Abstract
The recent study classe of five-membered heterocyclic compounds is thiazoles. Several thiazoles, both synthetic and natural, and their derivatives exhibited strong biological activity. Thiazole derivatives exhibit strong antibacterial action against a variety of bacterial species & diseases because of their special characteristics. As a result, the current study assigns different thiazoles and there derivatives' antimicrobial activity. Through the use of many databases, every pertinent piece of literature has been examined. The important studies on the antibacterial activity of thiazole derivatives served as the basis for discussion during the review. The thiazole and its near relatives are an important class of heterocyclic compounds whose study has been beneficial to the chemical and biological sciences. They are vital to the pharmaceutical sector because of their special qualities. Considerable antituberculosis, antioxidant, anticancer, and anti-inflammatory properties are demonstrated by thiazole and it’s recently prapared analogues. The current works will support to design & synthesis of diverse biologically active molecular probes, enabling scientists to investigate their antibacterial characteristics against a range of bacteria and disease through a variety of procedures.
Keywords
Thiazole, Anti-Microbial Activity, Pathogens, Resonating Structure, Bacteria, Heterocyclic Compound.
Introduction
In clinical world many bacterial pathogens are currently exhibiting resistance to current antibiotics, considering the possibility of dangerous bacteria's regression towards them is becoming an increasing global issue in the clinical arena.20-25 As a result, gram+ and gram- bacteria illnesses could appear to dying at an increasing rate. The primary causes are excessive population increase and excessive modernization for comfort. Therefore, the quick emergence of microbial resistance contributes to a creation of novel strong antimicrobial pharmacological agents that lower the rate of microbe death26-31 to revolutionize the failure of the synthesis of selective antibacterial drugs; researchers have used many important insights in recent decades. There is a need to create a new effective scaffold for effective control against bacterial viruses. Research on the development of new antimicrobial drugs has mainly focused on two aspects, such as the addition of drugs to potent bacterial antigens and the emergence of new bacterial pathogens. The most important aspect in synthesizing effective drugs is their structural property and speed of action. Initially, the use of a heterocyclic compound as a starting compound to synthesize an effective antibacterial drug was considered. Heterocyclic compounds have been fundamental to the area of medicine, according to an evaluation of the extensive data on antibacterial literature.32-37 The unique regulating qualities of heterocycles, like as its soluble nature, lipophilicity, and polarity, have made them highly attractive in the field of pharmacological. These characteristics have additionally driven repeated research efforts to find the desired active compounds.38-40 The drug development and the creation of new antibacterial for significant biological importance for bacterial infections and the 5-membered aromatic heterocycles under close examination are thiazoles. Previously the thiazole scaffold exhibit a variety of active biological properties, such includes antioxidant, antibacterial, ant-iviral, diuretic, anticancer, & anti-convulsant properties, and extensive investigations published on the thiazole ring over the last twenty to thirty years.41-42 An electron-releasing element (-S-) and an electron-withdrawing element (-N-) are present inside the thiazole. The delocalization of a non-bonded pair of electrons transfer to the –S- atom for fill the unoccupied Six-pi (6p

Thiazole's Resonating Structure
A numerous changes to the thiazole heterocyclic ring could result in the production of a significant medicinal molecule with antibacterial properties for use in human health. Heterocyclic thiazole compounds are widely used in medicine for treating a variety of bacterisidall illnesses or disease in humans since they are naturally occurring.43-44 This kind of biological exploration opens up new avenues for our study in the design and devlopment of novel therapeutic agent’s among potent biological activity. The death rate from bacterial diseases has fallen significantly over the past several years. The need to identify dangerous bacteria that are resistant to antibiotics quickly has led to new research into the synthesis and design of newer thiazole analogues, which have strong antibacterial properties. Because thiazole-containing drugs have a number of applications as therapeutic drugs, the thiazole nucleus is an active nucleusHantzsch and Weber provided the first description of it in 1887. Antimicrobials like myxothiazole (10), sulfathiazole (6), ethaboxam (8), abafungin (7), and ravuconazole (9), as well as antibiotics like penicillin (5), ceftrixone (3), ampicillin (1), cefotaxamine (4), and amoxicillin (2), share the thiazole nucleus. The medical chemists or researchers seem to be interest on the thiazole derivatives, according to a review of the literature.45-47 The cultivation of novel thiazole derivatives with significant structural modifications was aided by all of this knowledge. With the goal to create novel thiazole derivatives with antibacterial potential in different types of cells, this article highlights the impact of a variety of the substituents on heterocyclic thiazole heterocyclic rings by looking at the SAR correlation and MOA of multiple derivatives.
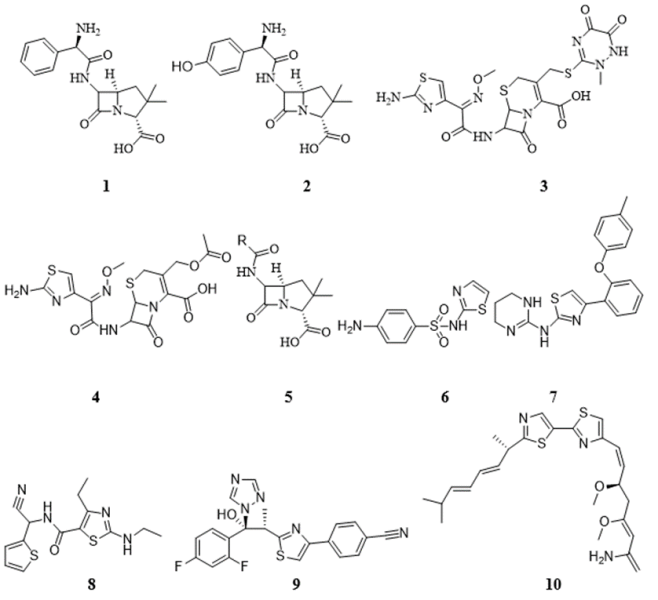
Thiazole Chemistry
A heterocyclic molecule with a five-member ring that has both nitrogen and sulfur heteroatoms across the entire cyclic structure, thiazole derivative is also known as 1,3-thiazole. Its chemical formula is C3H3NS. Sulpur(S) is located at the I-position while Nitrogen (N) is located in the III-position in the 5-membered thiazole structure. In 1920s, the thiazole aromatic property was understood, and the structure of the compound with stable double bonds at positions 4,5 and 2,3 was designed.48

