Abstract
Oxadiazoles are a class of heterocyclic aromatic chemical compound of the azole family.1,3,4-oxadiazole is a nitrogen and oxygen containing heterocyclic derivative in which nitrogen in 3rd and 4th positions and oxygen in 1st position with chemical formula, C2 H2N2O and molecular weight 70.051g.mol-1. 1,3,4- oxadiazole is also known asOxdiazole,1-Oxa-3,4diazacyclopentadiene. 1,3,4- oxadiazole and its derivatives were used by several chemists for therapeutic conditions because it posses many pharmacological activities. 1,3,4- oxadiazole and its derivatives have great significance in medicinal chemistry. 1,3,4-oxadiazole have found extensive applications in the pharmaceutical industry. It is correlated with many pharmacological effects like anti-cancer, anti-bacterial activity, anthelminthic activity, anti-convulsant, anti-inflammatory activity, anti-microbial, anti-viral activity, antioxidant, anti-analgesic, thyrosinase inhibitor and anti-tubercular activity. This review explores the various pharmacological effects of novel 1, 3, 4-Oxadiazole derivatives, different method of synthesis of 1,3,4- oxadiazole and their importance in biomedical research. In this review, the highlights in biological behaviour, synthesis and versatile activities of 1,3,4-oxadiazole and its derivatives are described.
Keywords
1,3,4- oxadiazole, Anti-cancer, Anti-convulsant, Anti-tubercular, , Tyrosinase inhibitory, Anti-allergic, Anti-inflammatory.
Introduction
Oxadiazoles are the heterocyclic compounds containing one oxygen and two nitrogen atoms in a five membered ring have been notable in having various promising medicinal effects. Oxadiazoles are a class of heterocyclic aromatic compound belongs to azole family. It is found to have anticancer, anti-bacterial activity, anthelminthic activity, anti-convulsant, anti-inflammatory activity, antimicrobial, anti-viral activity, anti-oxidant and anti-tubercular activity.[1,2,3]

Fig no 1; 1,3,4-oxadiazole nucleus
1,3,4-oxadiazole seems to be a ‘privileged structure’ for further screening and synthesis of the new drug analogues against life threatening HIV and cancer like
diseases.[4]
Table no:1; Physical properties of 1,3,4-oxadiazole

CHEMISTRY OF OXADIAZOLES
Oxadiazoles are compounds that are composed of a five membered heterocyclic ring that contain two nitrogen atoms and one oxygen atom. Oxadiazoles exists in different isomeric forms due to the difference in the arrangement of heteroatoms. E.g.; 1,3,4-oxadiazole, 1,2,3-oxadiazole, 1,2,4oxadiazole and 1,2,5-oxadiazole. Aromatic systems are also known as azoxins and the same number of nitrogen and oxygen atoms containing five membered cyclic molecules that have been partially reduced are known as furoxanes. 1,2, 3-oxadiazole is an extremely unstable structure due to the presence of open ring i.e., the diazo-ketone tautomer.1,2,4-oxadiazoles are stable thermodynamically and their reactivity is influenced by aromaticity.[5,6]
SYNTHESIS OF 1,3,4-OXADIAZOLE
Ainsworth in 1965 first described the preparation of unsubstituted 1,3,4oxadiazole carried out by applying thermolysis at atmospheric pressure to formyl hydrazone ethylformate.

Ethyl(2E)-(2-formylhydrazinyildene)acetate 1,3,4-oxadiazole
Ethanol
Fig no:2; synthesis of 1,3,4-oxadiazole
POSSIBLE METHODS FOR THE PREPARATION OF THE 1,3,4-OXADIAZOLES

Fig 3; Methods for the synthesis of 1,3,4-oxadiazole [7,8]
BIOTIC EFFECT
ANTI-CANCER ACTIVITY
N. Yadav et al designed, synthesized a series of new 1,3,4-oxadiazole-2(3H)-thione analogues and evaluated their anticancer activity. The four different cancerous cell lines like HeLa (cervical), U-87 (glioblastoma), MCF-7 (breast)and Panc (pancreatic) were used to assess the potency of the synthesized compounds as anticancer agents.
Among them fig 4a and 4b showed cytotoxicity against HeLa cell line. Further, as visualized by Annexin V APC and DNA fragmentation assay, it was found that 4a and 4b make the cell cycle progression inhibited and displayed cell death in HeLa cells via apoptosis. 4a and 4b induced caspase-3 activation, PARP cleavage, high expression of proapoptotic protein Bax and low expression of antiapoptotic protein Bcl-2. Also, 4a and 4b induced overexpression of p21 and low expression of cyclin B1 indicating the arrest of cells in G2-M phase of the cell cycle. Therefore, new lead compounds having anticancer activity through cell cycle inhibition and apoptosis are being suggested.[9,10,11,12]

