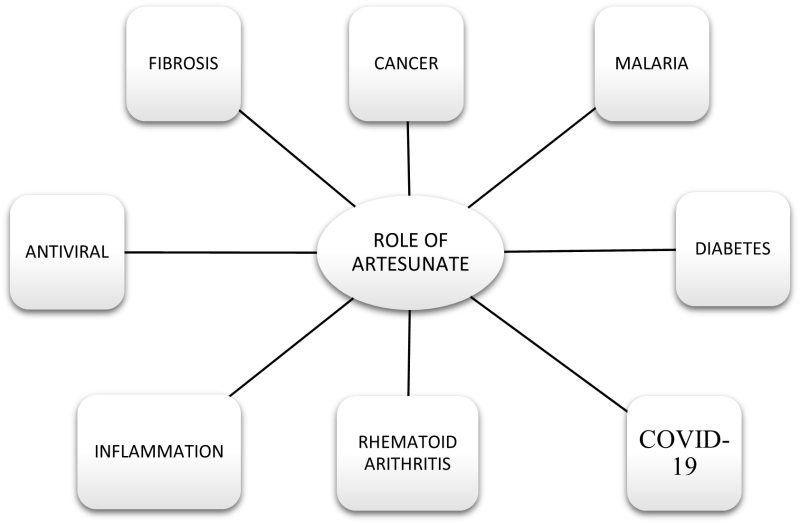
Artesunate is a derivative of Artemisinin . Artemisinin is extracted from the Artemisia annua plant. Artemisinin and its derivatives have been the most useful antimalarials in human history. Artesunate has the advantage of having a hydrophilic group compared to other Artemisinin , making it a more potent drug .On an industrial scale, Artemisinin is synthesized semi synthetically. The 1,2,4-endoperoxide bridge of Artemisinin is responsible for the antimalarial activity of the drug. Resistance to Artemisinin s has been demonstrated in Plasmodium falciparum, primarily p. There is evidence that it is caused by a mutation in the kelch13 protein. Tropical plants. Data from clinical trials suggest that Artesunate is more effective than quinine and other Artemisinin s in treating patients with severe malaria. There is evidence that Artemisinin could be developed as an anti-cancer agent. The mechanism of action of Artemisinin as an anticancer agent includes oxidative stress, DNA damage and repair, and various types of cell death. Artesunate has a wide range of therapeutic effects. It is mainly used as an antimalarial agent. It is used to treat cancer, diabetes, autoimmune diseases, atherosclerosis and infection with the COVID-19 virus. And systemic lupus erythematosus.
Artesunate , Artemisinin , cancer , Malaria , Diabetes
3. The main function of Artesunate
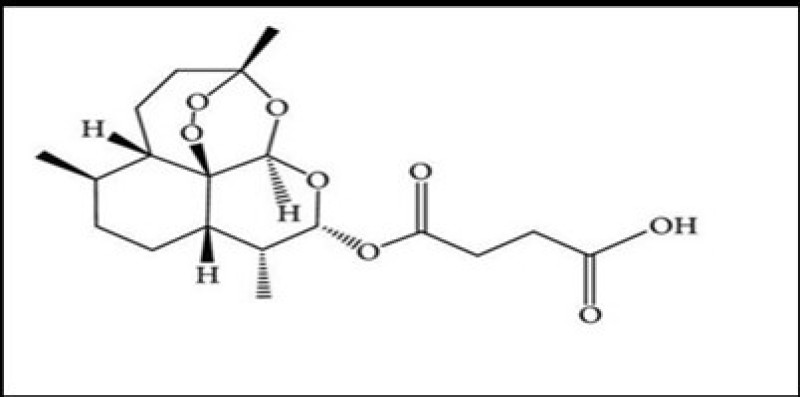
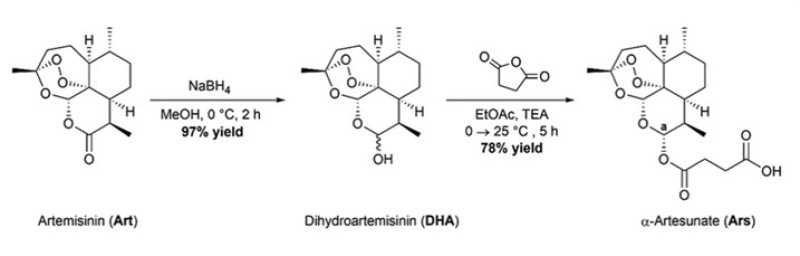
Artesunate is prepared by reacting DHA with succinic anhydride in basic medium. Like Artemisinin , it has four main functions: anti-parasitic, anti-tumour, anti-inflammatory and antibacterial functions. Among these four, the anti-parasitic function is the most classic and important role of Artesunate due to its killing effect on Plasmodium falciparum. Malaria remains a major cause of morbidity and mortality in children and adults in malaria-endemic countries. And combating it requires a comprehensive approach, including prevention and prompt treatment with effective antimalarials. The third edition of the Malaria Guidelines emphasizes the importance of intravenous or intramuscular Artesunate as the recommended treatment for adults and children (including infants, pregnant and lactating women) with severe malaria. Against malaria. The antimalarial mechanism of Artesunate works mainly by completely inhibiting the activity of Plasmodium cytochrome oxidase and promoting the formation of the active substance [10, 11]. Also, schistosomiasis is considered the second most devastating parasitic disease from a socioeconomic point of view (after malaria). In 2014, more than 61.6 million people were treated for schistosomiasis . Extensive in vivo and in vitro studies have