Abstract
The papaya, or Carica papaya Linn., is a member of the Caricaceae family and is renowned worldwide for its medicinal and nutritional qualities. Since ancient times, the various parts of the papaya plant have been utilized for medicinal purposes. In this article, we reviewed papaya leaf's antiviral, antidiabetic, anticancer, and anti-inflammatory properties. The data reported in this review paper about the use of Carica papaya leaf extract for therapeutic purposes was gathered by searching through a number of electronic sources, including PubMed, Google Scholar, Scopus, and Web of Science. Up until December 2023, the following keywords were investigated: Carica papaya, anticancer, anti-inflammatory, immunomodulatory, and phytochemicals. The papaya plant—fruit, leaves, seeds, bark, latex, and all of its components—plays a significant part in controlling the course of illness. The active ingredients in Carica papaya leaf—alkaloids, glycosides, tannins, saponins, and flavonoids—are what give it its therapeutic properties. In addition, those with dengue fever have higher platelet counts when they consume papaya leaf juice. The main conclusions showed that papaya leaf extract had potent antimicrobial, antiviral, anticancer, hypoglycaemic, and anti-inflammatory effects. Moreover, research on papaya leaf's medicinal potential requires clinical trials.
Keywords
Papaya Carica. anti-cancer. anti-inflammatory. Immunostimulating,
Introduction
Since ancient times, people have used plants and plant-based products to prevent a variety of human ailments. Approximately 80% of the world's population receives their basic healthcare straight from plants. It has been reported that over 45,000 plant species in India have therapeutic qualities. Natural products or isolated plant chemicals have demonstrated a number of advantages over synthetic medications, including being easily accessible, affordable, and having little adverse effects. The use of medicinal plants for the treatment of a wide range of diseases has been documented in numerous research. Carica papaya Linn, a member of the Caricaceae family, is native to South America and Central America. It is widely grown in India and is used all over the world for medical purposes. The papaya plant is a perennial that often grows up to 20 meters tall. It has a smooth, unbranched stem and long, stalked leaves with five or six lobes. Various parts of the papaya plant, including the fruit, bark, roots, seeds, peel, pulp, and leaves, have numerous documented medicinal use worldwide (Table 1).

Table 1: Table 1: Medicinal uses of different part of papaya plant
The papaya plant is a good provider of calcium and iron and a nutritionally plentiful supply of vitamins A, B, and C. It contains the digestive enzyme papain, which is also used to heal ulcers and certain microbial disorders. At greater doses, papain is particularly efficient against gram-negative bacteria. Benzyl isothiocyanate, found in papaya fruit seed extract, has bacteriostatic, fungicidal, and bactericidal properties at a single effective dose of 4-5 g seeds (25–30 mg BIC). Papaya has high levels of antioxidant activity, which helps to neutralize the production of free radicals and stop the pathogenesis. Papain, glycyl endopeptidase, chymopapain, and caricain are among the most significant components of latex, which varies in quantity depending on the part of the papaya plant. Papaya leaf has been the subject of numerous scientific studies in recent years to investigate its potential medical applications. Papaya leaves have been used as medicine for a number of illnesses, including asthma, fever, beriberi, colica, and jaundice. Papaya leaf extract (PLE) is currently prepared using a variety of techniques; however, aqueous extract, ethanol extract, methanol extract, and freeze-dried papaya leaf juice are most frequently used for disease prevention. Papaya leaf can be used as a dietary supplement because it is high in proteins, fats, carbs, and vitamins. These substances range in concentration from 38.6% ascorbic acid, 5.6% protein, 0.225% phosphoric acid, 8.3?rbs, 0.0064% iron, and 0.035% minerals per 100 g of leaf part. Aqueous PLE was found to contain 0.001% tannins, 0.022% saponins, 0.013% flavonoids, 0.011% phenolic, 0.019% alkaloids, and 0.004% steroids, according to the quantitative phytochemical examination. Furthermore, PLE offers a treatment alternative for preventing the viral disease dengue. Outski and associates have recently documented the impact of PLE on tumour cell proliferation and demonstrated elevated human lymphocyte production of TH1-type cytokines. According to current reports, papaya leaf contains a number of active ingredients, including ascorbic acid, alpha-tocopherol, chymopapain, cyanogenic glucosides, cystatin, flavonoids, glycosinolates, and papain, that can increase blood antioxidant capacity and lower lipid peroxidation levels. Fig. 1 displayed the structures of bioactive secondary metabolites obtained from PLE. There have been numerous reports of anecdotal references for the treatment of lung adenocarcinoma, osteosarcoma, hepatocellular carcinoma, pancreatic epithelioid carcinoma, cervical carcinoma, mesothelioma, and breast cancer. Even a number of studies on the anticancer qualities of PLE made by Australian aboriginal people have since been published. Numerous scientific investigations have been conducted to identify and evaluate the bioactive components found in papaya leaves. According to the photochemical investigation, young leaves have antibacterial, anti-inflammatory, antiviral, hypoglycaemic, anticancer, and many other medicinal qualities because they include alkaloids, saponins, tannin, flavonoids, and glycosides.
