Abstract
Background : Several circumstances such as accidents, surgery, traumatic hemorrhagic shock, and other causalities cause major blood loss. Allogenic blood transfusion can be resuscitative for such conditions; however, it has numerous ambivalent effects, including supply shortage, needs for more time, cost for blood grouping, the possibility of spreading an infection, and short shelf-life. Hypoxia or ischemia causes heart failure, neurological problems, and organ damage in many patients. To address this emergent medical need for resuscitation and to treat hypoxic conditions as well as to enhance oxygen transportation , researchers aspire to achieve a robust technology aimed to develop safe and feasible red blood cell substitutes for effective oxygen transport.
Area covered in This review article provides an overview of the formulation, storage, shelf-life, clinical application, side effects, and current perspectives of artificial oxygen carriers (AOCs) as red blood cell substitutes. Moreover, the pre-clinical (in vitro and in vivo) assessments for the evaluation of the efficacy and safety of oxygen transport through AOCs are key considerations in this study. With the most significant technologies, hemoglobin- and perfluorocarbon-based oxygen carriers as well as other modern technologies, such as synthetically produced porphyrin-based AOCs and oxygen-carrying micro\nanobubbles, have also been elucidated.
HISTORY
1. William Harvey discovered blood pathways in 1616 .Many people tried to use fluids such as beer, urine, milk and animal blood as a blood substitute.[1]
2. The first approved oxygen carrying blood substitute was a Per fluorocarbon based product called Fluosol-DA-20 .Manufactured by Green cross of Japan.
It was approved by the food and drug administration in 1989.[2]
3. In 1990’s Haemoglobin based oxygen carriers called Haemopure was approved for phase-3 trail.[3] 4. In December 2003 new Haemoglobin based oxygen therapeutic called Polyheme was introduced.[4,5]
Keywords
RBC, blood, White Blood Cells, hemoglobin.
Introduction
With the phenomenal increase in the number of surgical procedures (both elective and emergency) and trauma cases ,the demand for human blood for transfusion has seen an overwhelming rise. The number of units collected from blood donors is insufficient to cope up with the increasing requirements of human blood which modern day medicine and surgery demands. Additionally, donated human blood is fraught with concerns related to short storage life, possibility of transmission of blood borne infections, allergic reactions and increasing costs of collecting, processing and crossmatching. To mitigate this ever expanding disparity between the demand and supply of blood and the difficulties associated with stored human blood, artificial blood has emerged as a promising option. Artificial blood serves to provide a substitute of conventional blood transfusion where blood or blood products derived from one person is infused into another. The term artificial blood is often used interchangeably with blood surrogate or blood substitutes and all of them are actually misnomers as artificial blood lacks. numerous essential attributes of human blood like haemostatic processes, typing and immunologic defense of the body, nonetheless it serves to carry out the important function of transporting oxygen and carbon dioxide throughout the body. Thus, the appropriate terms for these substances can be Red Blood Cell (RBC) substitutes or Artificial Oxygen Carriers (AOC)[6]
COMPONENTS OF BLOOD
- Red Blood Cells (Erythrocytes)
- White Blood Cells (Leucocytes)
- Platelets (Thrombocytes)
- Haemoglobin
COMPONENTS OF ARTIFICAL BLOOD
1.Perflurocarbons
2.Modified Haemoglobins
3.Plasma Expanders
How to make artificial blood
Artificial blood can be produced in a variety of ways using synthetic production, chemical separation, or recombinant biochemical techniques. Synthetic hemoglobin products are made from hemoglobin collected from strains of E. coli. Hemoglobin is grown and fermented in seed tanks. [13] This artificial blood is used to reflect the function of biological blood as much as possible so that it is accepted by the body as if it were natural. [15] Premium hemoglobin-producing blood substitutes are based on molecular modifications of hemoglobin, which either chemically cross-link the molecules or modify them using recombinant DNA technology. So-called bifunctional drugs can cross-link hemoglobin molecules to form polyhaemoglobin. [12] Special techniques have been developed during surgery to minimize the need for blood transfusions. These techniques included the use of erythropoietin, drug therapy, surgical techniques, and the minimum acceptable levels of hemoglobin and blood substitutes. [8] Unlike red cell and blood substituent can be sterilized by filtration, pasteurization, and chemical cleaning. These steps eliminate the microorganisms that cause diseases such as AIDS and hepatitis. Surrogate does not have cell membranes containing blood group antigens, so no typing or crossover is required before use.
