Abstract
Itraconazole and Terbinafine are widely used antifungal agents with diverse applications in the pharmaceutical industry. The simultaneous estimation of these two drugs in bulk form and dosage formulations is essential for quality control and regulatory compliance. This review comprehensively evaluates various analytical methods reported in the literature for the simultaneous estimation of Itraconazole and Terbinafine. The review highlights the significance of method development in achieving accurate and precise quantification of these drugs. Different analytical techniques such as spectrophotometry, chromatography (high-performance liquid chromatography, ultra-performance liquid chromatography), and capillary electrophoresis have been explored for this purpose. Additionally, various sample preparation techniques including liquid-liquid extraction, solid-phase extraction, and derivatization have been employed to enhance sensitivity and selectivity. Additionally, recent advancements in analytical instrumentation and method optimization strategies are discussed, offering insights into the future direction of method development for simultaneous estimation of Itraconazole and Terbinafine. Overall, this review serves as a valuable resource for researchers and analysts involved in pharmaceutical analysis, providing guidance for the selection and optimization of suitable methods for the simultaneous determination of these important antifungal agents.
Keywords
Itraconazole, Terbinafine, RP-HPLC, UV Spectroscopy, Force Degradation Method Development, Quality by Design, DOE
Introduction
Itraconazole and Terbinafine are widely used antifungal agents with diverse applications in the pharmaceutical industry. The simultaneous estimation of these two drugs in bulk form and dosage formulations is essential for quality control and regulatory compliance. This review comprehensively evaluates various analytical methods reported in the literature for the simultaneous estimation of Itraconazole and Terbinafine. The review highlights the significance of method development in achieving accurate and precise quantification of these drugs. Different analytical techniques such as spectrophotometry, chromatography (high-performance liquid chromatography, ultra-performance liquid chromatography), and capillary electrophoresis have been explored for this purpose. Additionally, various sample preparation techniques including liquid-liquid extraction, solid-phase extraction, and derivatization have been employed to enhance sensitivity and selectivity. Additionally, recent advancements in analytical instrumentation and method optimization strategies are discussed, offering insights into the future direction of method development for simultaneous estimation of Itraconazole and Terbinafine. Overall, this review serves as a valuable resource for researchers and analysts involved in pharmaceutical analysis, providing guidance for the selection and optimization of suitable methods for the simultaneous determination of these important antifungal agents.
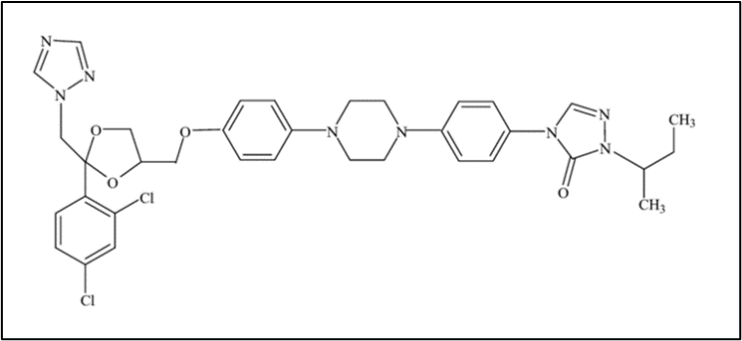
Figure 1. Chemical Structure of Itraconazole.

Figure 2. Chemical Structure of Terbinafine.
DRUG PROFILE
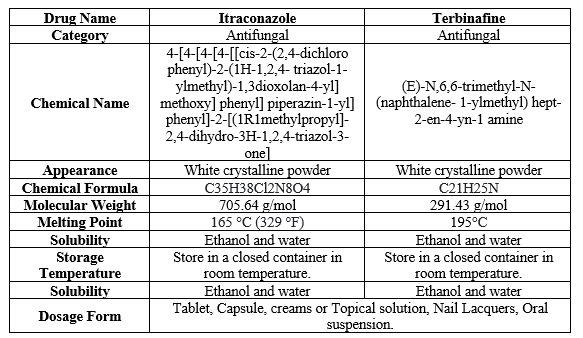
Table 1: Drug Profile of Itraconazole and Terbinafine.