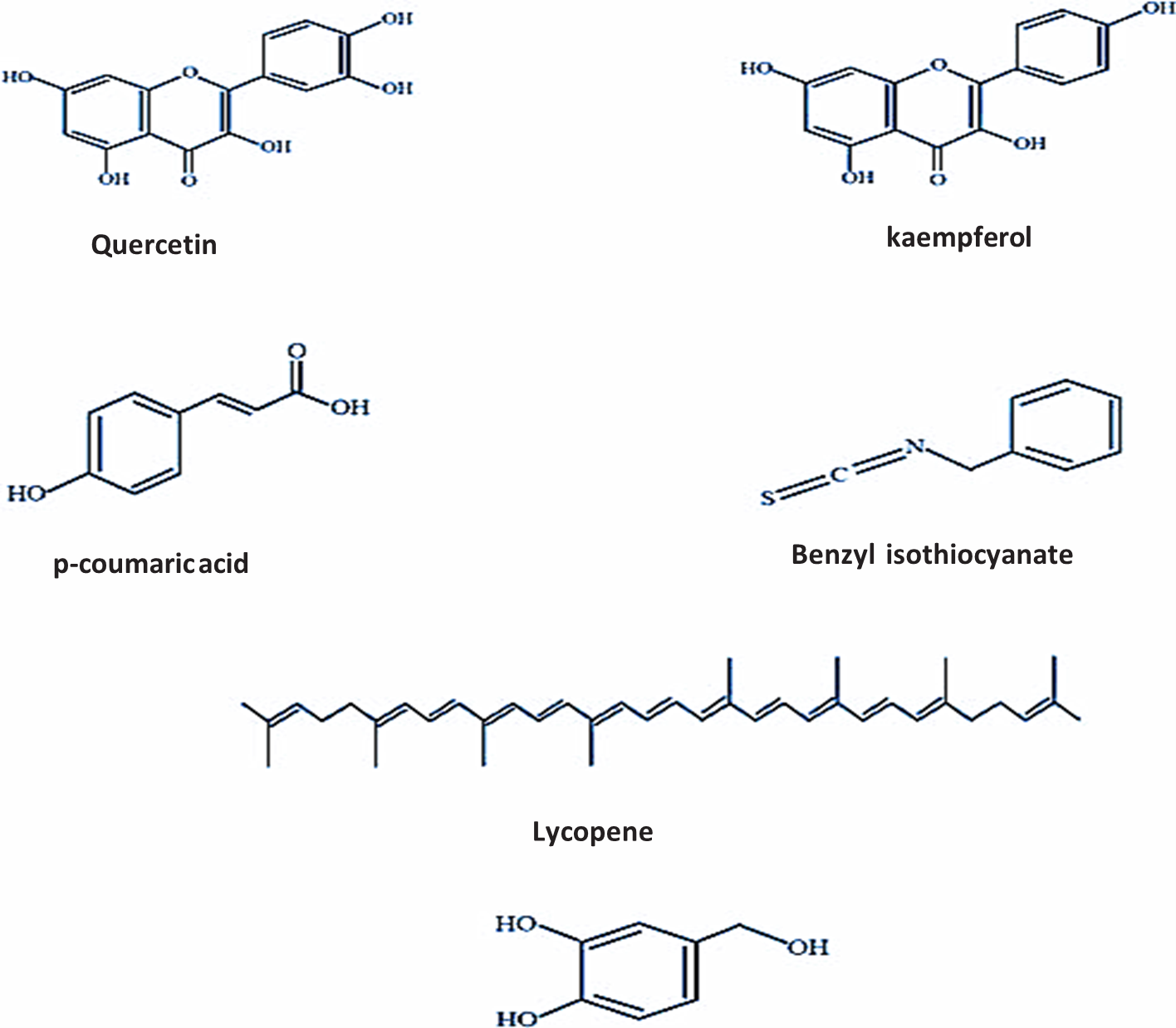
Figure 1 Carica papaya leaf derived compounds
The review primarily focuses on the medicinal qualities of Carica papaya leaf in managing and preventing the advancement of illness. Because papaya leaf extract (PLE) is a rich source of vitamins, minerals, and phytochemicals, it can be used medicinally to treat a variety of human ailments. Since ancient times, the implications of PLE for the management of several disorders have been chronicled in numerous literary works. Moreover, the present scientific studies support the possible significance of leaves in illness prevention, which is outlined below.
Mechanism of action of papaya leaf in health management
It is well known that PLE interacts with a wide variety of molecular targets and has anti-disease effects. The key molecular targets in the prevention of anticancer include DNA topoisomerase I/II activity suppression, signal pathway modifications, downregulation of Bcl-2 and Bcl-XL gene expression, upregulation of Bax, Bak, cleaved caspase 3, and upregulation of P53 gene expression. Nitric oxide (NO), costimulatory receptor (CD80), tumour necrosis factor-alpha (TNF-?), IL-12p40, IL-6, IL-12p70, IFN-?, and secretion of IL-2 and IL-4 were all enhanced during PLE treatment. Moreover, PLE regulates the release of chemokines CCL7, CCL2, and CCL8, as well as pro-inflammatory cytokines such as IL-1?, IL-6, IL-1?, and IL-8. According to reports, CPE therapy helps dengue patients by activating the expression of the PTAFR and ALOX 12 genes. The molecular pathways by which PLE treats different disorders are depicted in Figure 2. Moreover, in dengue virus-infected mice AG129, freeze-dried Carica papaya leaf reduces the production of inflammatory cytokines CCL6/MRP-1, CCL17/TARC, CCL12/MCP-5, CCL8/MCP-2, IL1RN/IL1Ra, IL1R1, PF4/CXCL4, and NAMPT/PBEF1. In diabetic rats, PLE also controls the release of insulin from ?-cells.