Composition of artificial blood
Perfluro-octyl bromide – 28%
FO-9982 – 12%
Yolk lecithin – 2.4%
DSPE -50 H - 0.12%
Distilled water - 48%
On the other hand, these first generation blood substitutes remain in the body's circulation for only20-30 hours (normal red blood cells last a few days). We use this term because of the long circulation time. It also does not contain the enzymes needed to protect the body from oxidants such as oxygen radicals[12]. The most promising blood products developed as blood substitutes are oxygen transporters based on perfluorocarbons and hemoglobin. PFC is a long chain compound similar to Teflon and has the ability to carry oxygen [16]. Even in the second generation alternative, the hemoglobin molecule is not protected by the red blood cell membrane. Therefore, researchers are working on a more complex third-generation blood substitute that encapsulates the hemoglobin contained in artificial red blood cells and the necessary enzymes [12]
Perfluorochemicals (PFCs)
Perfluorinated chemicals (PFCs) are colorless, non-abrasive, and are clearly water-resistant at low temperatures for fragrance and cannot be washed off with water and alcohol. In addition to particle size, PFCs containing carbon 9-11 should be used as a substitute for abnormal blood to be completely removed from the body after parental control [7] PFC has vertical or cyclic hydrocarbon fibers containing the general chemical structure of CnF2n+2, and the vertical type is oxygen better than the cyclic type. [9] The PFC can cause flu in some patients when 'They move these compounds. [7] The advantage of perfluorocarbons is that they allow increased solubility of oxygen in plasma, PFC does not react with oxygen. PFC allows oxygen to be transported more rapidly into the body and reduces the effects of factors such as pH and blood temperature.[11] perfluorinated chemicals are an inert substance that can break down oxygen 50 times more than blood plasma , a fluid that surrounds red blood cells. Perfluorinated chemicals are inexpensive to produce and are completely free of organic matter. Therefore, there is no risk of contamination from infectious bacteria. [12] Through a chemical process called polymerization, more than two cells combine to form a large HBOC molecule. HBOC is smaller than natural red blood cells. Although naturally occurring red blood cells remain in the bloodstream for up to 100 days, HBOC only circulates in human blood for one day. their ability to support long life.[13] The first (and only) FDA-approved PFC, called Fluosol-DA-20, was manufactured in Japan. [13]PFC stays in the bloodstream for about 48 hours. Because of their ability to break down oxygen, pfcs are the first group of artificial blood products researched by scientists. [7] It contains two pfcs, perfluorodecalin (PFD) and perfluorotripropylamine (FTPA). PFD is the main component in the oxygen carrier, whereas FTPA provides critical stability. Each of the two had a half-life with PFD only 3 to 6 hours due to its rapid elimination. FTPA, on the other hand, remains in the tissue. [13] The desirable characteristics of the second generation PFC include high oxygen depletion, rapid detoxification and minimal tissue depletion, no significant side effects, malaria increased purity, large production and availability.[13]
Hemoglobin-Based Oxygen Carriers (HBOC)
Hemoglobin is obviously a need for blood substitutes that exhibits many of the most desirable traits. [8] HBOC is made from strong hemoglobin and looks a little like real blood. Red blood cells are often used to represent red blood cells from damaged human blood, cow's blood, hemoglobin-producing bacteria, or human placentas. and at the same time seems to be successful. [7] Oxygen containing hemoglobin can be divided into two broad categories; solution of pure hemoglobin and modified hemoglobin. Modified hemoglobin can also be divided into polymerized hemoglobin, hemoglobin binding to intramolecular cross-linked, hemoglobin recombinant, and hemoglobin vesicles. [13] Oxygen-dependent hemoglobin (HBOCs) carry the same molecular protein molecule oxygen as found in the blood. Oxygen binds chemically to the hemoglobin molecule, where it dissolves only in perfluorocarbon emulsion. HBOC is different from red blood cells because hemoglobin is not present in the skin. [13] Overall, these findings suggest that HBOC caused only a small amount of blood loss during and after surgery, there is no improvement in mortality and increased adverse effects. [14] Some products have come close, no HBOC is recommended for hospital use in the United States or Europe. This study focuses on the history and clinical trials of three HBOCs that passed the Phase II or Phase III clinical trials: HemAssist, PolyHeme, and Hemopure. [14] The process is called ?