confirmed that Artesunate is effectively effective against s. Japanese mansion. These findings open up new options for treating schistosomiasis. Artesunate is also effective against myofascial hepatitis, clonorchis sinensis, babesiosis, leishmaniasis, and Toxoplasma gondii. It is also effective against other parasites such as like fasciola hepatica, clonorchis sinensis, babesia, leishmaniasis and toxoplasma gondii, and so forth which fully illustrated that Artesunate had broad antiparasitic effects
4. Pharmacokinetic activity of Artesunate
Pharmacokinetic studies in the field primarily use plasma samples, some using whole blood, serum, saliva or other biological samples. This technology has excellent physical and chemical properties and can be formulated into injections, suppositories, tablets, liposomal microcapsules, intravenous, intramuscular, oral, rectal and other dosage forms. After oral administration, the tablets must disintegrate, dissolve and be absorbed in the gastrointestinal tract.[6]The main site of absorption is the small intestine. Some ART work undergoes chemical hydrolysis by acids in the intestines to form DHA, which is then absorbed into the bloodstream. The unchanged portion enters the liver directly through the portal vein, and some is converted to DHA by hepatic metabolism. When drugs are measured directly in the blood, the proportion of drug absorbed into the blood is small and bioavailability is also low. Assessing the bioavailability of a work of ART by measuring DHA in the blood indicates high bioavailability. The half-life of ART in major tissues and organs is not the same. Bone marrow and spleen have the longest half-lives, approximately 140 to 222 hours, followed by lungs (105 to 123 hours), he ART (91 to 109 hours), muscle (96 to 106 hours) and kidney (94 to 106 hours). 102 hours) , blood (82-99 hours) . Plasma protein binding rates of prototype ATR or its active metabolite DHA were higher after administration by different routes of administration. In human and rat plasma, the plasma protein binding ratio was 73%–81%, and the DHA binding ratio was 76%–82%. The rate of plasma protein binding in patients infected with malaria parasites is 93% Intramuscular injection has a faster absorption rate and shorter peak time than oral administration. Absolute bioavailability, based on concentration, is 86.4% in adults and 88% in children with falciparum malaria [85]. The absolute bioavailability of ART work after oral administration was 61% (95% confidence interval: 52%–70%) . After ingestion, part of the domain is absorbed into the blood and converted to DHA, with a bioavailability of 80%-85%. Another part of the ART is absorbed into the liver through the portal vein and metabolized by the liver into DHA.[13][14].