Anti-cancer effect of PLE
One of the deadliest diseases, cancer is caused by the unchecked division of genetically unstable cells and is one of the leading causes of mortality globally. Lung cancer is the most common cancer in men, followed by breast cancer in women, among the various malignancies that have been documented in humans, including colon, cervix, liver, stomach, lung, pancreatic, and breast cancer. There are currently a variety of cancer therapies available, depending on the type, stage, and location of the disease. These treatments include surgery, chemotherapy, radiotherapy, immunotherapy, vaccines, and combination therapy. Chemotherapy is a common treatment for cancer that has spread widely. Chemotherapeutic medications, such as vinblastine, doxorubicin, oxaliplatin, melphalan, carboplatin, cisplatin, cyclophosphamide, docetaxel, vincristine, and paclitaxel, among others, have demonstrated remarkable efficacy against a diverse array of cancer types and have demonstrated encouraging outcomes when used either in isolation or in conjunction with other cancer treatments. These medications do, however, come with several drawbacks, such as toxicity, quick elimination, non-specificity, and low absorption. High cytotoxicity, neutropenia, sensory neuropathy, cardiovascular toxicity, pulmonary and hematologic toxicity, gastrointestinal toxicity, diarrhoea, and nephrotoxicity are among the side effects that are commonly associated with these medications. As a result, experts are increasingly concentrating on employing alternative cancer treatments that have few or no adverse effects. Plant extracts and the equivalents created from them are considered the most promising option for treating cancer without causing any visible side effects during therapy, according to significant research findings. Researchers have recently become interested in various tropical plants, including papaya, since they have a rich phytochemical-based medicinal system. Scientists from all around the world are collaborating to develop a potential phytochemical-based cancer treatment with negligible side effects. After eating aqueous PLE, patients with blood, lung, liver, pancreatic, and stomach cancers had improved survival rates in numerous cases, according to a 2008 study by Morimoto et al. This discovery was subsequently patented by him. The most popular method used worldwide to make extracts from different papaya plant sections is cold juicing, which involves using a mortar and pestle. When compared to its aqueous and ethanol extracts, this approach has been evaluated for its ability to release bioactive chemicals that have substantial cytotoxic effects on cancer cells. When applied in vitro, the extract obtained using this method exhibits a strong anticancer impact against benign, malignant, and normal cells originating from the prostate; however, an oral dose may not yield the same result in an animal. Research has demonstrated that in vitro-digested PLE maintained its anti-proliferative response, albeit with less strength and efficacy than crude PLE, by navigating important pathways that are responsible for physiochemical alterations during human digestion. Table 2 summarizes the many cancer cell types that PLE has been tested against in vitro to determine its anticancer efficacy. Additionally, PLE demonstrated a possible anticancer effect by reducing the proliferation of prostate cancer cells, but with little effects on normal cells, perhaps through death and cell cycle arrest. There have been reports that the anticancer impact of PLE is attributed to the activation of the p53-dependent mitochondrial pathway and caspase-3/7. However, another study shown that PLE's antitumor activity is mediated by the arrest of PCa cells in the S phase, which is followed by cell death.
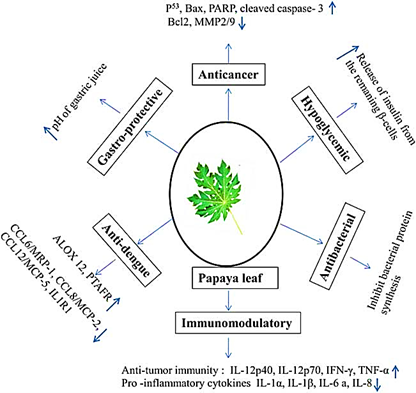
Figure 2 Molecular mechanisms of action of underlying therapeutic properties of papaya leaf
Furthermore, by lowering extracellular matrix (ECM), which serves as a chemo-attractant for PC-3 cell adhesion and migration, PLE has been demonstrated to reduce features of metastatic cancer, such as adhesion, migration, and invasion. To investigate the anti-metastatic potential of PLE and its compound(s) against various malignancies, however, more research is needed. An in vivo acute toxicity investigation discovered that rats given PLE orally at various doses ranging from 5 to 2000 mg/kg body weight (BW) did not exhibit any discernible adverse effects. Furthermore, rats did not experience any adverse effects after consuming PLE at a dose of 2 g/kg BW for 13 weeks. In comparison to the control group of C57BL/6 male mice, we have observed that oral administration of PLE (0.25%, 0.5%, and 1% v/v) in drinking water has not significantly changed BW, water consumption, or food intake.
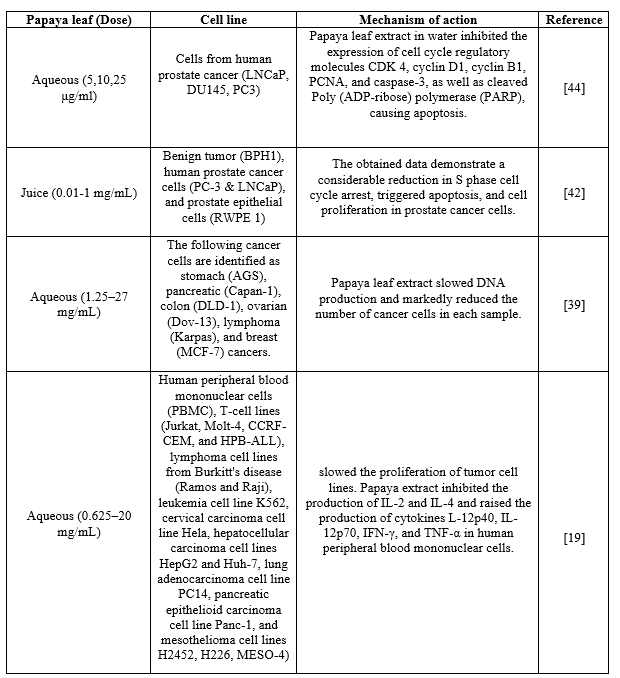
Table 2: In vitro anticancer effect of papaya leaf extract
Immunomodulatory effect of PLE
The results of experiments evaluating the various parts of the papaya plant indicate that it has great medicinal value and is helpful in treating a wide range of pathological illnesses, such as cancer, dengue fever, cardiovascular diseases, and wound healing. Strong immunomodulatory, anticancer, and anti-inflammatory effects of PLE on various cancer cell lines have been documented recently. However, there aren't many reports on mononuclear cells in peripheral blood (PBMC). A 2010 study demonstrated the immunomodulatory potential of PLE and assessed the cytokine profile of human peripheral blood mononuclear cells using ELISA. The study found that PLE dose-dependently downregulates the secretion of IL-4 and IL-2 in culture supernatants, raising the possibility that PLE may also cause apoptosis in PBMC, akin to its effect on cancer cells. Though there was only a minor impact on the production of IL-15, IL-6, IL-5, and IL-10, the secretion of Th1 type cytokines relevant to anti-tumour immunity, such as IL-12p70, IL-12p40, TNF-?, or IFN-?, was surprisingly elevated at a low dose of PLE. The results of this study indicate that there may be a strong relationship between increased Th1 type cytokine secretion and increased