neurotransmitters such as dopamine, serotonin, and norepinephrine in the brain, causing depression and dissatisfaction. Depression is one of the main symptoms of Parkinson's disease (PD) and affects up to 50% of patients, causing increased anxiety and attacks. Apathy and constant fatigue are also thought to be some of the early symptoms that can lead to depression. There are a variety of measures that can be used to assess depression, including the Montgomery-Asberg Depression Rating Scale (MADRS) and the Geriatric Depression Scale (GDS-15), but completing the assessment using one of the following methods requires expertise. [41, 42, 43]
OLD TREATMENT APPROACHES:
1.Dopaminergic pharmacological targets:
The main mechanism behind the initial symptoms of Parkinson's disease (PD) is the loss of dopaminergic neurons in the substantia nigra pars compacta, leading to striatal dopamine depletion. More than fifty years ago, the dopamine precursor amino acid levodopa was administered to reverse striatal dopamine depletion. This is one way to treat Parkinson's disease. Since then, significant advances in our understanding of the pharmacological control of nigrostriatal dopaminergic transmission have led to the identification of many new targets for dopaminergic therapy in Parkinson's disease. These can be broadly classified as therapeutically targeting presynaptic or postsynaptic function.
2.L-dopa:
Among anti-Parkinson drugs, levodopa remains the gold standard in treating symptoms. Eventually, nearly all Parkinson's patients will need to be treated with these drugs. [44] However, the emergence of movement disorders such as drug-induced dyskinesia and motor response oscillations hinders its management. We do not yet fully understand the underlying mechanisms that cause dyskinesia, especially after levodopa replacement. Presynaptic and postsynaptic mechanisms act, ultimately causing a non-motor pulsatile effect on striatal dopamine receptors and eliciting a series of maladaptive neuronal responses. [45, 46] The main reason for this is the short half-life of levodopa. The difference between absorption from the digestive system and transport to the blood-brain barrier ensures regular intake of the drug. To solve this problem, new formulations of levodopa with continuous administration (subcutaneous injection using a minipump or enteral injection through a PEG-J tube) have been developed166. Clinical evidence shows that levodopa bowel gel infusion can reduce pre-existing dyskinesia; This supports the idea that the application of dopaminergic receptor stimulation may prevent the development of drug-induced dyskinesia. [47, 48]
3.COMT-Inhibitors:
Current formulations of levodopa contain aromatic amino acid decarboxylase (AADC; carbidopa or benserazide) inhibitors to increase the bioavailability of dopamine and prevent peripheral metabolism. Therefore, activation of the central metabolic process associated with ortho-methylation of levodopa by catechol ortho-methyltransferase (COMT) is involved in the peripheral metabolism of levodopa. Peripheral inhibition of this enzyme will increase the half-life and bioavailability of levodopa, which will particularly benefit individuals with on-off dyskinesia. In these patients, COMT inhibitors have emerged as first-line therapy to prolong the effects of a single dose of levodopa, and there are currently three agents approved for clinical use. [49,50,51]
4.MAO-B Inhibitors:
The main mechanism of removal of synaptically produced dopamine from glial cells is oxidation by monoamine oxidase type B (MAO-B) followed by presynaptic reuptake by dopamine transporters. Selegiline is a selective inhibitor that was shown to be effective against levodopa in the 1970s. Inhibiting this enzyme increases and prolongs synaptic dopamine concentrations. [53] Recent studies have demonstrated the antiparkinsonian efficacy of selegiline and rasagiline (more MAO-B inhibitors) alone or in combination with levodopa in patients with motor impairment. 169 While both rasagiline and selegiline irreversibly block the MAO?B enzyme (also known as “suicide”), safinamide is a recently approved MAO?B inhibitor and acts as a reversible inhibitor. [54]
5.DA-Agonists:
Two dopamine receptors mediate the effects of dopamine on striatal spiny neurons. Direct striatopallidal projections are activated by the physiological dopamine activity of D1 receptors, but the firing of indirect striatopallidal pathways is inhibited by the stimulation of D2 receptors. Increased glutamatergic transmission from the motor nuclei of the thalamus to the prefrontal motor cortex is the end result of striatal dopamine release, which aids motor function. Since the 1970s, when the ergot alkaloid bromocriptine was first used in the treatment of Parkinson’s disease, dopamine mimetics (DA receptor agonists) have been an important pharmacological agent in the treatment of PD symptoms. [50, 55] Ergoline structure and activity of the 5-HTB2 receptor, the first member of this drug family, has been associated with heart and pleuropulmonary valve fibrosis, causing serious safety concerns. Currently, available drugs do not have this effect and are all non-ergoline drugs. Dopamine agonists are more effective than levodopa, one of which has a longer half-life. This makes them an attractive treatment option for patients with physical disabilities. [56] Additionally, rotigotine, a non-ergoline agonist, is available as a transdermal patch that provides a sustained dose. Dopamine agonists are generally thought to cause less pulsatile striatal dopamine receptor activation than levodopa; This helps explain why there is a potential for serious complications when dopamine agonists are used as primary monotherapy for the relief of Parkinson’s disease. [55,56] The overall effect of levodopa is smaller. They can also be tiring and uncontrollable. The latter may be due to their preference for D3 receptors in the ventral striatum and thus overstimulation of the brain’s reward system. [57] Apomorphine is unique among other dopamine agonists in that it is equivalent to levodopa and acts on D1 and D2 receptors. [58] Continuous subcutaneous apomorphine infusion is associated with a reduction in levodopa-induced dyskinesia before correcting changes in motor reactivity. [59]
NEW TREATMENT APPROACHES FOR PARKINSON DISEASE:
1.Gene Therapies:
Gene therapy is a rapidly developing genome-editing technology that treats diseases by genetically modifying cells that are malfunctioning or potentially reducing symptoms of the disease. [60] The basis of this technology is the addition of DNA, RNA, antisense oligonucleotides, or enzymes that deliver DNA or RNA into target cells via vectors to alter gene expression. [61, 62] Studies in animal models have demonstrated the safety and efficacy of two viral vectors, the lentivirus (LV) and adeno-associated virus (AAV) families, with very low drug resistance and in vivo recognition of intermediate genes. Thus, increasing clinical evidence suggests that vector-based gene therapy can be used to treat Parkinson's disease (PD). [63] AAV is frequently used as a vector for central nervous system (CNS) infections. [64] While other AAV serotypes are used to target various cells in the central nervous system, such as stellate cells and microglia, AAV serotype 2 (AAV2) exhibits a specific tropism for neurons there. [65,66] Viral vectors are used in gene therapy to treat Parkinson's disease (PD), altering genes to target protein expression in specific regions of the brain. Over the past decade, three main approaches have been used to move gene therapy for Parkinson's disease into clinical trials:
Three methods are used to preserve and repair nigral DAergic neurons:
- Administration of the enzyme glutamic acid decarboxylase (GAD) in the STN;
- Delivery of synthetic enzymes to raise striatal DA levels; and
- Local infusion of neurotrophic substances.[67,68]
The rationale for the first strategy is to use adeno-associated viral (AAV) vectors to introduce GAD, an enzyme that limits the rate of GABA synthesis, into glutamatergic neurons of the STN. This gene therapy strategy aims to alter STN activity by changing the phenotype of STN neurons from predominantly excitatory (glutamatergic) to predominantly inhibitory (GABAergic). This will normalize the striatopallidal circuit. [69,70] Additionally, clinical studies using viral vectors to modulate the expression of key dopamine synthesis pathway enzymes are ongoing. For this reason, tyrosine hydroxylase (TH) and its cofactors and amino acid decarboxylase (AADC) have been introduced using lentiviral (LV) and AAV vectors in various studies, and preliminary safety studies have been reported. [71, 72] The plan is to modify the striatal testis to produce and release dopamine from tyrosine locally, peripherally derived dopamine, or levodopa. Animal studies have shown that this strategy is possible and not only reduces symptoms associated with dopamine-dependent physical activity but also reduces the risk of future motor changes through stimulation of dopamine receptors. [73, 74] Since the results of sham surgery are encouraging, another strategy is to target the subthalamic nucleus of AAV2 vector-mediated GAD to promote GABA inhibition of STN activity. Control phase 2 testing. [75]