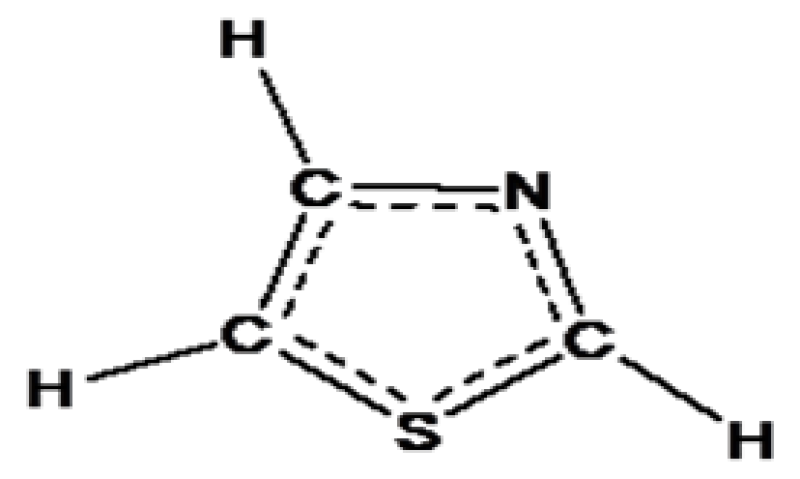

Many diverse biologically active series of chemicals are produced by the thiazole molecule. The thiazole cyclic ring also serves as a part of the structure of the B1-vitamin (thiamine). The chemicals thiazole and imidazole share a structural similarity, with thiazole being a member of the azoles group. It's possible that the thiazole ring structure is planar and aromatic. The field of thiazole investigation has been steadily developing thanks to the original work of Hantsch Bogert and Hofmann & also colleagues invested a great deal of effort to advance this field. Hoffman's discovery of benzothiazole during his studies in 1879 marked the starting point of the thiazole era. The Hantzsch the laboratory has been credited with establishing the existence of thiazole heterocyclic compounds and their derivatives in 1887.20

Physical and Chemical Properties
Reaction of Thiazole
Nucleophilic aromatic substitution reaction often needed leaving group such as Chlorine attached on the position C2 with that the reaction is:

Electrophilic aromatic substitution reaction often requires activating group such group is methyl at C5 followed by bromination:

Oxidation of thiazole at the position of nitrogen atom gives the thiazole-N-Oxide:
General Method For Synthesis Of Thiazole
Synthesis of 5-aryl Thiazoles: The Reaction Proceed from the N-formyl-N-(2-oxo-2-phenylethyl)formaide react with Trimethylamine and Phosphorus Pent-sulfide at require temperature 60 degree centigrade for 45-60 minutes now oDbtain 5-phenylthiazoles.16

Synthesis of 4-Substiuted 2-aminothiazoles: Reaction between potassium thiocynate and vinyl azides with using palladium(III)acetate as an catalyst and require temperature 80oc for the 12 hours after reaction completion the formation of 4-substiuted 2-aminothiazoles.15

Synthesis of 2-aminothiazoles:
The reaction proceeds through the hantzsch condensation method between 2-bromoacetophenones with thiourea and without use of any catalyst to formation of 2-aminothiazoles.17

Synthesis of 2-arylbenzothiazoles:
The operation beginning from the 2-aminothiophenol and aryl aldehyde in air anDd DMSO oxidant system are simple absence of catalyst to form 2-arylbenzothiazoles.

One Pot Three Component Synthesis:
The three component reaction between 1-ido-2-nitroarenes with aldehyde and sodium sulfide in the presence copper as a catalyst to form 2-substiuted benzothiazoles.Reaction proceed at temperature 100oc still for 12 hours.

Synthesis of 2-aryl-4,5-Dihydeothiazole-4-Carboxlic Acid:
The reported product 2-aryl-4,5-dihydrothiazole-4-carboxlic acid obtained through condensation reaction between L-cystine and aryl nitriles time duration for the 24-48 hours. Recemization in a NaHCO3/NaOH buffered aqueous alcoholic medium.

The Hantzsch Thiazole Synthesis:
Formation of N,N,5-trimethyl-4-phenylthiazole-2 amine the reaction between 2-methylpropanethioamide and 2-chloro-1-phenaylpropane-1-one with the use of methyl hydroxide(CH3OH), the reaction proceed time duration for 3 hours to give good yield product.18

Preparation of 2-amino-4-methylthiazole followed by codensation reaction:
The reaction between chloroacetone and thiourea after reaction completion addition of solid sodium hydroxide (NaOH) to form 2-amino-4-methylthiazole.19

Abeer M. El-Naggar et al (2022) has research on an increase in infections in cancer patients brought on by harmful germs has led researchers to focus on the creation of drugs with combined antibacterial and antitumor capabilities. In this study, several kinds of thiazole analogues products are synthesized and screened for antitumor and antibacterial properties. Using elemental and spectroscopic investigations, the newly designed structure of the generated hydrazinyl thiazole derivatives were identified. The MTT test technique was used for the in vitro cytotoxic biological properties of all newly produced compounds 5a-p against all tumor cell lines of colorectal cancer (HCT-116), breast carcinoma (MCF-7) and hepatocellular carcinoma (HePG-2), and was assessed. In comparison to the reference medication Roscovitine(IC50 = 9.32 – 13.82), Structure 5g and 5h demonstrated broad spectrum actions against three cancer cell lines, with values for the IC50 that ranged from 3.81 to 11.34 mM. The most promising substances were screened for their inhibitory biological action against the EGFR and ARO enzymes as well as for their ability to suppress apoptosis and stain with Annexin V/PI.1