5. Antimalarial activity of Artesunate
Artesunate is an antimalarial drug that works by increasing oxidative stress on Plasmodium in red blood cells Artesunate is active against strains of Plasmodium falciparum resistant to chloroquine and mefloquine, but in vitro susceptibility and resistance thresholds have not been established. Tropical plants Artesunate is the most potent in vitro compared to other qinghao derivatives (Arteether, Artemisinin , and artemether).Results of in vitro experiments using red blood cells from individual with the genetic variant of ?-thalassemia suggest that Artesunate is less effective in treating malaria in this population than in other patient groups. However, this has not yet been clinically proven. Animal models have demonstrated the efficacy of artesunate in experimental treatments. Bergey infection in rats and induced cerebral malaria in rhesus monkeys. Paired isolates isolated from patients with recurrent infections after artesunate monotherapy or combination therapy with mefloquine or doxycycline did not show a pattern of increased resistance to artesunate in vitro.[1][17]
Sodium Artesunate : Sodium artesunate (5a)is a basic salt as opposed to the acidic form .It is recommended form for the treatment of uncomplicated and severe malaria due its solubility [1]
6. Mechanism of Artesunate in diabetes
Effects and mechanisms of Artesunate in the treatment of diabetes, islet cell protection Islet cell protection Insulin produced by islet cells is essential for maintaining the normal physiological range of blood sugar, especially for the survival and functional integrity of islet cells. The survival and function of islet ? cells is impaired, leading to decreased insulin secretion, a major cause of diabetes.[18][19]Therefore, protecting pancreatic islet ?-cells and stimulating insulin secretion is an effective method for treating diabetes.[20]Interleukin-1? (il-1?) has been shown to play an important role in early ?-cell injury, with ?-cells themselves producing il-1? upon high glucose stimulation.[21]Studies have shown that il-1?-independent factors can cause dysfunction of glucose-stimulated insulin secretion by activating the NF-?B pathway.[22] Inhibition of the NF-?B pathway can protect human and rodent islets from il-1?-stimulated ?-cell damage.[23] Sirtuin1 (SIRT1) is a nicotinamide adenine dinucleotide-dependent deacetylase that regulates a variety of cellular activities, including cell survival, anti-stress potential, and cellular self-regulation. Studies have shown that sirt1 plays a role in reducing insulin resistance and protecting islet ? cells.[24] In addition, sirt1 can protect islet ? cells by deacetylating p65.[25] Therefore, sirt1 is expected to become a new target for diabetes treatment.[26] It is found that ART inhibited il-1?-induced NF-?B pathway activation and attenuated insulin secretory dysfunction.[27] ART reduced Il-1?-induced islet ?-cell damage and sirt1 expression.[28] The protective effect of arts on islet ?-cells was partially abolished after sirt1 inhibition. In conclusion, ART fully activates sirt1 expression in islet ?-cells, inhibits the NF-?B-INOS-no signalling pathway, promotes insulin secretion, and reduces apoptosis. In addition, this method improves the viability of mouse islet ? cells treated with streptozotocin, reduces the number of late apoptotic cells and pyroptotic cells, and modulates the expression of proteins related to the pyroptotic pathway. It is a way to protect islet ? cells.[29]
7. The role of Artesunate in fibrosis
Artesunate (ART) is a water-soluble derivative of Artemisinin which is used to treat malaria, is often given orally or by injection. It is currently listed on the WHO List of Essential Medicines and was developed as first-line treatment for children and adults with severe malaria artesunate also targets myofibroblasts in several ways. I.e. Action on fibroblast activation Induction of a gene expression paradigm in fibroblasts that downregulates many myofibroblast-related and profibrotic genes and regulates genes that degrade ECM and reduce collagen deposition. and reduction of fibroblast proliferation Although the use of Artesunate may be prescribed as a more effective way of delivering it into the body, studies have shown that the administration of Artesunate is more effective than Artesunate in treating malaria. The exact reason for this is still under investigation. However, compared to Artemisinin, oral administration or tea infusion of the plant shows increased bioavailability and pharmacological efficacy of Artemisinin and may be more effective in inhibiting fibrotic cells than pure dry leaf powder preparation or hot water infusion of leaves.[30]
8. Anti-inflammatory activity of Artesunate
Artesunate, a water-soluble derivative of the traditional Chinese herb Artemisinin, has been reported to have anti-inflammatory and immunosuppressive effects.[31] Artesunate inhibits proinflammatory cytokines [tumour necrosis factor-? (TNF-?) and interleukin-6 (il-6)] and anti-inflammatory chemokines (tumour necrosis factor-? (TNF-?) and interleukin-6 (il-6) suppresses.[32] It can inhibit the formation of atherosclerotic lesions alone or in combination with rosuvastatin. Day-8). However, the mechanism of Artesunate in ash still remains a mystery.