Abstract
The current study covers the invention of an isocratic RP-HPLC method that is straightforward, affordable, quick, accurate, and precise for estimating the amount of bulk azelnidipine and in its commercial products. An evaluation was conducted at 260 nm, which was determined to be Azelnidipine's ?max. The Cosmosil C18 Column, measuring 250 mm by 4.6 mm and 5µm, was used to establish the straightforward, isocratic, and selective RP-HPLC method for azelnidipine. The mobile phase was made up of methanol at pH 6 with 1% formic acid with OPA Water (58: 42% V/V) with a 1.0 ml/min flow rate and 260 nm detecting wavelength. ICH Q2 (R1) requirements were successfully followed in the validation of the developed approach. With r2 values of 0.9996, the chromatographic techniques demonstrated an excellent linear response. The approaches were determined to be precise when there was less than a two percent relative standard deviation. It was possible to infer from the findings that the established approach was robust, selective and specific. The examination of bulk azelnidipine and its commercial formulation could be effectively conducted using this technology.
Keywords
Azelnidipine, Methanol, RP-HPLC, Validation and ICH.
Introduction
Hydrophobicity is the basis for molecular separation in RP-HPLC. Outstanding resolution that is achievable in a variety of chromatographic settings [1]. It is possible to control chromatographic selectivity by altering the properties of the mobile phase. Excellent production and quick recoveries. Outstanding repeatability of repeated separations performed over an extended length of time. A new dihydropyridine derivative that acts antagonistically against calcium (Fig. 1). Azelnidipine has only been measured using a few number of analytical techniques, such as HPLC, LC-MS, and LC-ESI-MS [2-4].
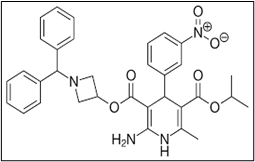
Figure 1: Azelnidipine
MATERIALS AND METHODS:
Materials:
The source of the pure medication Azelnidipine was Swapnroop Drug and Pharmaceutical Ltd. India. Methanol was taken from Merck Ltd., India. And all other chemicals utilized were of laboratory grade.
Instrumentation:
The HPLC apparatus that was employed was an Agilent Tech. Gradient System equipped with a (DAD) & Gradient Detector. Chemstation 10.1 software was used to manipulate the instrument’s settings. Azelnidipine was separated by chromatography using a inverted phase column Cosmosil C¬18 (250mmX 4.6mm,5µm) and a mobile phase that included Methanol + 1 % Formic Acid (pH 6 adjusted with OPA) water (58:42% v/v) and isocratic elution mode at a flow rate of 1.0 mL/min. Room temperature was maintained for the column and the injection volume was set at 20 ?L.
Preparation of sample and standard solution
After carefully weighing 10 mg of powdered Azelnidipine, it was put in a 10-milliliter volumetric flask to provide a 1000 ?g/ml solution. After adding a mobile phase, the mixture was exposed to sonication for 10 min. while being periodically shaken. The 0.1 ml stock solution that was left over was put into a 10 ml flask. A 0.22 ?l syringe filter was used to filter the resultant combination [5-7].
Getting the mobile phase ready
Prepare a mixture of Methanol +1 % Formic Acid (pH 6 adjusted with OPA) in the ratio of 58:42 % v/v respectively, and mix well. Resulting mixture used as diluent and blank [8].
Validation of Method:
Linearity
The volume was adjusted by adding a diluent. After 10 mg of azelnidipine had been placed to a 10 mL flask. For ten minutes, sonicated with sporadic swirls. Diluents were used to dilute 0.1 ml of this solution up to a 10 ml volumetric flask. Aliquots of the standard solution were collected to create sample solutions with drug ranging from 10 to 50 ?g/ml. The solutions were examined one by one. A linear fit was proven using Linear Regression analysis by graphing the peak area versus the Azelnidipine concentration [9,10].
Accuracy (recovery):
A common way to express accuracy is as a percentage of recovery from an assay using known additional analyte levels. Azelnidipine, which is identical to 20.18 mg was used to calculate the accuracy of the quantity to be equal to the average weight of tablets that are sold. After being triturated, this powder containing 10 mg of azelnidipine was subjected to the procedure indicated for chromatographic analysis. For three days, the outcome was examined in duplicate. The percentage of additional drug recovered was used as an accuracy metric [11-13].