Huda K. Mahmoud et al (2020) was made numerous bis-1,3,4-thiadiazoles 7, 10a,b, 13a-c, and bis-thiazoles 16a-d, 19a-d have been produced via the reaction of bis(hydrazonoyl)chloride-4 with different number of compounds of methyl carbodithioate and thiosemicarbazone. And using six designated microbes, their antimicrobial activities were evaluated. Comparing the synthesized derivatives to the reference, some of them demonstrated greater antibacterial activity. Additionally, using the MOE 2014.010 software package, and evaluations have been carried out on the molecular docking of the targeted enzymes' active region with the greatest active substance is 10b.2
10b
Dr. Md. Fakruddin Ali Ahammed et al (2020 were reported the hunt for new, effective drug candidates never stops as research on infections that are resistant to several drugs emerges. The wide range of biological activity that heterocycles are identified for indicates that they are search promising target for discover of novel therapeutic agent. This work aimed to obtain new powerful antibacterial chemicals. This is consistent with the synthesis, characterization, and evaluation of 2,5-Thiophene dicarboxylic acid-2-Thiophenecarboxamide and the complexes it forms (Co (II), Cu (II), Pb (II), Zn (II), and Hg (II)) against bacterial strains. Using the disk diffusion method, the ligand and its complexes were tested against four different bacterial strains. The outcome showed that, when it came to Staphylococcus aureus and Escherichia coli, the Hg (II) complex exhibited greater bioactivity than gentamicin, while the Cu (II) complex was more effective than the Zn (II) complex against bacillus subtilis. The bactericidal activity of the complexes of Co (II), Cu (II), Pb (II), Zn (II), and Hg (II) are potent than those of the freely ligand.3

Hg(II)
Sherif M. H. Sanad et al (2020) are here in reported for the one-pot, three-component methodology used to synthesize new hybrid thiazoles. The process includes halogen-containing reagents reacting with new aldehydes and thiosemicarbazides without the need for a catalyst or solvent. Using elemental analysis and spectroscopic data, the structures of the novel thiazoles were clarified. Assays for the novel thiazoles included both the MurB enzyme inhibition and in-vitro antibacterial screening. As compared to the reference antibiotic drugs compound 6d shows potent activity against S. aureus, S. mutans, and E.coli correspondingly at 8.1 ?M and its IC50 value for the MurB enzyme indicates the maximum level of inhibition. The impact of newly discovered molecules' structures on the potency of their antimicrobial activities was investigated by an analysis of the SAR. Additionally, molecular docking was used to forecast how the novel thiazoles will bind to the E. coli Mur B enzyme's sites of activity.4
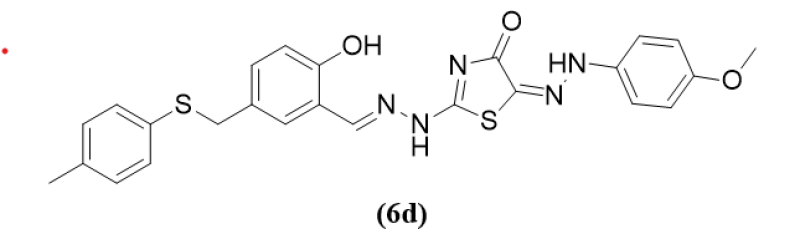
(6d)
Abdelsattar M. Omar et al (2020) has work on Gram+ and Gram- bacterial disease and infections were targeted with a chain of new 2-amino-4-methylthiazole derivatives that were created by a three step reaction. The most successful analogous 4d, showed incredible antimicrobial activity and significantly more selectivity than 100 times that of reference medications with extremely little toxicity to mammalian cells. Cell wall lysis and fast bactericidal action were demonstrated by the 4D biphenyl a component under a microscope, which also disrupted the bacterial membrane. Moreover, an intriguing in vitro study that demonstrated potency in the nanomolar range had been carried out toward GlcN-6-P Synthase Inhibition. This study, however, this is the first to apply a bio molecule making technique for create strong thiazole-carboxamide derivative that function as antimicrobial drugs via targeting GlcN-6-P Synthase. Significantly, a molecular modeling was performed for the potent active compound 4d with the goal to investigate its interaction with glucosamine bonding site, displaying a strong binding propensity this supports in vitro evidence.5
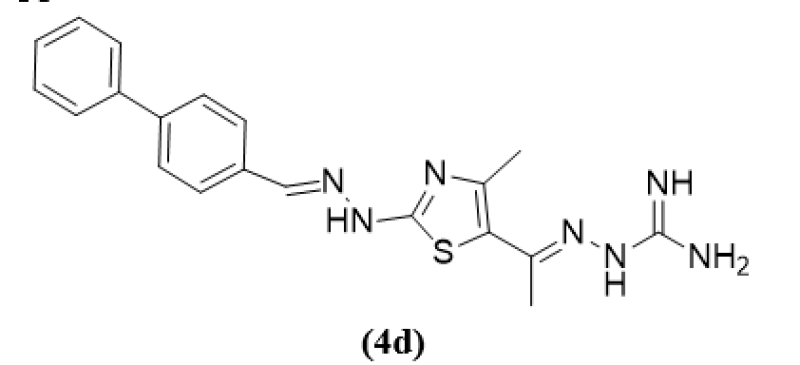
(4d)
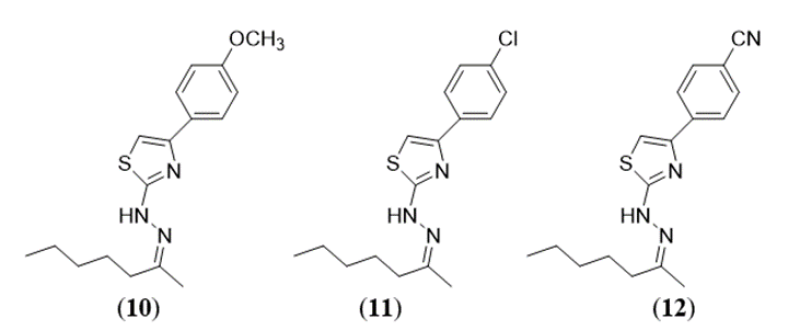
Cleudiomar Inacio Lino et al (2018) are reported in this research for newer antifungal drugs, an exclusive set of 15 hydrazine-thiazole derivatives was created & tested in-vitro against Paracoccidioides brasiliensis, 6 clinically significant species of Cryptococcus and Candida with least inhibitory values ranging between 0.45 to 31.2 ?M, eight compounds, 1-6 and 10-12, demonstrated potential antifungal activity. Some of them were as active as or even extra potent comparison to refrence drug amphotericin B and fluconazole. When the active ingredients were examined in vitro against HEK-293 cells, none of them showed any appreciable cytotoxicity, suggesting that they were very selective. The results of molecular modeling studies that these findings might prove useful in the development of novel antifungal drugs.6
Asaf Evrim Evern et al (2021) were introducing about where the emergence of drug resistance to antimicrobials has become a global concern. Antimicrobial medications used in clinics, most likely every one of them, are expected to shortly be removed from treatment regimens. As a result, scientists are focusing more on creating novel antimicrobial medications. We created and manufactured novel 4-methylthiazole-(benz)azole compounds in order to achieve this. By using spectral and elemental analyses such as 1H-NMR, 13C-NMR, HSQC, NOESY, HMBC, and LC/MS-IT-TOF, the compounds' structures were clarified. They were then evaluated for antibacterial efficacy against strains of microbes and fungus. The findings were combined with the outcomes of molecular docking to explain the structure-activity relationship (SAR). Therefore, SAR indicated that the products might be used in the discovery of new antimicrobial agents that act is due to the allosteric impact, in the creation of novel antimicrobial medicines that be able to be used as gyrase DNA inhibitors; drug 3f out performed the other produced compounds, although its effect was less significant than that of the reference drug. Furthermore, compound 3f exhibits a superior allosteric action, making it a promising lead candidate for the synthesis of novel, high-quality active hits. Furthermore, comparison with the reference medications showed that the potency for all the produced compounds against P. aeruginosa was halved. Conversely, no appreciable distinction was observed between the chemicals used to combat gram+ and gram- bacteria. During this research survey are helpful knowledge was offered regarding the interaction of the SAR with thiazole-(benz)azole the hybridization molecule. Future research endeavors can utilize the aforementioned concepts to create novel chemicals with enhanced antibacterial efficacy, specifically against species that have developed resistance.7