cytotoxicity, as IFN-?, TNF-?, and IL-12 have the ability to activate cell-mediated cytotoxicity, which in turn may lead to increased anti-tumour immunity. By inducing a shift in the immune response from Th2 to Th1 type, it further increases the likelihood that PLE may aid in the treatment of Th2-mediated allergic conditions such as bronchial asthma and allergic rhinitis, or function as an adjuvant for different vaccinations. The function of the bioactive components found in PLE extracted using polar solvents, primarily alcohol and water, in modifying immune-inflammatory indicators has been highlighted by a number of in vitro studies. Bertrand et al. (2014) demonstrated that the bacterial endotoxin lipopolysaccharide (LPS) stimulates innate immunity by influencing the production of many inflammatory mediators in monocytes/macrophages, including IL-6, IL-1?, IFN-?, and TNF-?. The pathophysiology of inflammation is significantly influenced by the pro-inflammatory cytokines IL-6, IL-1?, and IFN-?, which are released by monocytes or macrophages and are stimulated by TNF-?. This discovery inspired the researchers to identify the substance that may inhibit TNF-? production in the context of persistent inflammation. Bertrand et al. (2014) demonstrated that an ethanolic PLE markedly reduced the release of TNF-? in LPS-induced dendritic cells when isopentenyl pyrophosphate (IPP) was applied. Furthermore, it was observed that in LPS-stimulated human PBMCs, papaya leaf methanol extract reduced the secretion of pro-inflammatory IL-6, IL-1?, IL1?, TNF-?, and IL-8 by 42.9%, 27.4%, 12.5%, 10.8%, and 8.4%, respectively. Furthermore, methanolic PLE was shown to reduce nitric oxide (NO) release in IFN-? or LPS-activated murine macrophage cells (RAW 264.7 cell line) in 2011. An investigation revealed that in rats exposed with acrylamide, an aqueous extract of unripe papaya fruit markedly increased the levels of immunoglobulin IgG and IgM and modulated the levels of catalase, glutathione, superoxide dismutase, and malondialdehyde. All of the in vitro research presented here collectively suggests that papaya extracts may be able to change how inflammatory markers are expressed in a variety of stressed cell types. Since papaya extracts' polar solvent has been used in numerous in vitro experiments, more research is necessary to determine whether nonpolar extracts have anti-inflammatory properties. To comprehend the biological effects and possible applications of plant extracts for their pre-clinical importance, animal studies are necessary. When papaya is consumed in the form of fruit, peel, or leaf, Table 3 shows how it affects antioxidant enzymes, platelets, and inflammatory markers in both humans and animals. Additional in vivo research on humans and animals has also supported the anti-inflammatory and platelet-stimulating properties of PLE. Although there aren't many in vitro studies that have examined the immunomodulatory properties and processes of papaya fruit, the fruit has been the subject of several animal trials.
Anti-dengue effect of PLE
Dengue is a dangerous illness that strikes individuals all over the world; there are thought to be between 50 and 100 million cases annually. The dengue virus (DENV) 1-4, which is a member of the Flaviviridae family, is the cause of dengue and is spread via the bite of an Aedesaegypti mosquito. Four to seven days after the dengue virus incubates, the disease's symptoms—which include a high fever, rash, headache, vomiting, and muscle soreness—appear. One of the primary symptoms of dengue is thrombocytopenia, which is a decrease in platelet count that is used to diagnose dengue patients. According to the World Health Organization (WHO), thrombocytopenia is defined as a significant decline in platelet count, which is verified by a platelet count of less than 150,000 per microliter of blood. There isn't yet a vaccination or antiviral medication available to treat dengue fever. Only patients who require blood, blood components, and fluids for maintenance therapy or illness prevention are given supportive treatment. A vaccine to prevent dengue is on the horizon thanks to multiple ongoing clinical trials. As an alternative, further solutions must be investigated in order to combat the dengue nemesis. Numerous research projects have been undertaken to investigate the potential of herbal medicine as a complementary treatment for dengue complications. Numerous research has looked into the potential use of PLE in the treatment of dengue-related thrombocytopenia. These investigations showed that using papaya leaf extract to treat dengue patients considerably raises their platelet counts, which are normally lowered. Five dengue patients' platelet counts increased within twenty-four hours of receiving PLE medication, according to a research. Papaya leaf syrup was found to increase the mean platelet count in 285 patients as compared to control participants in a similar randomized controlled research. Comparable outcomes were noted in a Malaysian research including 228 participants. According to studies, the primary causes of bleeding in dengue patients are linked to a drop in platelet count, which is accompanied by an increase in plasma leakage and vascular permeability.
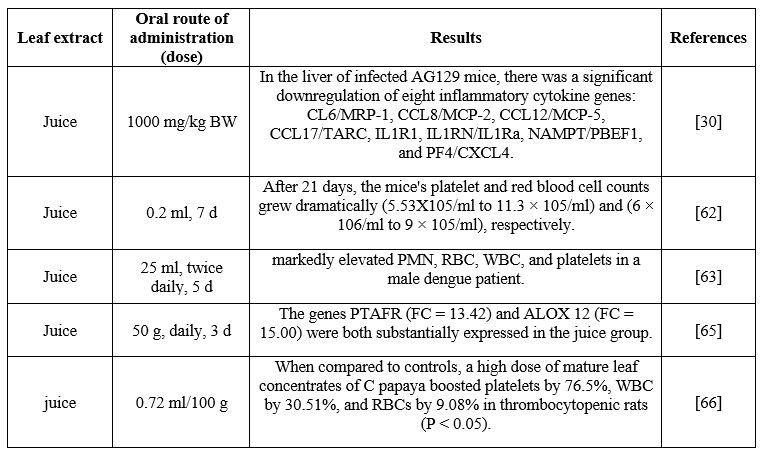
Table 3: In vivo studies of effect of papaya leaf extracts on dengue fever
It has been documented that the membrane-stabilizing abilities of PLE shield blood cells from damage brought on by stress. Patients with dengue can avoid platelet lysis by engaging in such activities. In dengue virus-infected mice AG129, freeze-dried Carica papaya leaf juice has also been shown to drastically reduce the production of inflammatory cytokines, such as CCL12/MCP-5, CCL8/MCP-2, CCL17/TARC, CCL6/MRP-1, IL1RN/IL1Ra, IL1R1, PF4/CXCL4, and NAMPT/PBEF1. It has been demonstrated that PLE causes an elevation of IFN-? expression in THP-1 cells and a decrease in the expression of DENV NS1 envelop protein. These findings are encouraging and indicate that in order to standardize the use of PLE for the prevention and treatment of dengue infection, comprehensive studies using animal models and clinical trials are required.