Precision:
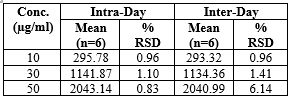
Intra-day precision:
Three distinct concentrations of Azelnidipine (10 mg/ml, 30 mg/ml, and 50 mg/ml) were present in the sample solutions. On the same day, azelnidipine was evaluated. Percentage R.S.D. was determined [14].
Inter-day precision:
The percentage R.S.D. was computed for sample solutions containing 10 mg of azelnidipine at three distinct concentrations (10µg/ml, 30µg/ml, and 50µg/ml) in HPLC and on various days. Typically, standard deviation are used to express it [14-16].
Robustness:
Using a 30-µg/ml solution of Azelnidipine, the mobile phase composition was changed in a proportion and the varie in detection wavelength (±1 ml/min-1) [14].
Limit of Detection &Quantification:
The following formula was used to determine LOD and LOQ in accordance with ICH regulations. LOQ = 10×? /S and LOD = 3.3 × ?/S [14-18].
RESULT & DISCUSSION:
Method Development for Azelnidipine By RP-HPLC
The mobile phase flowed one milliliter each minute. 20 µl was the injection volume and the wavelength used was 260 nm. The sample and column temperatures were at room temperature. The 15-minute sample run produced a chromatogram that was deemed good, and the system suitability parameters were acquired correctly. Enhanced chromatographic conditions (Fig.2) and system suitability parameters were developed
Figure 2: UV Spectrum for Azelnidipine
Optimized Chromatographic Conditions
The mobile phase that was chosen and optimized was Methanol : (1% formic Acid)pH 6 with OPA Water (58: 42% V/V) and Parameters optimized were the wavelength (260 nm), flow rate (1.0 ml/minute), and run time (15 minutes). Better results were displayed by the separated peaks in this instance. It was determined that the suggested chromatographic conditions (Fig. 3) were suitable for the drug's quantitative determination.
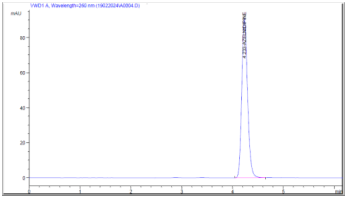
Figure 3: Chromatogram of Azelnidipine in Optimized Condition
Linearity
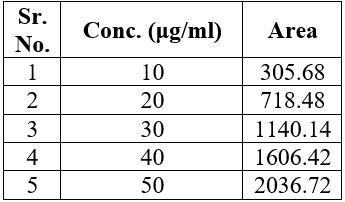