2.Fetal Cell Transplantation:
In the 1990s, cell transplantation was considered a treatment for brain damage in Parkinson's disease (PD). According to an open-label study, immature dopamine obtained from aborted embryos or fetuses can improve striatal dopamine transmission and connectivity as well as reduce physical symptoms (as seen in in vivo positron tomography and postmortem morphological results). [76,77] However, the treatment was abandoned after the results of two double-blind, placebo-controlled studies funded by the NIH were published in 2001 and 2003. [78] These studies did not provide evidence of clinical benefit, and retrospective analysis of patients in open-label studies supports the notion that some patients experience a lack of control over impaired dyskinesia (GID). [79] Although clinical trials have been stopped, the current period of research on stem cell therapy coincides with the beginning of research in the post-GID period. It is now believed, based on animal studies and research, that the number of serotonergic neurons in the dissected graft tissue is kept to a minimum and that if the patient is selected to be a patient not suffering from levodopa-induced dyskinesia (GID) before surgery. It is unlikely to happen. Tracing. [80] Concerns were raised about the longevity of cell transplants, as a 2008 study found that transplanted neurons showed signs of Lewy disease more than a decade after surgery. It is now generally accepted that transplanted neurons show signs of developmental degeneration, but it takes more than a decade for pathological changes that affect functional changes to appear. There are at least two cases where there is evidence showing a positive effect of changes in open-field shape up to 15 to 18 years after surgery. [81] The EU-funded TRANSEURO team launched an open study of fetal dopamine neuron implantation in 2015, and they hope to perform the procedure on 20 patients, each in the early stages of the disease, by the end of 2017. This experiment will help pave the way for future research using stem cell-derived neurons to replace dopamine neurons and show whether GPD can be prevented. [82,83]
3.Stem cells as donor tissue:
Stem cell therapy for Parkinson's disease has made significant progress in the last ten to fifteen years. Human embryonic stem cells (hESCs) and human induced pluripotent stem cells (hiPSCs) are two types of human pluripotent stem cells that can now be used to generate dopamine neurons with midbrain properties. According to recent research, they form axons innervating the brain, survive transplants in experimental animals, and promote functional recovery of defects caused by injuries. The main aim of the current research is to find solutions such as simplifying the use of mobile phones, making the products safe, and meeting the requirements to control the growth of biological products. Although many companies sponsor various treatments for Parkinson's disease, the scientific basis for the study of these cell types is often inadequate. [84] The International Society for Stem Cell Research has developed standards that must be followed when developing stem cell-based clinical trials. [85] Within two to three years, it is expected that products derived from rigorous research on stem cells will begin to be used in cell transplantation research for Parkinson's disease (PD). An international research collaboration called G-Force-PD was established to focus on the clinical translation of tumor cell therapy for Parkinson's disease (PD) and to promote progress in this field, holding monthly meetings to discuss processes and technological advances. [82]
4.Targeting alpha?synuclein:
The brain is rich in alpha-synuclein (?-syn), a 140 amino acid protein found only in the presynaptic terminals of neurons. This protein is encoded by the SNCA gene. Although its exact purpose is unknown, it appears to play a role in neurotransmitter release and recycling of synaptic vesicles. [86] The pathological feature of Parkinson's disease (PD) is the accumulation of ?-syn and its accumulation in cytoplasmic inclusions called Lewy bodies. Targeting the production, accumulation, isolation, and distribution of ?-syn is the main target of current disease-modifying drugs, but the exact mechanism of toxicity remains unclear. [87]