Hypoglycaemic effect of PLE
A set of metabolic disorders characterized by hyperglycaemia and a failure in insulin synthesis are called diabetes mellitus.
Diabetes is becoming more common as a result of a number of reasons, including poor diets, age, obesity, sedentary lifestyles, and causes linked to malnutrition. In 2017, 1.3% of people worldwide were expected to have diabetes, according to the American Diabetes Association. Because there are more and more people with diabetes in India, the country is regarded as the global centre for diabetes. By 2025, there will be 69.9 million individuals with diabetes worldwide, according to the International Diabetes Federation, which projected 40.9 million currently living with the disease. Many studies demonstrate the potent antidiabetic qualities of medicinal herbs, such as psyllium seeds, garlic, and bitter melon.
Papaya leaf has anti-hyperglycaemic and hypolipidemic properties in diabetic rats, according to a study. In a similar vein, the aqueous PLE increases the amount of insulin that the remaining ?-cells produce. The plasma concentrations of triacylglycerol and glucose were significantly reduced to 1.5 and 0.75 g/100 mL, respectively, upon the administration of the PLE [31]. Moreover, research indicates that fermented papaya improves the lipid profile and lowers basal and postprandial glycaemia. Moreover, to investigate the antidiabetic effect and molecular alterations brought on by PLE, mechanistic investigation and clinical studies are needed.
Sickle cell anaemia and PLE
A genetic condition that alters red blood cells' (RBCs') size and shape is sickle cell anaemia. The shape of normal red blood cells is changed to a sickle shape by a mutation in haemoglobin that results in the substitution of valine for glutamic acid at the sixth position in the beta-globin chain, according to molecular research. An essential component of oxygen delivery is haemoglobin. The three main signs of sickle cell disease are headaches, anaemia, and blood vessel obstruction. The prevalence of sickle cell disease has been steadily rising in developing nations. According to a 2012 WHO report, Nigerians account for a major portion of sickle cell disease cases worldwide. According to a study, pre-treating SS cell suspensions with PLE inhibited the growth of sickle cells in extreme hypoxia, resulting in 0-2% sickle cells compared to 60% sickle cells in untreated SS cell suspensions. In a different comparative study, the aqueous crude extract fraction at different treatment concentrations (1, 3, 5–10 mg/ml) reduced the formation of sickle cells; the highest concentration demonstrated a significant ant sickling property when compared to the crude methanol extract and the HbSS–sodium met bisulphite control. While 80% of the SS cells exhibit a sickle form, the extract concentrations of 5 and 10 mg/ml preserved the discoidal shape of the cells. Human crescent RBCs' osmotic fragility and level of haemoglobin polymerization were both reduced by the PLE. Additionally, studies on PLE revealed the presence of minerals like K, Mg, Ca, Fe, Mn, and Na, as well as amino acids like cysteine, glycine, and glutamic acid that shield the RBC membrane from lysis and destruction. It is not possible to investigate the mechanism of PLE for sickle cell disease prevention further.
Antibacterial activity of PLE
Few investigations have demonstrated the bactericidal efficacy of papaya leaf extracts from Carica. According to a study by Suresh et al., the Carica papaya leaf extract has the strongest antibacterial properties out of five plant extracts. Gram-positive bacteria (Pseudomonas aeruginosa, Bacillus subtilis, and Staphylococcus aureus) were tested, and PLE significantly decreased their growth. Gram-negative bacteria (Klebsiella pneumonia and Escherichia coli) were less affected by PLE. Plant extract inhibitor substances cannot enter gram-negative bacteria's cells due to their thick murrain layer in the outer membrane. Another study found that papaya leaves extracted in hot water, ethanol, methanol, ethyl acetate, acetone, or chloroform had excellent antibacterial properties against Staphylococcus aureus, Micrococcus luteus, Escherichia coli, Pseudomonas aeruginosa, and Bacillus cereus. Furthermore, a methanolic extract of PLE demonstrated antibacterial efficacy against Staphylococcus aureus, Escherichia coli, and Candida albicans. These seem to be preliminary findings that call for additional research on the molecular changes linked to its antibacterial activity.
Gastro-protective effect of PLE
A widespread gastrointestinal ailment affecting a significant portion of the global population are ulcers. Numerous risk factors, including non-steroidal anti-inflammatory medicines (NSAIDs), alcohol, stress, smoking, dietary deficiencies, and infections (Helicobacter pylori), may be linked to this condition. Odo and associates. Examined the potential of papaya leaf ethanoic extract in treating rats' experimentally-induced stomach ulcers. The results demonstrated that in rats with stomach ulcers caused by aspirin, there were significant (P < 0>
CONCLUSIONS AND FUTURE PERSPECTIVES
The therapeutic medicinal potential of papaya leaf for a variety of ailments was summarized in this review overall. Studies like the ones mentioned above have shown that papaya leaves contain bioactive phytochemicals that may help prevent and treat certain ailments. Given the current conditions, phytomolecules are anticipated to completely transform cancer prevention and therapy within the next ten years, offering a viable and potent substitute for pharmaceuticals. Thorough in vitro or in vivo research is necessary to assess the potential therapeutic uses of these phytochemicals before moving on to the clinic. Despite the encouraging results from several biochemical, cell culture, animal, and human investigations, more comprehensive research and clinical trials are required to fully understand the potential role of papaya in the treatment of a range of human disorders.
REFERENCES
- Tripathi L, Tripathi JN. Role of biotechnology in medicinal plants. Trop J Pharm Res. 2003;2(2):243–53.
- Jain SK. Ethnobotany and research in medicinal plants in India. Ethnobot Search New Drugs. 1994;185:153–68.