Linearity was assessed at 10-50 ?g/ml concentrations. Figure 4 displays the Azelnidipine calibration graph. Table 2, displays the linearity data.
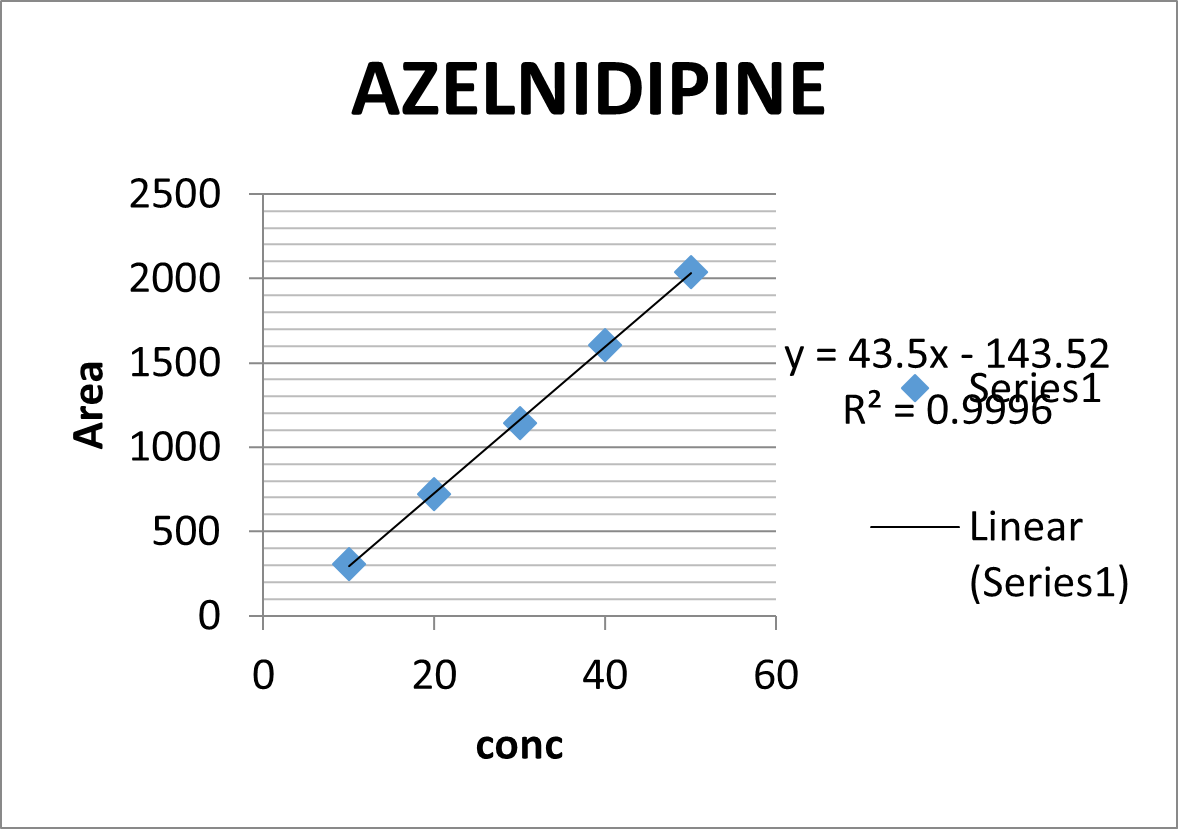
Figure 4: Calibration curve of Azelnidipine for HPLC method
Table 1: Linearity of Azelnidipine
Accuracy
Accuracy was assessed in the range of 80-120% of sample (table 3). The accuracy of the analytical method expressed as % recovery determines the degree of closeness between the obtained and the true values. The overall % recovery was observed to be in the range of 99-101% for Azelnidipine (Figure 5).
Table 3: Details of Accuracy
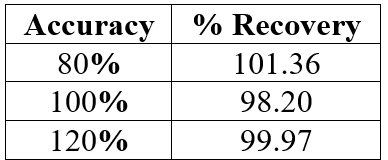
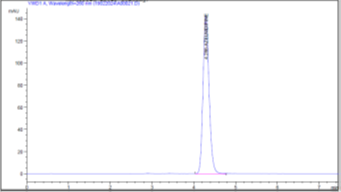
Figure 5 (a): Chromatogram of Accuracy 80%
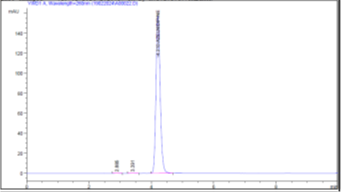
Figure 5 (b): Chromatogram of Accuracy 100%
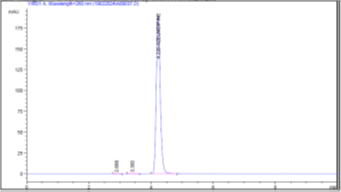
Figure 5 (c): Chromatogram of Accuracy 120%
Precision
Precision is expressed as % RSD, and % RSD NMT 2% is considered acceptable. Since the % assay and % RSD results were well within the acceptable range. The proposed method was found to have an overall % RSD for intraday and inter-day precision of less than 2% (table 4).
Table 4: Results of Precision
Robustness
Small and purposeful changes to chromatographic conditions and assay settings were made to test this. These modifications affected system appropriateness and injection results for standard and sample solutions. Table 5 shows that wavelength and flow rate adjustments had no influence on retention time, theoretical plate, or asymmetry. %RSD should be LT 2%. This indicated that the analytical approach was reliable and the created approach also satisfied the restrictions.
Table 5: Results for Robustness

LOD and LOQ
LOD = 3.3(Sy /S) = 3.3 X 2.27/43.5= 0.1722µg /ml
LOQ = 10(Sy /S) = 10 X 2.27/43.5 = 0.5218µg /ml
The values obtained for the LOD and LOQ indicated that the method was susceptible to detecting and quantifying the drug.