3f
Guda Mallikarjuna Reddy et al (2016) are reported the antibacterial activity of trisubstituted thiazoles was investigated and confirmed by theoretical computations. Furthermore, tests for MIC, MBC, and MFC were conducted. Comprehensive structure-activity connections were examined by SAR analysis. Their antibacterial and fungal assay was as well in actuality, reliably determined through LUMO orbital energy and orbital orientation. Due to its maximum the LUMO energy, the tri-methyl-substituted thiazole molecule out performed the other examined products in terms of antimicrobial activity and low MIC value.8
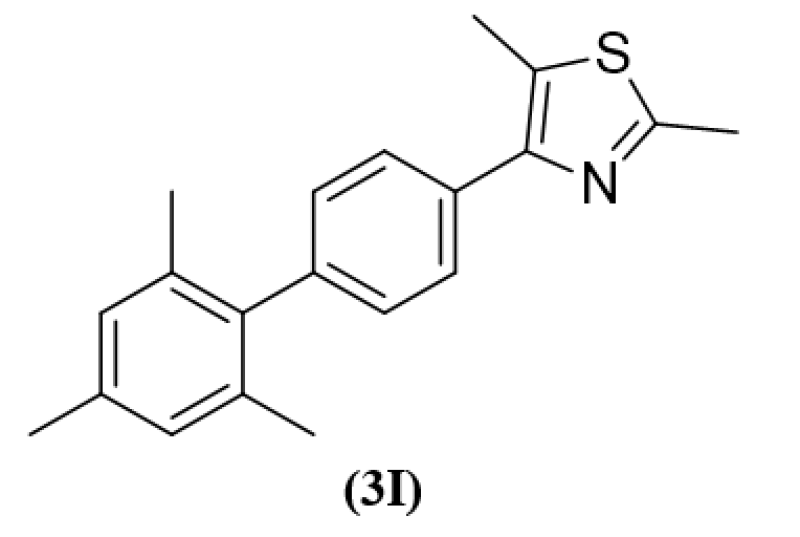
(3I)
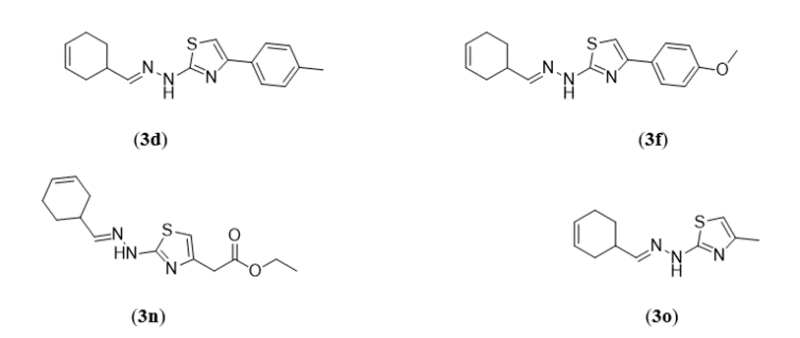
Anna Biernasiuk et al (2019) works with the design, synthesis, charecterization and antimicrobial activity and analysis of fifteen new thiazoles with a cyclohexene group are showcased. Compounds 3a-3d, 3n, 3o and 3f had the strongest efficacy among these derivatives against the reference strains pathogenic Candida species, among minium inbhitory concentration (MIC) = 0.015-3.91?g/ml. These are compounds showas higher activities than those of the positive control drug, nystatin. When it came to the majority of yeast isolated from clinical materials, compounds 3d, 3f, 3n, and 3o had the strongest activity and effect, with Maxium inhibitory concentration values ranging from 0.015-7.81 ?g/ml based on cytotoxicity experiments, the most active drugs suppressed the growth of Candida spp. in the mammalian L929 fibroblast at noncytotoxic concentrations. Furthermore, a strong association was found between the compounds' lipophilicity as assessed by reversed phase TLC and their antibacterial activity.9
Victor Kartsev et al (2022) was introduced the synthesis & estimation of novel hetero-aryl thiazole analogues as antibacterial efficacy. And the structure was predicated by a method of molecular hybridization. The evaluation conducted in vitro showed that few substances exhibited a modest level of antimicrobial activity like compound 3 exhibited the highest level of activities, with MIC and MBC falling within 0.23 and 0.7 and 0.47 and 0.94 mg/mL, correspondingly, and compound 2, 3 & 4 was screend against 3 resistant strains of methicillin-resistant such as S. aureus P. aeruginosa, and E. coli and they all showed a greater possibility than the marketed drug ampicillin. Compound 9 exhibited the highest activity, with maximal inbhitory concentration (MIC) and MFC values of .06– 0.23 & .11–0.47 mg/mL, correspondingly. Molecular docking studies suggest that the compounds' antibacterial action may be attributed to their predict inhibition of the E. coli MurB enzyme, whereas their antifungal activity could be attributed to their inhibition of 14a-lanosterol demethylase.11
Dovile Malukaite et al (2021) are study about the globally, substantial deaths and morbidity occur with rapidly developing antibiotic resistance in clinically important diseases involving bacteria and fungi. Thus, it is imperative to search for novel small compounds that specifically target diseases resistant to several drugs. This work presents the synthesis, antibacterial activity in vitro assessment, and characterization of newly discovered thiazole derivatives containing azole and other herocyclic moieties. While cytotoxicity experiments showed modest cytotoxic of Vero cells the ADME in-silico assessments demonstrated that compounds one to nine (1-9) with least two the Lipinski drug like features. The in vitro activity showed that 2a–c displayed strong and specific bactericidal pharmacological properties against Gram+ strains (MIC 1-64 g/mL), as well as significant activity against S. aureus (MIC 1-2 g/mL) that possessed genetically established resistance mechanisms and only the compound 2a and 5b shows potent activity as per reserch.1
Fitsum Lemilemu et al (2021) was study on the activity of a number of digestive enzymes associated with metabolism can be modulating with reported compounds with a Schiff base based on thiazoles are, which show great therapeutic promise. They also showed signs of antimicrobial, antifungal, anti-inflammatory, antioxidant, and antiproliferative properties. ZnO nanoparticles were used in this article as a catalyst to generating thiazole-based Schiff base compounds utilizing both traditional and environmentally friendly methods. Comparing the synthesized compounds to amoxicillin as (18.00 ± 0.01 mm and 17.00 ± 0.04), 11 of them had good activity against Gram- E. coli (14.40 ± 0.04) and Gram-positive S. aureus (15.00 ± 0.01 mm), respectively.13 This indicates that the compounds have good reactivities.
