Abstract
Obesity, a complex disorder associated with numerous health complications, has become a significant public health concern worldwide. Addressing this issue necessitates effective pharmacological interventions, one of which involves targeting fat metabolism and gastrointestinal lipases. Cetilistat, a novel lipophilic benzoxazinone, inhibits gastrointestinal and pancreatic lipases, offering promise for managing obesity. In this study, a robust and validated RP-HPLC method was developed for the quantitative determination of cetilistat in biological fluids. The method development involved optimization of chromatographic conditions, including selection of suitable solvents, wavelength, and mobile phase composition. The final method utilized a C18 column with a mobile phase consisting of methanol, water, and trifluoroacetic acid (85:15:0.1). The method exhibited good linearity over a concentration range of 25-75 µg/ml, with a correlation coefficient (R²) of 0.9996. The limit of detection (LOD) and limit of quantification (LOQ) were determined to be 1.48 µg/ml and 4.47 µg/ml, respectively. Validation of the method was performed according to ICH guidelines, encompassing parameters such as specificity, accuracy, precision, LOD, LOQ, and robustness. The method demonstrated satisfactory results in terms of system suitability, specificity, accuracy (% recovery ranging from 98.55% to 101.01%), and precision (%RSD within limits). Additionally, robustness testing confirmed the method's reliability against minor variations in analytical parameters. In conclusion, the developed RP-HPLC method offers a simple, accurate, and reproducible means for quantifying cetilistat in biological fluids. Its successful validation according to ICH guidelines indicates its suitability for routine analysis in pharmaceutical research and clinical settings, facilitating further investigation and potential therapeutic applications in the management of obesity.
Keywords
Fast Dissolving Tablet, Oral drug delivery system, super disintegrants.
Introduction
Obesity is a growing public health problem, and in many countries, it has reached epidemic proportions. It is a complex multifactorial disorder characterized by an excess accumulation of adipose tissue, and is associated with increased risk for a number of complications, including type-2 diabetes, cardiovascular disease, hypertension, dyslipidemia and cancer. A sustained weight loss of between 5 and 10% of initial body weight has been associated with clinically meaningful reductions in these obesity-related comorbidities.
One approach is to induce a negative energy balance by targeting fat metabolism and gastrointestinal (GI) lipases in particular. Inhibition of lipases reduces hydrolysis of dietary triglycerides, limiting the absorption of monoglycerides and free fatty acids, thereby enhancing weight loss. Cetilistat is a novel highly lipophilic benzoxazinone that inhibits GI and pancreatic lipases, which raises the possibility of a distinct clinical profile, and is in development for the management of obese patients with or without complications. Cetilistat is a drug designed to treat obesity. It acts in the same way as the older drug orlistat (Xenical) by inhibiting pancreatic lipase, an enzyme that breaks down triglycerides in the intestine. Without this enzyme, triglycerides from the diet are prevented from being hydrolyzed into absorbable free fatty acids and are excreted undigested.

Fig. 1:- Chemical structure of Cetilistat
Cetilistat, (2-hexadecoxy-6-methyl-3,1-benzoxazin-4-one). It is a white to off- white powder which is freely soluble in dichloromethane, chloroform, acetone, carbon tetra-chloride, chlorobenzene, n-hexane, diethyl ether. Slightly soluble in ethyl acetate and toluene, and insoluble in methanol and water.
MATERIALS AND EQUIPMENTS
The drugs, chemicals, reagents, instruments and filters used during the experiment. Cetilistat API were purchased from Bulat Pharmaceutical pvt. ltd. Gurugram, Haryana.