CONCLUSION
In this suggested development study, a straightforward, exact and accurate RP-HPLC method was established for the determination of Azelnidipine. The new HPLC method is simple to use, has a significantly shorter run time, and requires less time to prepare solutions. In order to optimize chromatography, all of the chromatographic parameters were tuned for a reliable and sturdy analytical approach. The approach was verified in terms of linearity, accuracy, precision, and repeatability in accordance with legal standards and the suggested methodology by the ICH. The validation data demonstrates that the RP-HPLC method is sensitive, accurate, precise, specific, and repeatable, and that the devised method has acceptable repeatability. The suggested technique can be applied to Azelnidipine routine analysis and quality control tests in medication samples and dosage forms. Since this approach may be simply adapted to estimate Azelnidipine in a variety of biological materials, it is advised for use in future bioanalytical analyses. The Azelnidipine method that was created is easy to use, quick, affordable, and suitable for industrial application. REFERENCES
- Buralla, K. K., & Varadarajan P. (2020). Validated RPHPLC Method Development of Pazopanib HCl in Bulk and its Pharmaceutical Dosage Form. Pharmaceutical Methods, 11(1), 21-24.
- Chaitanya, G., & Pawar. A. K. M. (2015). Development and Validation of UV spectrophotometric method for the determination of Pazopanib hydrochloride in bulk and tablet formulation. Journal of Chemical and Pharmaceutical Research, 7(12), 219-225.
- Escudero-Ortiz, V., Pérez-Ruixo, J. J., & Valenzuela, B. (2015). Development and validation of an HPLCUV method for pazopanib quantification in human plasma and application to patients with cancer in routine clinical practice. Therapeutic Drug Monitoring, 37(2), 172–179. https://doi.org/10.1097/FTD.0000000000000121
- Ghode, P. D., Dhaigude, P. U., Rathod, S. P., Sayare, A. S., Pachauri, A. D., Khandelwal, K., & Ghode, S. P. (2020). Stability Indicating HPTLC Method Development and Validation for the Estimation of Pazopanib HCL in Bulk and its Dosage Form. International Journal of Pharmaceutical Research, 12(3). https://doi.org/10.31838/ijpr/2020.12.03.518
- Koylu, B., Tekin, F., Aktas B. Y., Kilickap. S., & Koksal. D. (2022). Pazopanib HCl -induced chylothorax in a patient with renal cell carcinoma. Anti-Cancer Drugs, 33(1), 555-557. https://doi.org/10.1097/CAD.0000000000001172
- Ma, B., & Gou, X. L. (2016). Application of highperformance liquid chromatography in food and drug safety analysis. Journal of Food Safety and Quality, 7, 295-4298.
- Minocha, M., Khurana, V., & Mitra, A. K. (2012). Determination of pazopanib (GW-786034) in mouse plasma and brain tissue by liquid chromatography-tandem mass spectrometry (LC/MS-MS). Journal of Chromatography. B, Analytical Technologies in the Biomedical and Life Sciences, 901, 85–92. https://doi.org/10.1016/j.jchromb.2012.06.004
- Pressiat, C., Huynh, H. H., Plé, A., Sauvageon, H., Madelaine, I., Chougnet, C., Le Maignan, C., Mourah, S., & Goldwirt, L. (2018). Development and Validation of a Simultaneous Quantification Method of Ruxolitinib, Vismodegib, Olaparib, and Pazopanib in Human Plasma Using Liquid Chromatography Coupled with Tandem Mass Spectrometry. Therapeutic drug Monitoring, 40(3), 337–343.
- Patil SD, Bachhav RS, Udavant PB, Ahirrao SP. Deepak S.bhambere4, Development and Validation of Stability Indicating RP-HPLC Method for Azelnidipine for bulk drug. Natl Volatiles Essent Oils. 2021;8(5):11151-7.
- Gore MG, Pratap S. Dabhade, RP-HPLC method development and validation of azelnidipine, Gore and Dabhade, IJPSR. Vol. 7(12); 2016. p. 5111-4.
- Prabhakar D, Sreekanth J, Jayaveera KN. Method development and validation of azelnidipine by RP-HPLC. Int J ChemTech Res. 2017;10(10):418-23.