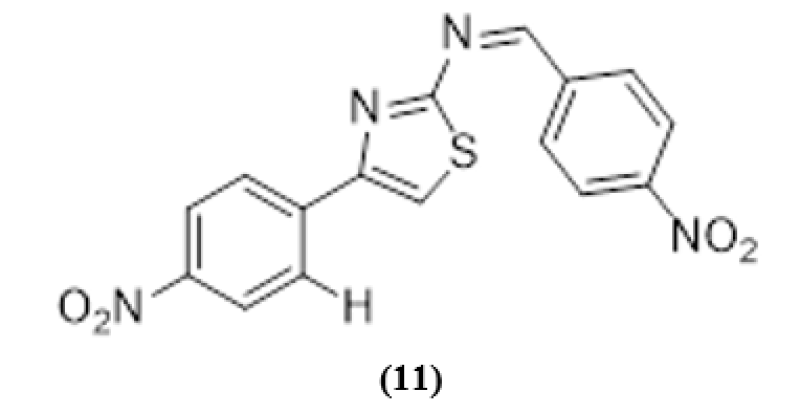
(11)
Mariam T. Sayed et al (2023) was an examination on a novel class of thiazoles produced via Hantzsch thiazole synthesis and connected to the 6-bromocoumarin structure. Therefore compound 1 reacted with hydrazonoyl halogen or halide 2b, 2a in triethylamine & presence of solvent methanol under reflux to generate the compounds 4 & 3, respectively in good yield. Further the structure elucidation of designed derivatives undergoes for elemental analysis and spectroscopic data helped to clarify the structures of the novel derivatives, using the agar diffusion well method, the primary antibacterial properties. The newly synthesise derivatives screend for 6-microorganism S. cerevisiae (ATCC-9763) and C. albicans (ATCC-10231) were employed as fungi, and the chosen pathogenic microbes were B. pumilis (MTCC-2296) and Streptococcus faecalis (MTCC-0459) as Gram + bacteria, Escherichia coli (ATCC-25955) and Enterobacter cloacae (ATCC-23355) as Gram – bacteria of Gram – bacteria. Penicillin G was employed as a typical Gram-positive antibiotic, Ciprofloxacin as a Gram-negative antibiotic, and Ketoconazole as an antifungal medication. The MIC, MBC, and MFC were measured using the broth dilution technique. The results of initial antimicrobial testing that thiazole analouge 3 which has a 5-phenyl azo group and the same IZ 5-position of the thiazole nucleus, was very effective against two Gram+ bacteria with IZs of 20 and 20 mm.14
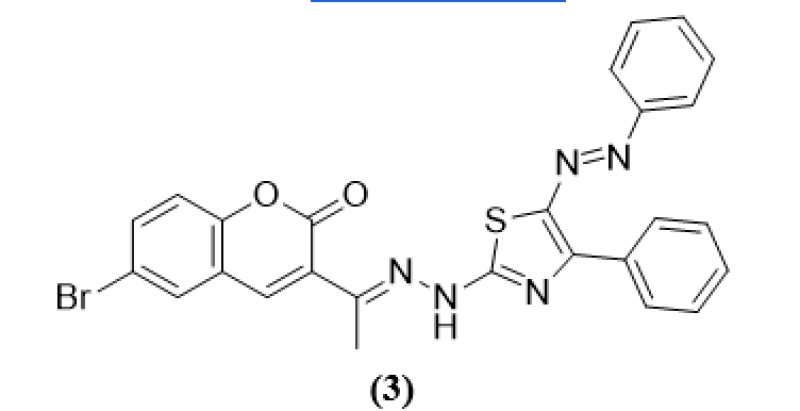
(3)
CONCLUSION
The medicinal Researcher having an appropriate stake in discovering effective and focused medications, Because thiazole derivatives are used as therapeutic agents to manage a variety of bacterial diseases that have effectively achieved a place within the field of pharmacological research. There are several the thiazole molecules undergoing clinical trials which could potentially be created as highly effective antimicrobial agents in the future. An excellent overview of thiazole analouges in the context of microbial medication effect against various microbes is given by this paper. The thiazole derivatives have excellent a heterocyclic constructs with significant antibacterial effects, according to data obtained from the many published articles. Additionally, this review indicates that the thiazole nucleus's ability to improve novel pharmacological therapies for bacterial infections and disorders is growing every day. Accordingly, this investigation is design and development of newer and selective thiazole derivatives based on their recent devlopments in microbial disorders. This article may intriduced an effective strategy for the designing of thiazole-based antimicrobial impactful active medicines. Further evidence showing the growing potential of the thiazole nucleus for developing novel therapeutic therapies for bacterial illnesses and medical disorders comes from the filing of numerous trademarks based on thiazole derivatives.
ACKNOWLEDGEMENT
I would like to express my sincere gratitude to my advisor, Professor Dr. NItin Mittal & co-advisor Professor Dr. Bhumika Yogi, as well as thanks to Professor Dr. Shikha Sharma and all Over supporting faculty of Lords University Alwar Rajasthan and JS Singh Institute of Pharmacy Sitapur for his continuous support of my PhD study and research, for his/her patience, motivation, and immense knowledge. His/Her guidance helped me in all the time of research and writing of this Review Article. I could not have imagined having a better advisor and mentor for my PhD study.
REFERENCE
- Abeer M. El-Naggar, Alaa Zidan, Eslam B. Elkaeed, Mohammed S. Taghour, Waleed A. Badawi, Design, synthesis and docking studies of new hydrazinyl-thiazole derivatives as anticancer and antimicrobial agents Journal of Saudi Chemical Society 26 (2022), 101488,pp-1-14. (https://doi.org/10.1016/j.jscs.2022.101488
- Huda K. Mahmoud, Ashraf A. Abbas & Sobhi M. Gomha, Synthesis, Antimicrobial Evaluation and Molecular Docking of New Functionalized Bis(1,3,4-Thiadiazole) and Bis(Thiazole) Derivatives Polycyclic Aromatic Compounds, 1040-6638(2020),pp-1-14. (https://doi.org/10.1080/10406638.2019.1709085)