Instruments Used:
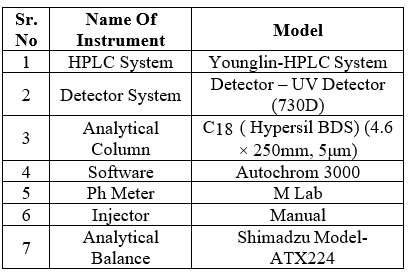
Table No 01:- Instruments Used In Method Development
Solvents And Chemicals:
- Methanol (gradient grade)
- Acetonitrile (gradient grade)
- Trifluoroacetic acid (HPLC grade)
- Water (HPLC grade)
- Human plasma
EXPERIMENTAL WORK
Optimization of chromatographic condition for the estimation of Cetilistat.
Solubility Studies:
As a first step of method development solubility of drugs was tasted in different solvents to obtain a suitable solvent which can be used for method development.
Selection Of Wavelength:
An UV spectrum of 10 ug/ml Cetilistat in methanol was recorded by scanning in the range of 200 nm to 400 nm. A wavelength which gives good response for the drugs to be selected. From the UV spectrum a wavelength of 222 nm was selected. Drugs showed optimal absorbance at this wavelength.
Selection of mobile phase:
The pure drug of Cetilistat was injected into the HPLC system and run in different solvent system. Each mobile phase was allowed to equilibrate with stationary phase until steady base line was obtained. Different mobile phase like methanol and water, acetonitrile and water, methanol and phosphate buffer, methanol and ortho phosphoric acid, methanol and Trifluloroacetic acid various proportions were tried to get a stable peak each mobile phase was filtered through 0.45µm membrane filter and sonicated on ultrasonic bath. After trials Methanol: Water: Trifluloroacetic acid, 85: 15:0.1 was found to be most satisfactory since it gave sharp peak with symmetry within limits and significant reproducible retention time.
Optimization of Chromatographic Condition
The following chromatographic conditions were established by trial by error and were kept constant throughout the experimentation.

Preparation of stock solution
Weighed accurately 50 mg of cetilistat standard and transfer to 100 ml volumetric flask, dissolve and diluted upto the mark with the help of diluent, shake weel, sonicate for about 5 min.
Preparation of standard solution
Pipette out 2ml from stock solution and transfer to 20 ml volumetric flask, diluted upto the mark with diluent, shake well, filter through 0.2 µm syringe filter.
Preparation of test solution
Prepare homogeneous mixture of 0.2 ml stock solution, 0.2 ml of plasma and 1.6 ml of diluent, shake well, centrifuge for 10 min at 6000 rpm, inject from supervent layer.
METHOD VALIDATION
Validation of RP-HPLC method was done as per ICH guidelines for parameters like linearity, accuracy, precision, robustness, LOD and LOQ.
System suitability
System suitability parameters were assessed by preparing standard solutions of Cetilistat. The solutions were injected six times at a concentration range of 50 µg/ml and various parameters like retention time, theoretical plates, tailing factor and peak area were calculated.
Specificity
Solutions of standard and sample were prepared and injected in to HPLC system and its respective peak area and retention time were observed.
Linearity
Suitable quantity of standard solution was transferred into a series of 10 ml volumetric flasks. The volume was made up to the mark with mobile phase to obtain the concentration of 25, 37.5, 50, 62.5, 75 µg/ml. Peak area of this solution was recorded and the graph was plotted against concentration. The correlation coefficient (R2) of least square linear regression of cetilistat was calculated.
Accuracy
Recovery studies were determined by standard addition methods by adding known amounts of cetilistat to pre-analyzed samples at three different concentration levels i.e. 80%, 100%, 120% of assay concentration and percent recovery was calculated. 3 ml of test solution was transferred to 3 different 10 ml volumetric flasks separately and 2.4, 3, 3.6 ml of 100 µg/ml standard solution was added to the flask and the volume was made up to the 10 ml with mobile phase. Peak area, standard deviation and % RSD was calculated.
Precision
The precision of the method was determined in terms of repeatability and intraday and interday precisions. Intraday and Interday precision was determined by analyzing the drugs in triplicate at concentrations 40, 60 and 80 µg/ml.