-Hb by at the hands of the US military and the dclhb or hemassist of Baxter. The source of hemoglobin is the red blood cells that humans collect, cleanse, lysed and filter. The product was then deoxygenated, combined with bis (3,5-dibromosalicyl) fumarate and re-oxygenated. One unit of Baxter dclhb was made from 25 g of hemoglobin and 250 ml to give a total of 10 g / dl
Dclhb solution forms P50 of 32 mmhg, colloidal osmotic pressure (COP) of 42 mmhg, as well asmethemoglobin content <5>
PolyHeme, MP4OX (Hemospan), Hemotech, Hemoglobin Engineered.4, None of the HBOCs are approved for clinical use in the United States. As shown in Table 4, all Hb disconnected for storage is discontinued. In a multicenter, randomized, single, small dose infusion of hemoglobin diaspirin-crosslinked hemoglobin
(DCLHb) for 3 days did not affect adverse outcomes in patients with severe stroke rule 1960.
[12]
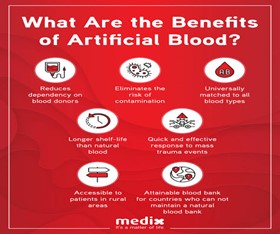
Advantages:
Improper blood flow has many benefits than human blood. Since blood substitutes belong to the same group of O blood, patients can be given regardless of their blood type. compatibility, can be injected through pasteurization. [17] The advantage of PFCs over living blood is that it can be stored for more than a year at room temperature. [17]
Disadvantages
It causes effects on the kidneys, neurotoxicity, platelet aggregation, antigenicity, increase in pancreatic and liver enzymes. They also cause the effects of intestinal obstruction, vasoactivity / hypertension, nephrotoxicity, coagulopathy, and anemia. [20] People who receive a blood transfusion are three times more likely to have a heart attack than those who receive blood transfusions. reduces the half-life distribution, disrupts certain physiological organs, especially the intestinal tract and red blood cells, hemoglobin. PFCs should be prepared as emulsions because they cannot remain in the liquid phase. [20] Side effects of HBOC may include high blood pressure, abdominal discomfort, and temporary redness of the eyes or 2 mouths. platelet count in the blood will decrease and it will cause flu like symptoms
LIMITATIONS OF HBOC’s
Certain factors are needed to be considered before widespread advocacy of HBOC’s. RBC’s do not exert any colloidal osmotic pressure whereas haemoglobin (like other plasma proteins) exerts the same. As a result a cellular haemoglobin can alter the intravascular volume and act as a plasma expander. HBOC’s circulation half life is shorter than normal RBC’s. Majority of HBOC remain in circulation for around 20-30 hours whereas whole blood transfusion lasts 34 days. They release free radicals inside the body from free haemoglobin and the breakdown products like haemandiron. Methaemoglobin concentrations also increase due to the oxidative properties of HBOC’s [19,18, 24, 25]. The first choice for obtaining haemoglobin is outdated human blood which has a limited supply. Thus bovine blood has to be utilized for procurement of haemoglobin. Bovine haemoglobin has the potential of harbouring the prion pathogen responsible for causing bovine spongiform encephalopathy (Creutzfeldt-Jackob disease). To overcome this problem and also toensure a steady supply of haemoglobin in future, genetically engineering bacteria to produce a recombinant source of hu-
man haemoglobin was attempted.Recombinant Haemoglobin (Optro): Recombinant DNA technologies can be utilized to produce modified haemoglo-
bin in organisms like E.coli and yeast. Here, certain segments of the amino acid sequence of human haemoglobin are replaced to prevent disassociation into dimers and maintain oxygen affinity. The haemoglobin gene is then transferred using a plasmid vector into E.coli cells. Expression of these genes leads to the production of haemoglobin proteins. This approach eliminates the concerns related to disease transmission through haemoglobin obtained from human or bovine sources. High costs of this techniques is, however, a major hindrance.