Fig 4a
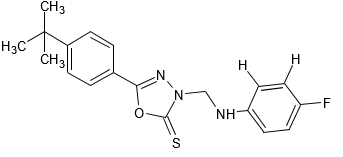
Fig 4b
ANTI-CONVULSANT ACTIVITY
Shafiee et al explained the synthesis of a series of 5-[2-(phenylthio) phenyl]1,3,4-oxadiazole, 1,3,4-thiadiazole and 1,2,4-triazole derivatives. Compounds were evaluated for their anticonvulsant and muscle relaxant activities (in vivo) using pentylenetetrazole (PTZ) and rotarod tests, respectively. However, most of the compounds were active in rotarod test and the most effective compound was 5-[2(phenylthio) phenyl]-1,3,4-oxadiazole-2(3H)-one (Fig 5) which had comparable activity with diazepam.

Fig 5

Fig 6
For the purpose of evaluating the effects of different substituents on pharmacological activity a new series of 1,3,4-oxadiazole, 1,2,4-triazole and 1,3,4thiadiazole derivatives by bioisosteric replacement of oxygen with sulphur in phenoxy group were synthesized. These compounds were performed by Rotarod and pentylenetetrazole (PTZ) induced lethal convulsion tests and the results were compared with a known BZ agonist, diazepam. In previous studies a new group 1,2,4-triazole and 1,3,4-oxadiazole rings (Fig 6) were designed, synthesized and are flexible BZ agonists . The designed structure had an aromatic ring and a coplanar proton-accepting group : BZ pharmacophores, number 2 or 3 nitrogen of 1,2,4-triazole or 1,3,4-oxadiazole rings. Phenoxy group, a second out-of-plane aromatic ring could potentiate binding to the receptor.

The derivatives that contains 1,3,4-oxadiazole ring in this study are amino-5[2-(phenylthio)phenyl]-1,3,4-oxadiazole(Fig 8), 15-[2(Phenylthio)phenyl]-1,3,4oxadiazole 2(3H)thione(Fig9),2alkylthio5[2(phenylthio)phenyl]1,3,4oxadiazoles(Fig 10), 1,3,4-oxadiazol-2-one(Fig 13), 3-alkyl-5-[2-(phenylthio) phenyl]-1,3,4oxadiazol-2ones (Fig 11) and they exhibit anti-convulsant activity.[13,14]
ANTI-MICROBIAL ACTIVITY
G.Sahin et al explained six new synthesis of 5-(1-/2-naphthyloxymethyl)-1,3,4oxadiazole2(3H)-thione,2-amino-5-(1-/2-naphthyloxymethyl)-1,3,4-oxadiazole5-(1-/2naphthyloxymethyl)-1,3,4oxadiazole-2(3H)-one derivatives from 1-and/or 2-naphthol. The structures of the compounds were confirmed by IR and 1H NMR spectral data and microanalysis. The antimicrobial properties of the compounds were investigated using micro broth dilution method against Staphylococcus aureus, Escherichia coli and Pseudomonas aeruginosa, Candida albicans, C. krusei and C. parapsilosis . 2-Amino-5-(2naphthyloxymethyl)-1,3,4-oxadiazole and 5-(2naphthyloxymethyl)-1,3,4-oxadiazole-2(3H)one show significantly (32 g/ml), compounds 5-(1-/2naphthyloxymethyl)-1,3,4-oxadiazole- 2(3H)-thione, 2-amino-5-(1-naphthyloxymethyl)-1,3,4oxadiazole and 5-(1naphthyloxymethyl)-1,3,4-oxadiazole-2(3H)-one moderately (64 g/ml) active against C. krusei. All the compounds were active against S. aureus, E. coli, P. aeruginosa, C.albicans and C. parapsilosis at 64 -256 g/ml concentration. 1,3,4-oxadiazole ring is associated with many types of biological properties like antiinflammatory (Fig 13-15), hypoglycaemic (Fig 16). Some 5-[isoxazolo [5, 4- d] pyrimidinyloxymethyl] - 2 – substituted phenylamino1,3,4-oxadiazole and 1,3,4-oxadiazole2(3H)thione derivatives were reported to have significant antimicrobial activity against Staphylococcus aureus and Candida albicans. Keeping the above facts in view, they considered the interest to synthesize some new derivatives such as 5-(1-2- naphthyloxymethyl) - 1,3,4 - oxadiazole 2(3H)-thione(Fig 14), 2-amino-5-(1-2-naphthyloxymethyl)-1,3,4-oxadiazole(Fig 15),5-(1-2-naphthyloxymethyl)-1,3,4-oxadiazole2(3H)-one (Fig 16) and their antimicrobial activity against S.aureus, Escherichia coli and pseudomonas aeruginosa, C. albicans, C. krusei and C. parapsilosis using micro broth dilution method. The reference compounds used were Ceftazidime and Fluconazole in antibacterial and antifungal activity studies.