[33] The inflammasome-containing LR family 3 (nlrp3) consists of the nlrp3 (cytoplasmic sensor molecule) pyrin domain, apoptosis-like protein, caspase accumulation domain (ASC; adapter protein), and procaspase-1. effector proteins)[34]. Artesunate-mediated inhibition of the nlrp3 inflammasome pathway has been proposed to ameliorate glomerular interstitial cell damage. The inhibitory effect of Artesunate occurs by increasing LPL expression, for example through the klf2/nrf2/tcf7l2 axis[35]. Activation of the Nlrp3 inflammasome contributed to the development of AS. In this study, we sought to determine the attenuating effect of Artesunate on the regulation of the Nlrp3 inflammasome and its downstream mechanisms. Inhibition of TXNIP has been reported to inactivate the NLRP3 inflammasome and attenuate the inflammasome. The ?-ARRESTIN protein, TXNIP, is involved in a variety of pleiotropic biological responses [36]. For example, TXNIP has been shown to mediate inflammatory responses by inhibiting thioredoxin activity[37]. Increased expression of TXNIP was observed in AS, and its inhibition promoted endothelial cell proliferation. The XNIP target scan database predicted MICRORNA(mir)-16-5p as a target gene. Miranas have been proposed to regulate many developmental and cellular processes involving approximately 21 nucleotides [38]. Several mirnas have been implicated in AS, including mir-122 (13) and mir-24 (14). mir-16-5p was downregulated in diabetic kidney disease compared with type 1 diabetes, which was confirmed by bioinformatics analysis, and mir-16-5p can also regulate genes.[39]
9. The role of Artesunate in COVID-19
There are in vivo and in vitro studies showing the positive effects of Artesunate in treating COVID-19. Sardar et al. reported a case co-infection with covid-19 and Plasmodium vivax and treated with ARTesunate.24 In an in vitro study of the anti-SARS-CoV-2 efficacy of Artemisinin -related compounds in VERO e6 African kidney cells in monkeys, Artesunate is seems as a SARS-Kov-2 service. The period of pulmonary injuries and the period of the disease was statistically reduced in the ART group compared to the control group. -KB) entry into cells such as paths and chlorokine. The nerve delivery of the nerves occurs during the infection with SARS-COV-2. 28 GLARS-COV-2 is the purpose of ACE-2 receptors. This is an Asen metal (MMP) metallic rookie of the viral infection reaction to complicate the complications of the central nervous system. Created by the cells of the central nervous system. 30.31 U-373mg test experience[40]
10. Anticytomegalovirus activity of Artesunate
The anticytomegalovirus activity of artesunate is not limited to HCMVS, but also applies to CMV of animal origin, especially CMV of rat origin. An important finding is that increasing the intracellular iron concentration enhances the anticytomegalovirus activity of artesunate.[41]This iron-enhanced efficacy has been supported by several observations, and some promising features of artesunate. anticytomegalovirus activity are outlined below. First, treatment of cmv-infected fibroblasts with Artesunate plus iron (ferrozanol, Mannheim) and/or soluble transferrin resulted in greater inhibition of viral replication[42] Since ferrozanol is a clinically approved drug, this drug can be safely combined with Artesunate in clinical practice. Second, the antiviral activity of Artesunate is additive when combined with existing drugs such as ganciclovir, cidofovir, and foscarnet. Combining drugs with different mechanisms of action can slow the development of drug resistance.[43] Third, the antiviral activity of Artesunate against CMV was also demonstrated in vivo using a murine CMV model. Importantly, the first successful clinical use of Artesunate for the treatment of HCMV was described in a patient who developed a drug-resistant infection during prophylactic antiviral treatment after stem cell transplantation.[44] Artesunate has been shown to be a highly effective and well-tolerated HCMV replication inhibitor, suggesting the need for further clinical evaluation of the role of Artesunate in the treatment of HCMV infection.[45]
11. Effects of Artesunate on cancer
ART is an emerging drug against cancers. Such as leukaemia , liver cancer ,lung cancer, ovarian cancer ,breast cancer[4][46]. The anticancer activity of artesunate works through affecting through various mechanism and pathways in cancer cells like arresting cell cycle,[46] inhibiting angiogenesis [47],inducing apoptosis[46],inhibiting proliferation[48] and inducing autophagy. ART restores the sensitivity of cancer by changing the sensitivity of the chemotherapy drug by controlling various signals and regulating other signals. (HCC) is used to improve kinase tyrosine inhibitor. Major treatment of liver cancer. ART is being used as a medication to treat cancer. ART blocks many signalling pathways involved in tumour growth, as demonstrated by numerous research investigations using cancer cell lines in vitro and in animals. Button mice bearing sk-7721 tumour cells were treated with SOR, ART or a combination of the two (ART-SOR). ART-sor treatment showed better tumour growth reduction than ART-sor treatment alone. In vitro effects of both showed that 50% inhibition of SC cells was achieved with 2.77 ?m ART-sor compared to 5.23 ?m sor required for the same effect. For cancer cells living in vitro, the observed inhibition of 11.43 ?m ART-sor was only 5.30 ?m sor. ART was found to produce a positive synergistic effect by increasing annexin and SC cell production and inducing pro-apoptotic processes by increasing parp and cleaved caspase-3.. ART has been shown to restore the sensitivity of a number of cancer types to chemotherapeutic drugs by modulating various signaling pathways; for example, ART can improve the apoptosis of HCC by inhibiting the PI3K/AKT/mTOR pathway. ART can increase liver cancer cell sensitivity to sorafenib via suppression of the MEK/ERK pathway.