CONCLUSION
The fast improvements in screening strategies of donor blood have decreased the incidences of transfusion associated infections like HIV and hepatitis, however the space be-tween the increasing call for of human blood and the limited deliver continues to widen. moreover, immunological out comes of blood transfusions result in multiplied wound infections ,behind schedule recovery and development of malignant diseases. Even though the present sorts of synthetic blood best serve for oxygen transport, transport and volume expansion, their concutting-edge usage with other blood salvaging strategies can significantly decrease (or keepaway from) the requirement of allogenicblood transfusion in the course of surgical tactics. Hyperoxicair flow in mixture with small boluses of percent’s canmaintain adequate tissue oxygenation intraoperatively insurgical procedures with predicted predominant blood loss [13] The scientificimplications of synthetic blood may be a long way attaining in particularconditions of mass military or civilian casualties (battle, herbalscrew ups) and in areas with shortage of safe blood for transfusion (like South Africa and Nigeria where a big population is HIV inflamed). hence, it will become a robust basis for promoting the development of artificial blood or blood substitutes.The extensive medical utility of these substances is currently impeded by using problems associated with protection, fee, lower intravascular stay instances, sufficient supply of uncooked materials, toxicity and prolonged tissue retention. additionally, modern blood substitutes do not possess immunologic or clotting homes which are essential properties of human blood. therefore, in addition trends inside the area of coaching of synthetic platelets, white blood cells and blood proteins are underway to mitigate this short coming of the available molecules and to make sure that the destiny blood substitutes incorporate the functionality of those elements. development of lyophilized platelets, infusible platelet membranes and fibrinogen coated albumin micro drugs are encouraging strides in that course to decorate the procoagulant effect and effectiveness of present platelets in patients with thrombocytopenia. Upcoming dimensions for further investigations consist of experimentation with stem cells (production of RBC’s of specific and rare groups by the approach referred to as as blood pharming) transgenic haemoglobin (from transgenic pigs to lessen rejection) and polyhaemoglobin enzyme complexes (reduction of ischaemic reperfusion injuries in strokes,myocardial infarctions,
haemmorhagic shock and transplant surgeries) [31].
REFERENCES
- http//curiocity.discovery.com
- http//en.wikipedia.org/wiki/blood. substitute #history
- http//www.euroblood substitute.com/
- A review article on artificial blood by takkar anjali. 8-15.
- A review on artificial blood by Ananddixith Vaddi, Asrar Ahmed Iqbal. International Journal of Pharmaceutical research and Review. 116 & 1366. A review article on artificial blood by Shalini S.International Journal of Pharmacy Practice and Drug Research.
- Kim, H.W.; Greenburg, A.G. Artificial oxygen carriers as red blood cell substitutes: A selected review and current status. Artif. Organs, 2004, 28(9), 813-828.
- Pacific Heart, Lung & Blood Institute, Artificial Blood Substitutes, A 501(C)(3)
- Keyhanian Sh Phd1 ,Ebrahim ifard M Msc, ,Zandi M Msc3, Investigation On Artificial Blood Or Substitute Blood Replace The Natural Blood, Iranian Journal Of Pediatric Hematology Oncology Vol4.No2.