- Wang MW, Hao X, Chen K. Biological screening of natural prod- ucts and drug innovation in China. Philos Trans R SocLond B Biol Sci. 2007;362(1482):1093–105.
- Aravind G, Bhowmik D, Duraivel S, Harish G. Traditional and medicinal uses of Carica papaya. J Med Plants Stud. 2013;1(1):7– 15.
- Owoyele BV, Adebukola OM, Funmilayo AA, Soladoye AO. Anti- inflammatory activities of ethanolic extract of Carica papaya leaves. Inflammopharmacol. 2008;16(4):168–73.
- Vij T, Prashar Y. A review on medicinal properties of Carica papaya Linn. Asian Pac J Trop Dis. 2015;5(1):1–6.
- Leader B, Baca QJ, Golan DE. Protein therapeutics: a summary and pharmacological classification. Nat Rev Drug Discov. 2008;7(1): 21–39.
- Tang CS. Benzyl isothiocyanate of papaya fruit. Phytochemistry. 1971;10(1):117–21.
- Krishna KL, Paridhavi M, Patel JA. Review on nutritional, medic- inal and pharmacological properties of papaya (Carica papaya Linn.). Nat Prod Radiance. 2008;7(4):364–73.
- Wall MM. Ascorbic acid, vitamin a, and mineral composition of banana (Musa sp.) and papaya (Carica papaya) cultivars grown in Hawaii. J Food Compos Anal. 2006;19(5):434–45.
- Rahmani AH, Aldebasi YH. Potential role of carica papaya and their active constituents in the prevention and treatment of diseases. Int J Pharm Pharm Sci. 2016;8(1):11–5.
- Paul BI, Nasreen MA, Sarker AN, Islam MR. Isolation, purification and modification of papain enzyme to ascertain industrially valu- able nature. Int J Biotechnol Res. 2013;3(5):11–22.
- Patil T, Patil S, Patil A, Patil S. Carica papaya leaf extracts–an Ethnomedicinal boon. Int J Pharmacogn Phytochem Res. 2014;6(2):260–5.
- Hu T, Guo YY, Zhou QF, Zhong XK, Zhu L, Piao JH, et al. Optimization of ultrasonic-assisted extraction of total saponins from Ecliptaprostrasta L. using response surface methodology. J Food Sci. 2012;77(9):C975–82.
- Longdet IY, Adoga EA. Effect of methanolic leaf extract of Carica papaya on plasmodium berghei infection in albino mice. Eur J Med Plants. 2017;20(1):1–7.
- Saran PL, Choudhary R. Drug bioavailability and traditional medi- caments of commercially available papaya: a review. Afr J Agric Res. 2013;8(25):3216–23.
- Bamisaye FA, Ajani EO, Minari JB. Prospects of ethnobotanical uses of pawpaw (Carica papaya). J Med Plants. 2013;1(4):171–7.
- Sarala N, Paknikar SS. Papaya extract to treat dengue: a novel therapeutic option?.Ann Med Health Sci Res. 2014;4(3):320–4.
- Otsuki N, Dang NH, Kumagai E, Kondo A, Iwata S, Morimoto C. Aqueous extract of Carica papaya leaves exhibits anti-tumor activ- ity and immunomodulatory effects. J Ethnopharmacol. 2010;127(3):760–7.
- Seigler DS, Pauli GF, Nahrstedt A, Leen R. Cyanogenicallosides and glucosides from Passifloraedulis and Carica papaya. Phytochemistry. 2002;60(8):873–82.
- Hadadi SA, Li H, Rafie R, Kaseloo P, Witiak SM, Siddiqui RA. Anti-oxidation properties of leaves, skin, pulp, and seeds extracts from green papaya and their anti-cancer activities in breast cancer cells. J Cancer Metastasis Treat. 2018;4:25.
- Liew SY, Stanbridge EJ, Yusoff K, Shafee N. Hypoxia affects cel- lular responses to plant extracts. J Ethnopharmacol. 2012;144(2): 453–6.
- Saranya V, Malathi N. Evidence-based review on anticancer effects of commonly used herbs. J AdvClin Res Insights. 2014;1(2):73–7.
- Webb LJ. The use of plant medicines and poisons by Australian aborigines. Aust J Anthropol. 1969;7(2):137–46.
- Nugroho A, Heryani H, Choi JS, Park HJ. Identification and quan- tification of flavonoids in Carica papaya leaf and peroxynitrite- scavenging activity. Asian Pac J Trop Biomed. 2017;7(3):208–13.
- Rumiyati S. Effect of the protein fraction of Carica papaya L. leaves on the expressions of P53 and BCL-2 in breast cancer cells line. Maj Farm Indones. 2006;17:170–6.
- Singh SP, Kumar S, Tomar MS, Singh RK, Verma PK, Kumar A, et al. Aqueous extract of Carica papaya leaf elicits the production of TNF-? and modulates the expression of cell surface receptors in tumor-associated macrophages. Biosc Biotech Res. 2019;4:1115– 22.
- Salim E, Kumolosasi E, Jantan I. Inhibitory effect of selected me- dicinal plants on the release of pro-inflammatory cytokines in lipopolysaccharide-stimulated human peripheral blood mononucle- ar cells. J Nat Med. 2014;68(3):647–53.
- Siddique O, Sundus A, Ibrahim MF. Effects of papaya leaves on thrombocyte counts in dengue–a case report. JPMA J Pak Med Assoc. 2014;64(3):364–6.
- Norahmad NA, Razak MR, Misnan NM, Jelas NH, Sastu UR, Muhammad A, et al. Effect of freeze-dried Carica papaya leaf juice on inflammatory cytokines production during dengue virus infec- tion in AG129 mice. BMC Complement Altern Med. 2019;19(44): 1–10.
- Juárez-Rojop IE, Díaz-Zagoya JC, Ble-Castillo JL, Miranda-Osorio PH, Castell-Rodríguez AE, Tovilla-Zárate CA, et al. Hypoglycemic effect of Carica papaya leaves in streptozotocin-induced diabetic rats. BMC Complement Altern Med. 2012;12(1):236.