METHOD DEVELOPMENT AND LITERATURE STUDY IN BULK AND PHARMACEUTICAL DOSAGE FORM:
1. Reverse Phase High-Performance Liquid Chromatography:
Reverse Phase High-Performance Liquid Chromatography is a term that is commonly used to describe liquid chromatography, which consists of a liquid mobile phase that is mechanically pumped through a stationary phase-containing column. An HPLC system consists of an injector, a pump, a column, and a detector [19] . The pump is in charge of regulating the flow of solvent through the system. After leaving the pump, the solvent passes through the injector, then through the section, and then through the optical unit of a detector HPLC columns are made up of spherical silica gel beads coated with the hydrophobic stationary phase and packed into the column. C4 (butyl), C8 (octyl), C18 (octadecyl), phenyl (phenylpropyl), and nitrile (cyanopropyl) columns are common stationary phases [20-24]
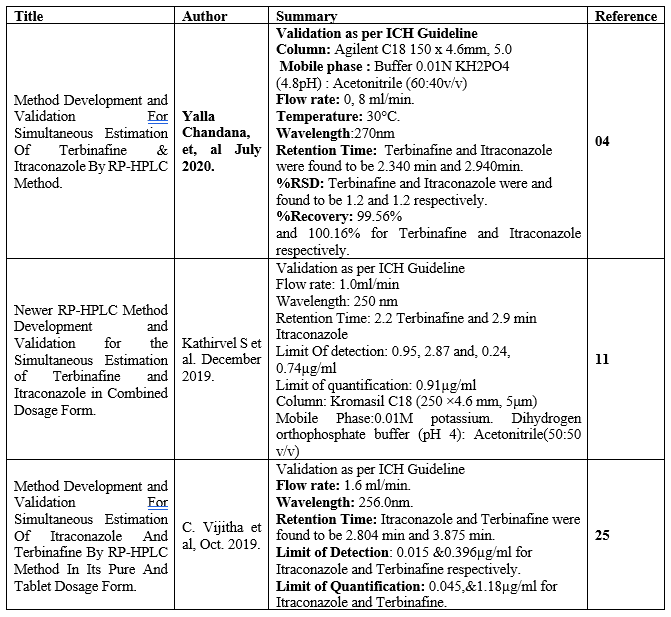
Table 2 – Summary of RP- HPLC method for determination of Itraconazole and Terbinafine:
UV Spectrophotometric Method:
The primary idea behind UV spectroscopy states that the excitation of electrons in individual atoms and molecules from lower to higher energy levels is responsible for the absorption of visible and ultraviolet light, or light in the 200–400 nm range [27]. The concentration of the absorbing species in the solution and the path length are proportional to the absorbance of a solution, as per the Beer-Lambert law [28].
Principle: Absorption in the visible or ultraviolet range happens when radiation causes an electronic change within the structure of a molecule or particle. Consequently, the electronic state of the molecules within a sample changes when it absorbs light in the visible or ultraviolet spectrum. Electrons can be promoted from their ground state orbitals to higher orbitals by energy[29].
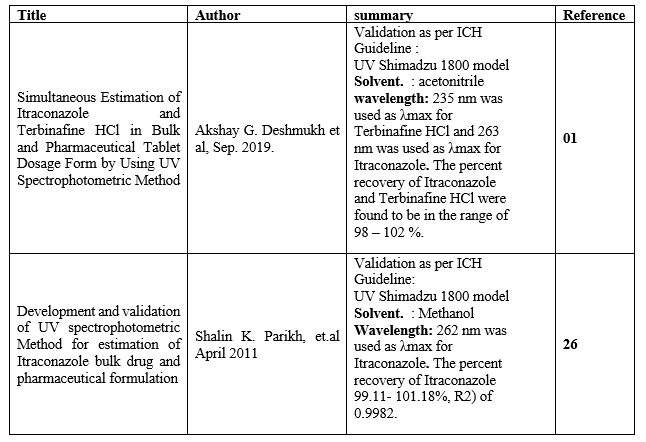
Table 3 – Summary of UV Spectrophotometric method for Itraconazole and Terbinafine:
Stability-Indicating Method Development And Validation :
The stability-indicating assay is a verified quantitative technique that aids in the examination of sample stability in the pharmaceutical industry and can identify changes over time by examining the characteristics of drug ingredients and medicinal products. As to the ICH, the aim of a stability-indicating assay method is to precisely measure the intact drug or medications along with any additional components or excipients and breakdown products. To ensure that there is no peak overlap between the excipients, degradants, and the active medication, it is ideal for every component in the formulation to be present [30,31-33].

Table 4– Summary of stability Indicating method for Itraconazole and Terbinafine:
CONCLUSION:
The USFDA approved Itraconazole and Terbinafine in 1992.As a result of the above information, it can be concluded that the numerous analytical procedures used to determine simultaneous estimation of Itraconazole and Terbinafine alone or in combination have been successfully utilized on a routine basis, allowing the drug to be quantified in various pharmaceutical dosage forms. These procedures are all simple, fast, accurate, sensitive, and reproducible, with high linearity and precision.