5.Decreasing the expression of a-synuclein:
Experiments have shown that altering ?-syn levels via RNA-interactive gene silencing is effective in restoring ?-syn performance and improving motor function; however, a careful balance is required to avoid overrestriction leading to nigrostriatal neurotoxicity. [88] One target that tightly regulates ?-syn expression is DNA methylation of SNCA intron 1, which regulates ?-syn transcription. The methylation level in PD differs from controls. Downregulation of SNCA expression in stem cell-derived dopaminergic neurons was successfully modulated using a novel technology of clustered regularly interspaced short palindromic repeats, suggesting a new strategy. [89]
6.Prevention of ? syn aggregation:
Recently, a novel oligomer modulator anle138b was developed through a combination of high-throughput screening of combinatorial libraries and chemical treatment. This combination has been shown to inhibit pathological oligomer formation in vitro and various mouse models of PD and prion disease. The first human clinical study (NCT04208152) in healthy workers was completed in August 2020 and found no harm at doses up to 300 mg. additionally, in animal models plasma levels were higher than required for efficacy and diet had no effect on absorption. Based on these findings, additional funding was provided for the diagnosis of Parkinson's patients. [90]
7.Immunotherapies targeting ? syn:
Vaccination techniques include attempts to create antibodies that specifically target the N- or C-terminus of ?-syn or its transcript. Phase 2 trials are currently ongoing to evaluate the efficacy of ?-syn mimetic peptides PD01A and PD03A. Early clinical studies in Parkinson's patients and healthy controls (NCT01568099, NCT01885494, NCT02216188, NCT02618941, and NCT02267434) demonstrated safety and tolerability with long-term immunosuppression. The theory behind vaccination is that long-term injections of antibodies can prevent the formation and spread of harmful ?-syn aggregates and modify diseases. While several Phase 1 studies (NCT03716570, NCT03272165, NCT03611569, and NCT04127695) have been completed and others are ongoing, the main problem is that only 0.1% - 0.2% of the vaccine has reached and has reached the central nervous system (CNS). It functions as extracellular diffusion but cannot enter the cell. [92,93,94]
8.Glucocerebrosidase targeting therapies:
A 497 amino acid lysosomal hydroxylase called glucocerebrosidase (GCase) breaks down glucocerebroside into ceramide and glucose. Increased glucocerebroside in the liver, spleen, bone, and bone marrow causes gaucher disease in individuals homozygous for deleterious variants of GBA, a GCase. Its genetics are known, but heterozygotes for this variant are more likely to develop dementia and Parkinson's disease. Additionally, Parkinson's patients carrying GBA mutations have lower survival, faster disease progression, and earlier age of onset. [97,98,99] GCase deficiency causes glucocerebroside accumulation in neurons, leading to glucocerebroside accumulation in neurons. This then leads to the formation of toxic oligomers and a decrease in lysosomal proteolysis, which mainly affects ?-syn.
9.LRRK2 targeting therapies:
LRRK2, also known as leukocyte-rich repeat kinase 2, belongs to the Ras complex (ROC) family of proteins. Mutations in the LRRK2 gene are particularly important in certain groups of people and often cause autosomal dominant Parkinson's disease (PDD). They also occur in patients with Parkinson's disease (PD), often presenting as late-onset Parkinson's disease (PD) with symptoms and levodopa responses similar to idiopathic Parkinson's disease. [100] Gly2019Ser is the most common mutation of LRRK2 and is located in the kinase domain of the protein, causing 1% of PD and 4% of familial PD worldwide. Based on genetic and biochemical evidence, the LRRK2 pathogenic polymorphism (e.g., Gly2019Ser) as well as mutations in the GTPase Roc and COR domains of the protein lead to overexpression of LRRK2 kinase, mediated by gain of activity. [101,102,103]
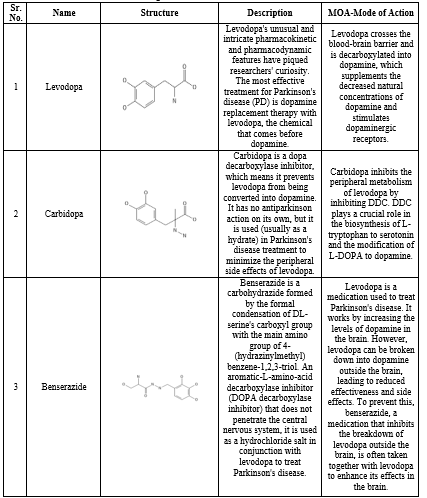
DRUGS USED IN TREATMENT OF PARKINSON DISEASE [104-110]
Table 1. Drugs used in treatment of Parkinson disease


 Siddhant M. Sawant*
Siddhant M. Sawant*
 Prashant J. Burange
Prashant J. Burange
 10.5281/zenodo.11351692
10.5281/zenodo.11351692