- Society AC. Cancer facts & figures. Am Cancer Soc. 2016.
- Weaver BA. How Taxol/paclitaxel kills cancer cells. Mol Biol Cell. 2014;25(18):2677–81.
- Caruso M, Colombo AL, Fedeli L, Pavesi A, Quaroni S, Saracchi M, et al. Isolation of endophytic fungi and actinomycetestaxane producers. Annales de Microbiologie. 2000;50(1):3–13.
- Patra CR, Mukherjee S, Kotcherlakota R. Biosynthesized silver nanoparticles: a step forward for cancer theranostics. Nanomed. 2014;9(10):1445–8.
- Chua LK, Lim CL, Ling AP, Chye SM, Koh RY. Anticancer poten- tial of Syzygium species: a review. Plant Foods Hum Nutr. 2019;74(1):18–27.
- Singh S, Sharma B, Kanwar SS, Kumar A. Lead phytochemicals for anticancer drug development. Front Plant Sci. 2016;7:1–13.
- Rasmann S, Agrawal AA. Latitudinal patterns in plant defense: evolution of cardenolides, their toxicity and induction following herbivory. Ecol Lett. 2011;14(5):476–83.
- C Morimoto, N Dang, inventors; Dang Nam H, assignee. Compositions for cancer prevention, treatment, or amelioration comprising papaya extract. United States patent application US 11/631,655. 2008.
- Nguyen TT, Parat MO, Hodson MP, Pan J, Shaw PN, Hewavitharana AK. Chemical characterization and in vitro cyto- toxicity on squamous cell carcinoma cells of Carica papaya leaf extracts. Toxins. 2016;8(1):1–11.
- Nguyen TT, Parat MO, Shaw PN, Hewavitharana AK, Hodson MP. Traditional aboriginal preparation alters the chemical profile of Carica papaya leaves and impacts on cytotoxicity towards human squamous cell carcinoma. PLoS One. 2016;11(2):1–15.
- Pandey S, Walpole C, Cabot PJ, Shaw PN, Batra J, Hewavitharana AK. Selective anti-proliferative activities of Carica papaya leaf juice extracts against prostate cancer. Biomed Pharmacother. 2017;89:515–23.
- Bouayed J, Hoffmann L, Bohn T. Total phenolics, flavonoids, an- thocyanins and antioxidant activity following simulated gastro- intestinal digestion and dialysis of apple varieties: bioaccessibility and potential uptake. Food Chem. 2011;128(1):14–21.
- SP Singh, SV Mathan, A Dheeraj, D Tailor, RP Singh, A Acharya. Anticancer effects and associated molecular changes of Carica papaya against prostate cancer. AACR; Cancer Res. 2019; 79(13): Abstract nr 3004.
- Cragg GM, Newman DJ. Plants as a source of anti-cancer agents. J Ethnopharmacol. 2005;100(1–2):72–9.
- Rumiyati S. Effect of the protein fraction of Carica papaya L. leaves on the expressions of P53 and BCL-2 in breast cancer cells line. Maj FarmIndones. 2006;17:170–6.
- Fauziya S, Krishnamurthy R. Papaya (Carica papaya): source ma- terial for anticancer. CIBTech J Pharm Sci. 2013;2(1):25–34.
- Nguyen TT, Shaw PN, Parat MO, Hewavitharana AK. Anticancer activity of Carica papaya: a review. Mol Nutr Food Res. 2013;57(1):153–64.
- Ismail Z, Halim SZ, Abdullah NR, Afzan A, Rashid A, Amini B, et al. Safety evaluation of Oral toxicity of Carica papaya Linn. Leaves: a subchronic toxicity study in Sprague Dawley rats Evid Based Complement. 2014;2014:1–10.
- Halim SZ, Abdullah NR, Afzan A, Rashid BA, Jantan I, Ismail Z. Acute toxicity study of Carica papaya leaf extract in Sprague Dawley rats. J Med Plant Res. 2011;5(10):1867–72.
- Cooper CR, McLean L, Walsh M, Taylor J, Hayasaka S, Bhatia J, et al. Preferential adhesion of prostate cancer cells to bone is medi- ated by binding to bone marrow endothelial cells as compared to extracellular matrix components in vitro. Clin Cancer Res. 2000;6(12):4839–47.
- Maniyar Y, Bhixavatimath P. Antihyperglycemic and hypolipid- emic activities of aqueous extract of Carica papaya Linn. Leaves in alloxan-induced diabetic rats. J Ayurveda Integr Med. 2012;3(2): 70–4.
- Imaga NA, Gbenle GO, Okochi VI, Adenekan S, Duro-Emmanuel T, Oyeniyi B, et al. Phytochemical and antioxidant nutrient constit- uents of Carica papaya and Parquetinanigrescens extracts. Sci Res Essays. 2010;5(16):2201–5.
- Gurung S, Škalko-Basnet N. Wound healing properties of Carica papaya latex: in vivo evaluation in mice burn model. J Ethnopharmacol. 2009;121(2):338–41.
- Anjum V, Arora P, Ansari SH, Najmi AK, Ahmad S. Antithrombocytopenic and immunomodulatory potential of meta- bolically characterized aqueous extract of Carica papaya leaves. Pharm Biol. 2017;55(1):2043–56.
- Foulds KE, Wu CY, Seder RA. Th1 memory: implications for vac- cine development. Immunol Rev. 2006;211(1):58–6.
- Baxevanis CN, Voutsas IF, Tsitsilonis OE, Gritzapis AD, Sotiriadou R, Papamichail M. Tumor-specific CD4+ T lymphocytes from can- cer patients are required for optimal induction of cytotoxic T cells against the autologous tumor. J Immunol. 2000;164(7):3902–12.
- Pandey S, Cabot PJ, Shaw PN, Hewavitharana AK. Anti- inflammatory and immunomodulatory properties of Carica papaya. J Immuno toxicol. 2016;13(4):590–602.