Limit of Detection and Limit of Quantification
Detection limit was determined based on the standard deviation of peak area and was calculated by formula LOD = 3.3(Standard deviation/Slope). Also Quantification limit was determined based on the standard deviation of peak area and was calculated by formula LOQ = 10(Standard deviation/Slope).
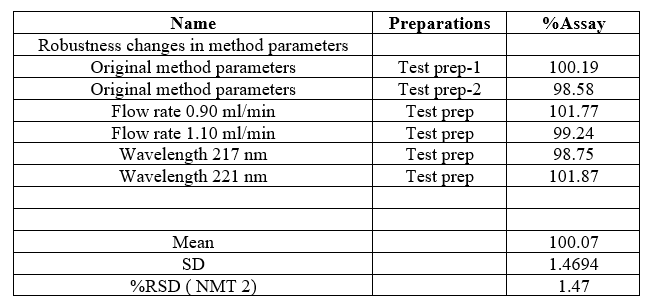
Robustness
Few parameters were deliberately varied for study of robustness. The Robustness was carried out by changing flow rate, wavelength and mobile phase composition. Flow rate, wavelength and mobile phase composition were varied by ±2% and the %RSD was calculated.
RESULT AND DISCUSSION
HPLC Method Optimization
For method optimization various mobile phases were tried in different ratios, such as acetonitrile: water (60:40), methanol: water: TFA (70: 30: 0.1), methanol: water: TFA (85: 15: 0.1). All these mobile phases were unacceptable due to tailing, fronting and no sharpness in the peak. After various trials mobile phase consisting of methanol: water: TFA (85: 15: 0.1) was selected which gave sharp peaks with no tailing and fronting. The chromatogram of standard cetilistat was shown in Fig 2.
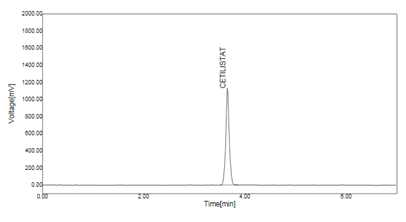