Artesunate exhibits multiple mechanisms of cancer treatment
13.1 Apoptosis. Apoptosis is a type of programmed cell death that does not cause an inflammatory response.[49]
13.2 Autophagy.
Autophagy is a conserved self-destruction system that is important for maintaining cellular homeostasis under stress conditions and, in combination with the autophagy-related protein family, has been shown to play an important role in cancer.[50]
13.3 Inhibition of angiogenesis
Blood vessels provide oxygen and a nutrient supply for the growth of tumours, which also facilitate the proliferation, migration and subsequent invasion of malignant tumour cells in the long term.[51]
13.4 Cell cycle arrest.
Aberrant cell division is one of the characteristic features of cancer cells. ART inhibits the proliferation of bladder cancer cells (RT4, RT112, T24 and TCCSup), which is associated with G0/G1 phase cell cycle arrest and downregulation of cell cycle regulatory proteins [cyclin D1 and CDK4 (required for entry into the G1 phase)[52]
13.5 Ferroptosis.
Ferroptosis is a recently identified form of regulated cell death, which is characterized by iron overload, lipid ROS accumulation and lipid peroxidation[53]
Artesunate is a very effective, less toxic, and well-tolerated drug for cerebral malaria and all other forms of severe malaria. Recent developments have also shown that medications with a single mechanism of action and significant adverse effects are probably not the best choices for treating neurological conditions.[5] As a result, artesunate's pluripotent effect makes it a valuable tool for treating and preventing various neurological conditions. Artesunate acts on central nervous system infection, subarachnoid hemorrhage (SAH)[65] intracerebral hemorrhage (ICH), ischemic cerebrovascular disease (ICD),[66]nervous tissue tumour ,metastatic nervous tissue tumour. Neurological Autoimmune Diseases and Neurodegenerative disorders. Epilepsy Traumatic Brain Injury and Spinal Cord Injury Ischemic Cerebrovascular Disease In the treatment of Central nervous system Antimicrobes is one of the primary role of artesunate Research reported artesunate could enhance the anti-MRSA activity of oxacillin ,when combined with ampicillin sodium-sulbactam sodium, it could further increase survival rate of mice challenged with S. Aureus. He main pathogens of the CNS parasitic diseases are cysticercus cellulosae, Paragonimus westermani, Schistosoma, Echinococcus and Toxoplasma gondii, and so forth. As the disease is part of systemic parasitosis, the researches for the CNS parasitic infection are few. Research indicated that artesunate had evident inhibitory effect on Toxoplasma gondii in vitro and in vivo . Moreover, artesunate-DHA combination could substantially reduce the size of the cerebral cysts of Toxoplasma gondii in mice after only 5 days of treatment[69]
In case of vascular disease of CNS in subarachoid hemorrhage artesunate could preserve blood-brain barrier (BBB) integrity and improve neurological outcome after SAH, possibly through activating S1P1, enhancing PI3K activation, stabilizing ?-catenin via GSK-3? inhibition, and then effectively raising the expression of Claudin 3 and Claudin 5[70]
Artesunate is the first line antimalarial drug which is mainly used as primary treatment for severe malaria .from this review we can conclude that artesunate is one of the drugs having a wide therapeutic range, due to its hydrophilic nature. ART has antiviral, anticancer, antidiabetic, and anti-inflammatory properties in addition to its primary use as an antimalarial medication. The production of reactive oxygen species (ROS), suppression of heme polymerization, and destabilisation of the parasite membrane are its modes of action for treating malaria. artesunate is the emerging and affordable drug for patients. In conclusion, the data that is now provided support the use of ART as an anticancer treatment. However, data obtained from comprehensive in vivo studies on both humans and animals will be needed in the future to further our comprehension of the anti-malarial and other activities of ART.


 Shital B.Bharambe*
Shital B.Bharambe*
 Shailesh Jawarkar
Shailesh Jawarkar
 10.5281/zenodo.10798299
10.5281/zenodo.10798299