- Dr. Md. Fakruddin Ali Ahammed, A. Sravani, K. Rameswari, V. Nagarathna, C.S. Shafiya, P. Samrin Khanam and O. Sravani Synthesis, Characterization And Antimicrobial Activity Of Some Metal Complexes Derived From Thiazole Schiff Base (2, 5-Thiophene Dicarboxylic Acid- 2-Thiophene Carboxylic Amide) World Journal of Pharmaceutical Research, 9(2020)4,800-813,pp-1-14. (Doi: 10.20959/wjpr20204-17021)
- Sherif M. H. Sanad, Ahmed A. M. Ahmed, Ahmed E. M. Mekky, Synthesis, in?vitro and in?silico study of novel thiazoles as potent antibacterial agents and MurB inhibitors Arch Pharm Dphg,10(2019)1002,pp-1-13.(Doi: 10.1002/ardp.201900309)
- Abdelsattar M. Omar, Saleh Ihmaid, EL-Sayed E. Habib, Sultan S. Althagfan, Sahar Ahmed, Hamada S Abulkhair, Hany E. A. AhmedThe Rational Design, Synthesis, and Antimicrobial Investigation of 2-Amino-4-Methylthiazole Analogues Inhibitors of GlcN-6-P Synthase, Journal Pre-proofs Biochemistry,45-2068(2020)30409,pp-1-24. (https://doi.org/10.1016/j.bioorg.2020.103781)
- Cleudiomar Inacio Lino, Igor Goncalves de Souza, Beatriz Martins Borelli, Thelma Tirone Silverio Matos, Iasmin Natalia Santos Teixeira, Jonas Pereira Ramos, Elaine Maria de Souza Fagundes, Philipe de Oliveira Fernandes, Vinicius Goncalves Maltarollo, Susana Johann, Renata Barbosa de Oliveira, Synthesis, molecular modeling studies and evaluation of antifungal activity of a novelseries of thiazole derivatives, European Journal of Medicinal Chemistry, 223-5234(2018)30326-X, pp-1-36. (DOI: 10.1016/j.ejmech.2018.03.083)
- Asaf Evrim Evren, Sam Dawbaa, Demokrat Nuha, Sule Aybuke Yavuz, Ulkuye Dudu Gul, Leyla Yurtta, Design and synthesis of new 4-methylthiazole derivatives: In vitro and in silico studies of antimicrobial activity Journal of Molecular Structure, 1241 (2021) 130692, pp-1-10. (https://doi.org/10.1016/j.molstruc.2021.130692)
- Guda Mallikarjuna Reddy, Jarem Raul Garcia, Vemulapati Hanuman Reddy, Ageo Meier de Andrade, Alexandre Camilo, Junior, Renan Augusto Pontes Ribeiro, SergioRicardo de Lazaro Synthesis, antimicrobial activity and advances in structure-activity relationships (SARs) of novel tri-substituted thiazole derivatives, European Journal of Medicinal Chemistry, S0223-5234(2016)30625-0, pp-1-19. (Doi: 10.1016/j.ejmech.2016.07.062)
- Anna Biernasiuk, Magdalena Kawczynska, Anna Berecka-Rycerz, Beata Rosada, Anna Gumieniczek, Anna Malm, Katarzyna Dzitko, Krzysztof Z. Laczkowski, Synthesis, antimicrobial activity, and determination of the lipophilicity of ((cyclohex-3-enylmethylene)hydrazinyl)thiazole derivatives,Medicinal Chemistry Research 28(2019)2023–2036,pp-1-14. (https://doi.org/10.1007/s00044-019-02433-2)
- Patitapaban Mohanty, Sunita Behera, Rubi Behura, Lipsa Shubhadarshinee, Priyaranjan Mohapatra, Aruna Kumar Barick, Bigyan Ranjan Jali, Antibacterial Activity of Thiazole and its Derivatives, Biointerface Research,12(2022)2,2171 – 2195,pp-1-25. (https://doi.org/10.33263/BRIAC122.21712195)
- Victor Kartsev, Athina Geronikaki, Alexander Zubenko, Anthi Petrou, Marija Ivanov, Jasmina Glamo bclija, Marina Sokovic, Lyudmila Divaeva, Anatolii Morkovnik and Alexander Klimenko, Synthesis and Antimicrobial Activity of New Heteroaryl(aryl)Thiazole Derivatives Molecular Docking Studies, Journal of MDPI,11(2022)1337, pp-1-20. (https://doi.org/10.3390/antibiotics11101337)
- Dovil Mal ukait, Birut Grybait, Rita Vaickelionien, Giedrius Vaickelionis, Birut Sapijanskait, Banevi, Povilas Kavaliauskas, and Vytautas Mickevi cius, Synthesis of Novel Thiazole Derivatives Bearing Amino Acid and Aromatic Moieties as Promising Scaffolds for the Development of New Antibacterial and Antifungal Candidates Targeting Multidrug-Resistant Pathogens, Journal of MDPI, 27(2022)74, pp-1-21. (https://doi.org/10.3390/molecules27010074)
- Fitsum Lemilemu, Mamaru Bitew, Taye B. Demiss, Rajalakshmanan Eswaramoorthy and Milkyas Endale, Synthesis, antibacterial and antioxidant activities of Thiazole?based Schiff base derivatives: a combined experimental and computational study, BMC Chemistry,15(2021)67,pp-1-18. (https://doi.org/10.1186/s13065-021-00791-w)
- Mariam T. Sayed, Salwa A. Elsharabasy & Anhar Abdel?Aziem, Synthesis and antimicrobial activity of new series of thiazoles, pyridines and pyrazoles based on coumarin moiety, Scientific Reports, 13(2023)9912, pp-1-9. (https://doi.org/10.1038/s41598-023-36705-0)