ACKNOWLEDGMENT:
I would like to express my sincere thanks to Mrs.Hemlata Bhawar Mam and Dr. Santosh Dighe for their valuable guidance and support for this review work.
REFERENCES:
- Akshay G. Deshmukh, “Simultaneous Estimation of Itraconazole and Terbinafine HCl in Bulk and Pharmaceutical Tablet Dosage Form by Using UV Spectrophotometric Method. Citation: Akshay G. Deshmukh. Ijppr.Human, 2019; Vol. 16 (2): 265-277.
- Vankayalapati Manjusha*1 , P. Sreenivasa Prasanna2 and K. Thejomoorthy3, Stability Indicating Method Development And Validation For The Estimation Of Terbinafine And Itraconazole In Api And Tablet Dosage Form By Rp-HPLC, wjpmr, 2021,7(6), 322-332.
- Kesharaju Shivaranjani, Priyanka Bikkasani, Titus Darsi, Damerakonda Kumaraswamy, Stability Indicating Rp-HPLC Method For Estimation Of Itraconazole And Terbinafine In Bulk And Tablet Dosage Forms, Citation: Kathirvel S et al. Ijppr.Human, 2019; Vol. 17 (1): 232-244.
- Yalla Chandana*, Dr. Devanaboyina Narendra, P. Venkata Kishore and Gadi Vijaya Lakshmi, Method Development And Validation For Simultaneous Estimation Of Terbinafine & Itraconazole By Rp-HPLC Method, wjpmr, 2020,6(8), 226-231.
- DEVYANI M RODE, Dr. NUTAN RAO Stability-Indicating Method Development And Validation Of Itraconazole And Terbinafine Hcl In Bulk And Pharmaceutical Tablet Dosage Form , Asian J Pharm Clin Res, Vol 12, Issue 9, 2019, 51-55.
- Analytical Quality-by-Design Approach to Stability Indicating RP-HPLC Method Development and Validation for Estimation of Favipiravir in Bulk and Formulation, Namita A. Joshi1*, Dr. Sunil V. Amrutkar2, Dr. Chandrasekhar D. Upasani3, Eur. Chem. Bull. 2023, 12(Issue 9), 118 – 133.
- Quality by Design Approach (QbD) for the Simultaneous Determination of Anti-Hypertensive Drugs (Candesartan, Irbesartan and Hydrochlorothiazide) by RP-HPLC, J Anal Pharm Res 2017, 4(5): 00118.
- Implementation of QbD Approach to the Analytical Method Development and Validation for the Estimation of Propafenone Hydrochloride in Tablet Dosage Vankayalapati Manjusha*1 , P. Sreenivasa Prasanna2 and K. Thejomoorthy3, Stability Indicating Method Development And Validation For The Estimation Of Terbinafine And Itraconazole In Api And Tablet Dosage Form By Rp-HPLC, wjpmr, 2021,7(6), 322-332.Form, Monika L. Jadhav and Santosh R. Tambe, Hindawi Publishing Corporation Chromatography Research International Volume 2013, Article ID 676501, 9 pageshttp://dx.doi.org/10.1155/2013/676501
- Ramalingam, P., & Jahnavi, B. (2019). QbD considerations for analytical development. In Pharmaceutical Quality by Design (pp. 77-108). Academic Press. https://doi.org/10.1016/B978-0-12-815799-2.00005-8
- An efficient RP-HPLC-PDA Method for Estimating Dolutegravir and Lamivudine in Combined Pharmaceutical Formulations using a Box Behnken Design Approach ,A. Ramyasree, S. Umadevi, *Author for Correspondence: umadevi.sps@velsuniv.ac.in
- Kathirvel S , Gayatri Ramya M , Rajesh Akki,Prem Kumar , “Newer RP-HPLC Method Development and Validation for the Simultaneous Estimation of Terbinafine and Itraconazole in Combined Dosage Form” Human Journals Research Article December 2019 Vol.:17, Issue:1 232-244.