- Sagnia B, Fedeli D, Casetti R, Montesano C, Falcioni G, Colizzi V. Antioxidant and anti-inflammatory activities of extracts from Cassia alata, Eleusine indica, Eremomastax speciosa, Carica papaya and Polyscias fulva medicinal plants collected in Cameroon. PLoS One. 2014;9(8):1–10.
- Lee KH, Padzil AM, Syahida A, Abdullah N, Zuhainis SW, Maziah M, Sulaiman MR, Israf DA, Shaari K, Lajis NH. Evaluation of anti- inflammatory, antioxidant and antinociceptive activities of six Malaysian medicinal plants. J Med Plant Res 2011 Oct 23;5(23): 5555–5563.
- Sadek KM. Antioxidant and immunostimulant effect of Carica pa- paya Linn. Aqueous extract in acrylamide intoxicated rats. Acta Inform Med. 2012;20(3):180–5.
- Dharmarathna SL, Wickramasinghe S, Waduge RN, Rajapakse RP, Kularatne SA. Does Carica papaya leaf-extract increase the platelet count? An experimental study in a murine model. Asian Pac J Trop Biomed. 2013;3(9):720–4.
- Ahmad N, Fazal H, Ayaz M, Abbasi BH, Mohammad I, Fazal L. Dengue fever treatment with Carica papaya leaves extracts. Asian Pac J Trop Biomed. 2011;1(4):330–3.
- Yunita F, Hanani E, Kristianto J. The effect of Carica papaya L. leaves extract capsules on platelets count and hematocrit level in dengue fever patient. Int J Med Aromat Plants. 2012;2(4):573–8.
- Subenthiran S, Choon TC, Cheong KC, Thayan R, Teck MB, Muniandy PK, et al. Carica papaya leaves juice significantly accel- erates the rate of increase in platelet count among patients with dengue fever and dengue haemorrhagic fever. Evid Based Complement Alternat Med. 2013;2013:1–7.
- Gammulle A, Ratnasooriya WD, Jayakody JR, Fernando C, Kanatiwela C, Udagama PV. Thrombocytosis and anti- inflammatory properties and toxicological evaluation of Carica pa- paya mature leaf concentrate in a murine model. Online Int J Med Plant Res. 2012;1(2):21–30.
- Oishi K, Saito M, Mapua CA, Natividad FF. Dengue illness: clin- ical features and pathogenesis. J Infect Chemother. 2007;13(3): 125–33.
- Kala CP. Leaf juice of Carica papaya L. a remedy of dengue fever. Med Aromat Plants. 2012;1(6):1–2.
- Srikanth BK, Reddy L, Biradar S, Shamanna M, Mariguddi DD, Krishnakumar M. An open-label, randomized prospective study to evaluate the efficacy and safety of Carica papaya leaf extract for thrombocytopenia associated with dengue fever in pediatric sub- jects. Pediatric Health Med Ther. 2019;10:5–11.
- Srikiatkhachorn A. Plasma leakage in dengue haemorrhagic fever. Thromb Haemost. 2009;102(12):1042–9.
- Ranasinghe P, Ranasinghe P, Abeysekera WK, Premakumara GS, Perera YS, Gurugama P, et al. In vitro erythrocyte membrane stabi- lization properties of Carica papaya L. leaf extracts. Pharmacogn. Res. 2012;4(4):196–202.
- Sharma N, Mishra KP, Chanda S, Bhardwaj V, Tanwar H, Ganju L, et al. Evaluation of anti-dengue activity of Carica papaya aqueous leaf extract and its role in platelet augmentation. Arch Virol. 2019;164(4):1095–110.
- Pandey SK, Sharma V. World diabetes day 2018: battling the emerging epidemic of diabetic retinopathy. Indian J Ophthalmol. 2018;66(11):1652–3.
- Gupta M, Prabhu K, Parijatham BO, Kalaiselvi VS, Rajendran SM, Rose J. Prevalence of diabetes mellitus in South India: a retro spective analysis. JIMSA. 2012;25(4):239–40.
- Fakeye TO, Oladipupo T, Showande O, Ogunremi Y. Effects of coadministration of extract of Carica papaya Linn (family Cariaceae) on activity of two oral hypoglycemic agents. Trop J Pharm Res. 2007;6(1):671–8.
- Aruoma OI, Somanah J, Bourdon E, Rondeau P, Bahorun T. Diabetes as a risk factor to cancer: functional role of fermented papaya preparation as phytonutraceutical adjunct in the treatment of diabetes and cancer. Mutat Res. 2014;768:60–8.
- Gardner RV. Sickle cell disease: advances in treatment. Ochsner J. 2018;18(4):377–89.
- Ambe JP, Mava Y, Chama R, Farouq G, Machoko Y. Clinical fea- tures of sickle cell anaemia in northern nigerian children. West Afr J Med. 2012;31(2):81–5.
- Imaga NO, Gbenle GO, Okochi VI, Akanbi SO, Edeoghon SO, Oigbochie V, et al. Antisickling property of Carica papaya leaf extract. Afr J Biochem Res. 2009;3(4):102–6.
- Nurain IO, Bewaji CO, Johnson JS, Davenport RD, Zhang Y. Potential of three Ethnomedicinal plants as Antisickling agents. Mol Pharm. 2017;14(1):172–82.
- Suresh K. Antimicrobial and phytochemical investigation of the leaves of Carica papaya L., Cynodondactylon (L.) Pers., Euphorbia hirta L., Meliaazedarach L. and Psidiumguajava L. Ethnobot Leaflets. 2008;12:1184–91.
- Nirosha N, Mangalanayaki R. Antibacterial activity of leaves and stem extract of Carica papaya L. Int J Adv Pharm Biol Chem. 2013;2(3):473–6.
- Baskaran C, Velu S, Kumaran K. The efficacy of Carica papaya leaf extract on some bacterial and a fungal strain by well diffusion method. Asian Pac J Trop Dis. 2012;2:S658–62.


 Waghmare Jagdish A.*
Waghmare Jagdish A.*
 Bankar A.S.
Bankar A.S.





 10.5281/zenodo.10938605
10.5281/zenodo.10938605