Fig. 2:- Typical chromatogram of standard solution.
Linearity
The linearity for cetilistat was determined in the range of 25-75µg/ml. The regression equation was found to be y = 106.9x - 26.248 R?2; = 0.9996. Data for calibration curve was shown in Table 3 and the calibration curve was shown in Fig 3.
Table no 3:- Calibration data of cetilistat
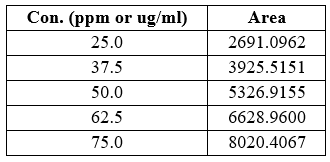
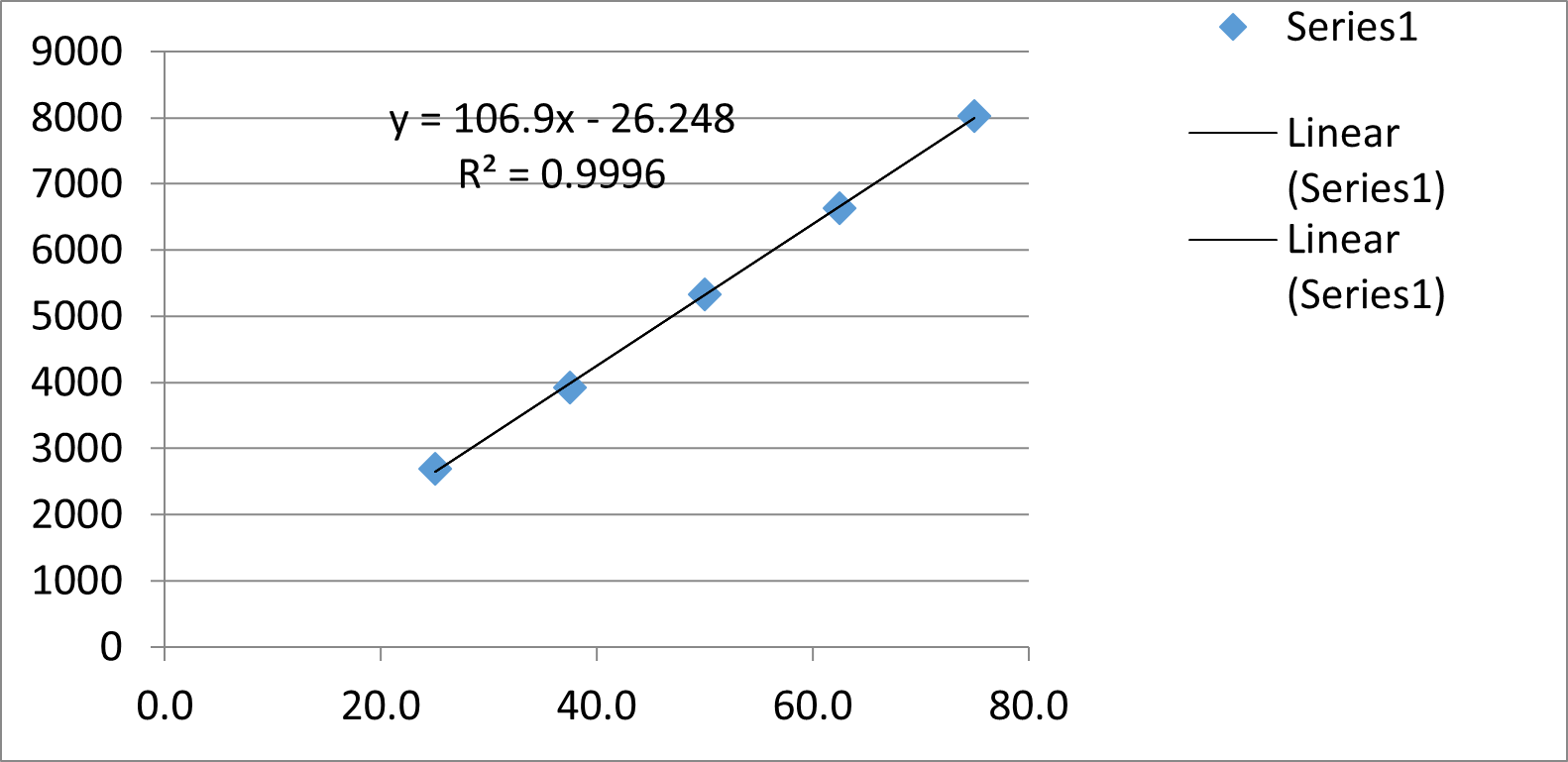
Fig 3:- Linearity graph of Cetilistat.
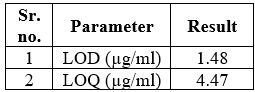
LOQ and LOD
LOD and LOQ were calculated from the equation and were found to be 1.48µg/ml and 4.47 µg/ml respectively shown in Table 4
Table no. 4:- LOD and LOQ of cetilistat
Accuracy
The accuracy of the analytical procedure for cetilistat was determined at 80%, 100% and 120% levels of standard solution. Results were expressed in terms of % recoveries. The % recovery was found in the range from 98.55% to 101.01% and was shown in Table 5.
Table no 5:- ?curacy

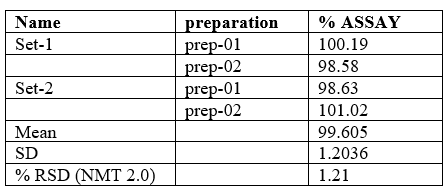
Precision
The precision results (measurement of intraday, interday, repeatability) showed good reproducibility and %RSD values were within limits which proved that method was highly precise. The results were shown in Table 6 and 7.