- Ubale S, Dr. Kalshetti MS, Habib B, Mittha J, Adlinge S. Development and validation of RP-HPLC method for quantification of azelnidipine in tablet. Int J Creat Res Thoughts (IJCRT). 2021;9 (7, July): 797-802.
- Jadhav, Lokhande, Narwade, Mahananda V. Ghodke, Rutuja. S. Desai and Prerna R. Mote, Method development & validation of stability indicating Rp-HPLC method for simultaneous estimation for azelnidipine & telmisartan in bulk & pharmaceutical dosage form, world. J Pharm Med Res Wjpmr. 2022;8(3):216-22.
- Zou Jian-Jun, et al, Determination of Azelnidipine by LC-ES-MS and its application to a pharmacokinetic study in healthy Chinese volunteers, Pharmazie 63, 2008, 568-570.
- Nilam Patel, et al, Spectrophotometric method for the simultaneous determination of Azelnidipine and Olmesartanmedoxomil by First Derivative Spectrophotometric method, Scholar Research Library, 2012, 1080-1084.
- Hiroyuki Daikuhara, et al, The combination of Olmesartan and a Calcium Channel Blocker (Azelnidipine) or candesartan and a Calcium Channel Blocker (Amlodipine) in Type 2 Diabetic hypertension patients: The OLCA study, Diabetes and Vascular Disease Research, 2012, 280-286.
- Kunti D. Raskapur, et al, UV Spectrophotometric method development and validation for determination of Azelnidipine in pharmaceutical dosage form, International Journal of Pharmacy and Pharmaceutical Sciences, Volume-4, Issue 1, 2012, 238-240.
- Raveendra Babu Ganduri, Stability indicating liquid chromatographic method for determination of Olmesartan Medoxomil and Azelnidipine in combined Tablet dosage form, International Journal of Pharma Science and Research, Volume-5, 2014, 275-282.
- Jayvadan K. Patel Validated stability indicating RP-HPLC method for the Simultaneous determination of Azelnidipine and Olmesartan in their combined dosage form, Sci Pharm, 2014, 541- 554
- Rele Rajan V. Spectrophotometric estimation of Azelnidipine in bulk and pharmaceutical dosage form by second order derivative method, Journal of Chemical and Pharmaceutical Research, 2014, 198-202.
- Selvadurai Muralidharan, et al. Simple validation of Azelnidipine by RP-HPLC Method, AIMST University, Malaysia, Volume-1, 2015, 43-45.
- Rajan V. Rele, et al., Development and validation of Stability Indicating Reverse Phase Liquid Chromatographic method for the assay of Azelnidipine in bulk and pharmaceutical formulation , International Journal of Pharma and biosciences, 2016, 270-275.
- Prabhakar D., et al., Development and Evaluation of Transdermal Patches of Azelnidipine, International Journal of Pharmacy and Pharmaceutical Sciences, Volume-5, 2013, 805.
- Sethi P.D., et al., HPLC-Quantitative analysis of Pharmaceutical formulations, CBS publisher, & distributors, New Delhi, 2011, 11.
- Yuri Kazakevich, Rosario Lobrutto, HPLC for Pharmacetical Scientists, A John Wiley & Sons, Inc., Publication, Canada, 2007, 10-22, 26-34.
- F. Settle, et al., Handbook of Instrumental Techniques for Analytical Chemistry, NJ: Prentice-Hall, 1997, 58.
- Validation of Analytical procedure; Text and methodology Q2 (R1), International Conference on Harmonisation of technical requirements for human use, ICH Harmonized tripartite guidelines, 2005.
- P. Ravisankar et al, A Review on Analytical Method Development, Indian Journal of Research in Pharmacy and Biotechnology. 2014, 1183-1195.
- Kiyoshi Kawabata, et al. Simultaneous determination of Azelnidipine and two metabolites in human plasma using liquid chromatography-tandem mass spectrometry, Drug metabolism and pharmacokinetics, Resaerch Laboratories Sankyo, Co. Ltd. 1- 2-58, Hiromachi, Shingawa-ku, Tokyo, Japan, 2006, 46-52.


 Akanksha D. Punekar*
Akanksha D. Punekar*











 10.5281/zenodo.13770597
10.5281/zenodo.13770597