- Samira Moradi, Ali Jahanian-Najafabadi, Mehryar Habibi Roudkenar, Artificial Blood Substitutes: First Steps On The Long Route To Clinical Utility, Clinical Medicine InsightsBlood Disord. 2016; 9: 33–41
- Jiin-Yu Chen, Michelle Scerbo, George Kramer, A Review Of Blood Substitutes: Examining The History, Clinical Trial Results, And Ethics Of Hemoglobin-Based Oxygen Carriers 2009 Aug; 64(8): 803–813
- Mayurya Krishna, Khatoon Ruqsana, Paswan Shravan, Artificial Blood: A Tool For Survival Of Human, International Research Journal Of Pharmacy, 3(5) : 119-123, May 2012.
- Robert M. Winslow,How To Scientists Makes Artificial Blood ? How Effective Is It Compared with Real Things, Octomber 21,1999
- Dr Izzuna Mudla Bt Mohamed Ghazali, Dr Mohd Aminuddin Bin Mohd Yusof , Health Technology Assessment Unit Medical Development Division Ministry Of Health Malaysia 008/07, Journal Of American College Of Surgeons. 2003;196(1): Pg 8.
- Winslow Rm. Hemoglobin-Based Red Cell Substitutes. Baltimore And London: Johns Hopkins University Press; 1992. The Results Of62 Large-Volume Hemoglobin Infusions In Man; Pp. 177–8.
- Trevor Bernier, Biomedical Engineering, University of Rhode Island BME 281 First Presentation, artificial blood, November 26, 2012
- L. Mohankrishna, G.Balammal, G.Aruna, A Review On Artificial Blood, International Journal Of Biopharmaceutics, 2011; 2(2): 80-88
- Krishna Veni R, Brindha Devi P, Ivo Romauld S, A Review On Artificial Blood: A Source We Need, Asian Journal Of Pharmaceutical And Clinical Research, Vol 10, Issue 9, 2017.
- Scott, M.G.; Kucik, D.F.; Goodnough, L.T.; Monk, T.G. Blood substitutes: Evolution and future applications. Clin. Chem., 1997, 43(9), 1724-1731.
- Jahr, J.S.; Nesargi, S.B.; Lewis, K.; Johnson, C. Blood substitutes and oxygen therapeutics: An overview and current status. Am. J. Ther., 2002, 9(5), 437-443.
- Krishna Veni R, Brindha Devi P, Ivo Romauld S, A Review On Artificial Blood: A Source We Need, Asian Journal Of Pharmaceutical And Clinical Research, Vol 10, Issue 9, 2017.
- Shinji Takeoka, Developmental Trend Of Artificial Blood ( Artificial Red Blood Cell), Journal Of The Japan Medical Association ,Vol. 131, No. 7, 2004, Pages 907–910.
- Trevor Bernier, Biomedical Engineering, University of Rhode Island BME 281 First Presentation, artificial blood, November 26, 2012
- L. Mohankrishna, G.Balammal, G.Aruna, A Review On Artificial Blood, International Journal Of Biopharmaceutics, 2011; 2(2): 80-88
- Anbari, K.K.; Garino, J.P.; Mackenzie, C.F. Hemoglobin substitutes. Eur. Spine J., 2004, 13(Suppl. 1), S76-S82.
- Shander, A.; Alalawi, R.; Seeber, P.; Lui, J. Use of a hemoglobin-based oxygen carrier in the treatment of severe anemia. Obstet. Gynecol., 2004, 103(5 Pt 2), 1096-1099.


 Harshal Bhagat*
Harshal Bhagat*
 Shivanand Morde
Shivanand Morde
 Mansi Shinde
Mansi Shinde
 Akshada Pawar
Akshada Pawar
 Amol Nathe
Amol Nathe

 10.5281/zenodo.12919389
10.5281/zenodo.12919389