Table no 6:- Interday Precision Studies

Table no 7:- Intraday Precision Studies
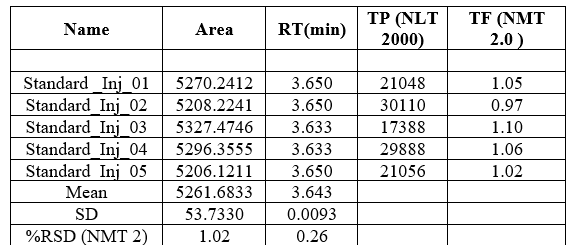
System suitability
These parameters were shown to be within specified limits. Column efficiency (theoretical plates), resolution factor and peak asymmetry factor, tailing factor, LLOQ are the system suitability parameters. These parameters of the optimized methods were found satisfactory. The results of the system suitability studies in plasma were shown in table 8. These parameters were shown to be within specified limits.
Table no 8:- System Suitability Studies
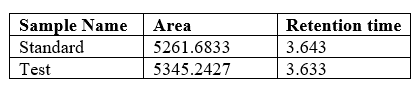
Specificity
Table 9 shows that retention time for standard and test sample of cetilistat are same. This shows that the method is highly selective.
Table no 9:- Specificity Studies of Cetilistat.
Robustness
Robustness was carried out by deliberate modification of analytical parameters, which indicated that retention time and peak area remained unaffected by small changes in wavelength, and flow rate. %RSD calculated was with in ICH limit thus indicating that method was sufficiently robust. The results were shown in Table 10.
Table no 10:- Robustness results of Cetilistat
CONCLUSION
The proposed RP-HPLC method was successfully validated for parameters such as linearity, specificity, accuracy, precision, LOD, LOQ and robustness as per ICH guidelines. The method was found to be simple, accurate, precise, highly sensitive, reproducible and. The proposed method was found suitable for determination of cetilistat. All the validation parameters were within the acceptance limits. Hence this method can be effectively applied to the routine analysis of cetilistat.
REFERENCE
- Kopelman, P., Bryson, A., Hickling, R., Rissanen, A., Rossner, S., Toubro, S., & Valensi, P. (2007). Cetilistat (ATL-962), a novel lipase inhibitor: a 12-week randomized, placebo-controlled study of weight reduction in obese patients. International journal of obesity, 31(3), 494-499.
- Kang, J. G., & Park, C. Y. (2012). Anti-obesity drugs: a review about their effects and safety. Diabetes & metabolism journal, 36(1), 13-25.
- Baheerati M, Devi G. Obesity in relation to Infertility. Research J. Pharm. Tech, 2018; 11(7): 3183-3185.
- Yamada Y, Kato T, Ogino H, et al. Cetilistat (ATL962), a Novel Pancreatic Lipase Inhibitor, Ameliorates Body weight Gain and Improved Lipid Profiles in Rats. Hormone and Metabolic Research, 2008; 40(8): 539-43.
- Padwal R. Cetilistat, a new lipase inhibitor for the treatment of obesity. Curr Opin Investig Drugs, 2008; 9(4): 414-21.
- ICH,. Q2 (R1) Validation of Analytical Procedures: Text and Methodology. ICH Harmonized Tripartite Guideline, 2005; 8–13.
- Kshirsagar S.A, Mane S.B, Hanchate Y.S, et al. UV Spectrophotometric Method Development and Validation for Determination of Cetilistat in API and in Pharmaceutical Dosage Form. Int. J. Pharm. Res. Scho, 2018; 7(1): 1-8.
- J Gras. Cetilistat for the treatment of obesity. Drugs Today (Barc), 2013; 49(12): 755-9.
- Padwal R. Cetilistat, a new lipase inhibitor for the treatment of obesity. Curr Opin Investig Drugs, 2008; 9(4): 414-21.
- ICH, Harmonized Tripartite Guideline. Stability Testing of New Drug Substances and Products (Q1AR2), in: Proceedings of the International Conference on Harmonization, Geneva, 2003.


 Omkar Bidkar *
Omkar Bidkar *
 V. M. Waghulkar
V. M. Waghulkar
 M. D. Game
M. D. Game













 10.5281/zenodo.11203334
10.5281/zenodo.11203334