- Guideline, I. H. T. (2009) Pharmaceutical development Q8 (R2). Current step, 4. https://database.ich.org/sites/default/files/Q8(R2) Guideline.pdf
- 13. 14. Guideline, I. H. T. (2005). Validation of analytical procedures: text and methodology. Q2(R1),1(20),05.https://database.ich.org/sites/default/files/Q2(R1) Guideline.pdf
- Nagar, P., Garg, M., Chauhan, C., Kumar, R., & Chaudhary, A. K. (2022). Analytical Quality by Design (AQBD) Approach for HPLC Method Development, Method Optimization and Validation. Quality Assurance, 13(2), 103-110. DOI: 10.25258/ijpqa.13.2.2
- Haque, M.A.; Bakshi, V.; Thippani, M.; Manda, R.M.; Boggula, N. Development and Validation of UV Spectrophotometric method for the determination of Dolutegravir by using Quality by Design (QbD) Approach. J. Adv. Sci. Res. 2021;12(1):113–119.
- Kumar, L. (2023). Quality-by-design driven analytical method (AQbD) development and validation of HPLC-UV technique to quantify rivastigmine hydrogen tartrate in lipidic nanocarriers: Forced degradation, and assessment of drug content and in vitro release studies. Microchemical Journal, 108944. https://doi.org/10.1016/j.microc.2023.108944
- Urmi, KF, Nawaz M, Islam SM (2022) Analytical quality by design approach to RPHPLC method development and validation for simultaneous estimation of esomeprazole and naproxen in modified-release dosage form. Future Journal of Pharmaceutical Sciences, 8(1), 1-16. https://doi.org/10.1186/s43094-021-00396
- Haque, M.A.; Bakshi, V.; Thippani, M.; Manda, R.M.; Boggula, N. Development and Validation of UV Spectrophotometric method for the determination of Dolutegravir by using Quality by Design (QbD) Approach. J. Adv. Sci. Res. 2021;12(1):113–119.
- PUSHPA D GOSWAMI*, stability-indicating rp-hplc method for analysis of terbinafine Hydrochloride in bulk and in tablet dosage form, Int J Pharm Pharm Sci, Vol 5, Suppl 3, 536-540.
- Andrea Weston and Phyllis R. Brown, HPLC and CE Principles and Practice, academic press, 1997, 01.
- Siddiqui, Masoom Raza, Zeid A. AlOthman, and Nafisur Rahman. "Analytical techniques in pharmaceutical analysis: A review." Arabian Journal of chemistry 10 (2017): S1409-S1421.
- Columns. From http://www.waters.com/watersdivision/pdf/Ic3AC.pdf.Accessed April 05, 2013.
- Wagaw S, Tedrow J, Grieme T, Bavda L, Wang W, Viswanath S et al. HPLC Guide; Department R450, R452, R45R.
- Weston A, Brown PR, HPLC and CE Principles and Practise, Academic press, California, 1997.
- C. Vijitha1, P Vivek Sagar, T Ravi Chander, Method Development And Validation For Simultaneous Estimation Of Itraconazole And Terbinafine By Rp-HPLC Method In Its Pure And Tablet Dosage Form, J Sci Res Pharm, 2019; 8(11): 99-102.
- Shalin K. Parikh, Ankit D. Patel, Dr. J. B. Dave, Dr. C. N. Patel and Dr. D. J. Sen,development and validation of uv spectrophotometric Method for estimation of itraconazole bulk drug and Pharmaceutical formulation, Int. J. Drug Dev. & Res., April-June 2011, 3 (2): 324-328.
- Bakshi M, Singh S. Development of Validated StabilityIndicating Assay Methods: Critical Review. J. Pharm. Biomed. Anal 2002; 28: 1011–1040.
- Cione AP, Tonhi E, Silva P. Stability Indicating Methods. Bioagri Laboratories, Quality Control of Herbal Medicines and Related Areas 25-36.
- Allen LV. Beyond-Use Dates and Stability Indicating Assay Methods in Pharmaceutical Compounding. ACPE 15.
- Allen LV. Beyond-Use Dates and Stability Indicating Assay Methods in Pharmaceutical Compounding. ACPE 15.
- Azhlwar S, Kochupappy RT. Stability indicating HPLC method for simultaneous determination of Drotaverine and aceclofenac. Int J Pharm Pharm Sci 2011; 3(1): 245-250.
- Mahajan MP, Sawant SD. Stability indicating RP-HPLC method for the estimation of zolpidem tartrate in bulk and tablet dosage form. Int J Pharm Pharm Sci 2012; 4(5): 268-274.
- Laha TK, Padhy DR, Sen S. A validated stability indicating reversed phase high performance liquid Chromatographic method of ranolazine dihydrochloride and Characterization of its degradation products. Int J Pharm Pharm Sci 2013; 5(1): 61- 66.


 Vanita B. Katore*
Vanita B. Katore*
 Hemlata S.Bhawar
Hemlata S.Bhawar





 10.5281/zenodo.10998827
10.5281/zenodo.10998827