- B. Chen, S. Guo, X. Guo, G. Zhang, Y. Yu. “Selective access to 4-substituted 2-aminothiazoles and 4-substituted 5-thiocyno-2-aminothiazoles Z”, J. Organic. Letters. (2015), 17, 4698-4701. (https://doi.org/10.1021/acs.orglett.5b02152)
- P.W. Sheldrake, Mizio Matteucci, Edward McDonald. ‘Facile Generation of a Library of 5-aryl-2-arylsulfonyl-1,3thiazoles”. J. Synlett. (206), 3, 460-462. (https://doi.org/10.1055/s-2006-926243)
- V. Facchinetti, M. M. Avellar, C. R. B Gomes, T. R. A Vasconcelos, Marcus V. N. de Souza. “An Eco-friendly, Hantzsch-Based, Solvent-Free Approach to 2-Aminothiazoles and 2-Aminoselenazoles”, Georg Thieme Verlag Stuttgart. New York-Synthesis, (2016), 48, pp-437-440. (https://doi.org/10.1055/s-0035-1560534)
- Schwarg, G. Dimethylthiazole. “Organic Syntheses”, A Publication of reliable methods for the prepration of organic compounds.(1945), pp-25-35. (DOI: 10.15227/orgsyn.025.0035)
- A. R Tatchell, A.J Hannaford, P.W.G Smith, B.S Furniss, “Vogel’s, Practical Organic Chemistry” 5th edition. Pearson education, (1989), 1153, 1053, pp-197-236. (https://faculty.ksu.edu.sa/sites/default/files/vogel-practical_organic_chemistry_5th_edition.pdf)
- Mishra, I. Mishra, R. Mujwar, S. Chandra, P. A retrospect on antimicrobial potential of thiazole scaffold. J. Heterocycl. Chem, 57 (2020)2304-2329, pp-1-26. (https://doi.org/10.1002/jhet.3970)
- Jali, B. R. Behura, R. Barik, S. R. Parveen, S. Mohanty, S. P. Das, R. A Brief Review: Biological Implications of Naphthoquinone Derivatives. Res J Pharm Techno, 11(2018), 3698-3702, pp-1-8. (https://doi.org/10.5958/0974-360X.2018.00679.0)
- Leoni, A.; Locatelli, A.; Morigi, R.; Rambaldi, M. Novel thiazole derivatives: a patent review (2008-2012; Part.1). Expert Opinion on Therapeutic Patents, 24(2013)201-216, pp-1-16. (https://doi.org/10.1517/13543776.2014.858121)
- Leoni, A. Locatelli, A. Morigi, R. Rambaldi, M. Novel thiazole derivatives: a patent review (2008-2012. Part 2). Expert Opinion on Therapeutic Patents, 24(2014)759-777,pp-1-19. (https://doi.org/10.1517/13543776.2014.910196)
- Mishra, C. B. Kumari, S. Tiwari, M. Thiazole A promising heterocycle for the development of potent CNS active agents. Europion Journal of Medicinal Chemistry, 92(2015), pp-1-34. (https://doi.org/10.1016/j.ejmech.2014.12.031)
- Jali, B. R. Kuang, Y. Neamati, N. Baruah, J. B. Selective binding of naphthoquinone derivatives to serum albumin proteins and their effects on cytotoxicity. Chem.-Biol. Interact. 214(2014), 10-17, pp-1-8. (https://doi.org/10.1016/j.cbi.2014.01.014)
- Behera, S. Behura, R. Mohanty, M; Dinda, R. Mohanty, P. Verma, A. K. Sahoo, S. K. Jali, B. R. Spectroscopic, cytotoxicity and molecular docking studies on the interaction between 2,4-dinitrophenylhydrazine derived Schiff bases with bovine serum albumin. Sensors International 1(2020), 100048. (https://doi.org/10.1016/j.sintl.2020.100048).
- Pathak, N. Rathi, E. Kumar, N. Kini, S. G. Rao, C. M. A Review on Anticancer Potentials of Benzothiazole Derivatives. Mini-Rev. Med. Chem, 20(2020), 12-23. (https://doi.org/10.2174/1389557519666190617153213).
- Siddiqui, N. Arshad, M. F. Ahsan, W. Alam, M. S. Thiazoles: a valuable insight into the recent advances and biological activities. Int. J. Pharm Sci. Drug Res, 1(2009), 136-143 (https://ijpsdr.com/index.php/ijpsdr/article/view/46).
- Kamal, A. Syed, M. A. H. Mohammed, S. M. Therapeutic potential of benzothiazoles: a patent review (2010-2014). Expert opinion on therapeutic patents, 25(2015), 335-349. (https://doi.org/10.1517/13543776.2014.999764).
- Kashyap, S. J.; Garg, V. K.; Sharma, P. K.; Kumar, N.; Dudhe, R.; Gupta, J. K. Thiazoles: having diverse biological activities. Med Chem Res, 21(2012) 2123-2132. (https://doi.org/10.1007/s00044-011-9685-2).
- Shi, Y.; Zhou, C. H. Synthesis and evaluation of a class of new coumarin triazole derivatives as potential antimicrobial agents. Bioorganic journal of Medicinal Chemistry Letter, 21(2011)956-960. (https://doi.org/10.1016/j.bmcl.2010.12.059)
- Behera, S, Behura, R, Mohanty, M, Sahoo, M, Ramakrishna, D. S, Jali, B. R. Study of Interaction Between Bovine Serum Albumin and Dolutegravir Intermediate: Fluorescence and Molecular Docking Analysis. Biointerface Research Applied Chemmistry, 11(2021)13102-13110. (https://doi.org/10.33263/BRIAC115.1310213110).
- Singh, I. P. Gupta, S. Kumar, S. Thiazole Compounds as Antiviral Agents: An Update. Med Chem 16(2020), pp-4-23. (https://doi.org/10.2174/1573406415666190614101253).
- Zhang L, Liu X. Lu, S. Liu, J. Zhong, S. Wei, Y. Bing, T. Zhang, N. Shangguan, D. Thiazole Orange Styryl Derivatives as Fluorescent Probes for G-Quadruplex DNA. ACS Appl. Bio Mater, 3(2020)2643-2650. (https://doi.org/10.1021/acsabm.9b01243).
- Soliman, N. N, Salam, M. A. EI, Fadda, A. A, Motaal, M. A. Synthesis, Characterization, and Biochemical Impacts of Some New Bioactive Sulfonamide Thiazole Derivatives as Potential Insecticidal Agents against the Cotton Leafworm, Spodoptera littoralis. J. Agric. Food Chem. 68(2020) 5790-5805. (https://doi.org/10.1021/acs.jafc.9b06394).
- Li. Y. Sun, N Ser, H.L. Long. W, Li Y. Chen, C. Zheng, B. Huang, X. Liu, Z. Lu. Y.J., Antibacterial activity evaluation and mode of action study of novel thiazole-quinolinium derivatives. RSC Adv. 10(2020), 15000-15014. (https://doi.org/10.1039/D0RA00691B).
- Sharma P, C. Bansal, K. K. Sharma, A Sharma, D. D. Aakash, Thiazole-containing compounds as therapeutic targets for cancer therapy. Eur. J. Med. Chem 188(2020), 112016. (https://doi.org/10.1016/j.ejmech.2019.112016).
- Sayed, A. R. Gomha, S. M. Taher, E. A. Muhammad, Z. A. El-Seedi, H. R. Gaber, H. M. Ahmed, M. M. One-Pot Synthesis of Novel Thiazoles as Potential Anticancer Agents. Drug Des Devel Ther, 14(2020), 1363-1375. (https://doi.org/10.2147/DDDT.S221263).
- Yu, B. Zhou, S. Cao, L. Hao, Z. Yang, D. Guo, X. Zhang, N. Bakulev, V. A. Fan, Z. Design, Synthesis, and Evaluation of the Antifungal Activity of Novel Pyrazole-Thiazole Carboxamides as Succinate Dehydrogenase Inhibitors. J. Agric. Food Chem, 68(2020), 27, 7093-7102. (https://doi.org/10.1021/acs.jafc.0c00062).
- Mohammad, H. Eldesouky, H. E. Hazbun, T. Mayhoub, A. S. Sellemm, M. N. Identification of a Phenylthiazole Small Molecule with Dual Antifungal and Antibiofilm Activity Against Candida albicans and Candida auris. Sci Rep 9(2019), 18941. (https://doi.org/10.1038/s41598-019-55379-1).
- Biernasiuk, A. Kawczy?ska, M. Berecka-Rycerz, A. Rosada, B. Gumieniczek, A. Malm, A. Dzitko, K. Laczkowski, K. Z. Synthesis, antimicrobial activity, and determination of the lipophilicity of ((cyclohex-3-enylmethylene)hydrazinyl)thiazole derivatives. Med Chem Res 28(2019), 2023-2036. (https://doi.org/10.1007/s00044-019-02433-2).
- Adole, V. A. More, R. A. Jagdale, B. S. Pawar, T. B. Chobe, S. S. Efficient Synthesis, Antibacterial, Antifungal, Antioxidant and Cytotoxicity Study of 2?(2?Hydrazineyl)thiazole Derivatives. Chemistry Select 5(2020), 2778-2786. (https://doi.org/10.1002/slct.201904609).
- Pricopie, A.-I. Focsan, M. Ionut, I. Marc, G. Vlase, L. Gaina, L.-I. Vodnar, D. C. Simon, E. Barta, G. Pirnau, A. Oniga, O. Novel 2,4-Disubstituted-1,3-Thiazole Derivatives: Synthesis, Anti-Candida Activity Evaluation and Interaction with Bovine Serum Albumine. Molecules 25(2020), 1079. (https://doi.org/10.3390/molecules25051079
- Kaddouri, Y. Abrigach, F. Yousfi, E. B. Kodadi, M. E. Touzani, R. New thiazole, pyridine and pyrazole derivatives as antioxidant candidates: synthesis, DFT calculations and molecular docking study. Heliyon, 6(2020), e03185. (https://doi.org/10.1016/j.heliyon. 2020.e03185).
- Hossan, A. S. M. Synthesis, modelling and molecular docking of new 5-arylazo-2-chloroacetamido thiazole derivatives as antioxidant agent. J. Mol. Struct, 1206(2020), 127712. (https://doi.org/10.1016/j.molstruc.2020.127712).
- Muluk, M. B. Patil, P. S. Kasare, S. L. Kulkarni, R. S. Dixit, P. P. Choudhari, P. B. Haval, K. P. Synthesis and molecular docking studies of novel pyridine-thiazole-hydrazone conjugates as antimicrobial and antioxidant agents. Eur. Chem. Bull, 9(2020), 184-192. (http://dx.doi.org/10.17628/ecb.2020.9.184-192).
- Ramalingam, A.; Sarvanan, J. Synthesis, Docking and Anti-cancerous Activity of Some Novel Thiazole Derivatives of Biological Interest. Int. J. Pharm. Investigation, 10(2020), 594-603. (http://www.jpionline.org/index.php/ijpi/article/view/815/520).
- Ram Sevak Verma, Shobhit Srivastava, Bhumika Yogi and Sujeet Kumar Gupta, synthesis and anticonvulsant activity of 2-(4-methylthiazole-2-ylamino)-1-(substitutedphenyl)-3-phenylpropane-1-one derivatives, World Journal of Pharmaceutical and Research, 10(2021)5,1345-1353, pp-1-9. (DOI: 10.20959/wjpps20215-18885).


 Ram Sevak Verma *
Ram Sevak Verma *
 Nitin Mittal 2
Nitin Mittal 2





























 10.5281/zenodo.10893122
10.5281/zenodo.10893122