By using micro broth dilution method 5-(1-2-Naphthyloxymethyl)1,3,4oxadiazole2(3H)thione(14), 2-Amino-5-(1-2naphthyloxymethyl)-1,3,4-oxadiazole(3a–b), 5(1-2-Naphthyloxymethyl)-1,3,4-oxadiazole2(3H)one (Fig 16) prepared in the scheme1 were tested against some Gram-positive and Gram-negative bacteria like S. aureus (ATCC 25923), E. coli (ATCC 25922) and P. aeruginosa (ATCC 27853) .The antifungal activities of the compounds were evaluated in vitro against some yeast like fungi such as Candida albicans (ATCC 90028), C. Krusei (ATCC 6258) and C. parapsilosis (ATCC 22018). Ceftazidime and Fluconazole were used as reference compounds in antibacterial and antifungal activity studies.[15,16,17]
ANTI-TUBERCULAR ACTIVITY
Sambhaji T. Dhumala et al explained the synthesis and anti-tubercular activity of new 1,3,4-oxadiazoles bearing pyridyl and thiazolyl rings, 2-pyridinyl substituted thiazolyl-5-aryl-1,3,4-oxadiazoles(Fig 17), also have been designed and synthesized. Compounds were screened for their invitro antitubercular activity against Mycobacterium tuberculosis H37Ra (MTB) and Mycobacterium bovis BCG. These compounds shows low cytotoxicity towards four human cancer cell lines. Tuberculosis posses a significant health concern and the treatment requires longer duration to address the patterns effectively. Amino thiazoles and thiazolylhydrazones are compounds with potential pharmacological applications due to their diverse chemical structures. Their reported antitubercular activity suggests they could be useful in the fight against tuberculosis, a significant global health concern. Research into the synthesis and evaluation of novel compounds for their biological activities, such as antitubercular activity, is crucial for discovering new drugs or improving existing treatments. The work done by Balkan and co-workers could contribute valuable insights into the development of therapies for tuberculosis. 1,3,4-oxadiazoles are a class of compounds known for their diverse biological activities such as antibacterial, antitubercular, antitumor, antifungal, anti-inflammatory. Combining 1,3,4-oxadiazoles with thiazoles, pyridines, indoles, quinolines and pyrroles has demonstrated increased efficacy against
tuberculosis .[18,19]

Fig 17a
Fig17; 2-pyridinyl substituted thiazolyl-5-aryl-1,3,4-oxadiazole
TYROSINASE INHIBITOR ACTIVITY
H. Khalilullah et al carried out the various biological activities of 1,3,4oxadiazole derivatives. A series of 2,5-disubstituted-1,3,4-oxadiazoles were synthesized and evaluated for their inhibitory effects on the enzyme tyrosinase, revealing insights into their structure-activity relationship (SAR). It was concluded that the presence of electronegative substituents and specific positioning within the molecule are crucial for effective inhibition, likely due to interactions with hydrophobic sites in the enzyme's active site. Compound 3-[5-(4-bromophenyl)1,3,4-oxadiazol-2-yl] pyridine (18b) demonstrated the most potent inhibition (IC50 =2.18 M) against tyrosinase, surpassing the standard inhibitor L-mimosine in potency.[20,21]
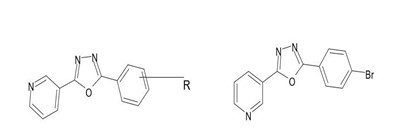
(18a) (18b)
R=NO2, Br, CH3
Fig18. Chemical structure of 1,3,4-oxadiazole derivatives studied as tyrosinase inhibitors.
ANTI-HIV ACTIVITY
Z. Li, P. Zhan et al explained 1,3,4-oxadiazole in antiviral agents. The review highlights recent developments in synthesizing the 1,3,4-oxadiazole ring and explores various classes of 1,3,4-oxadiazoles renowned for their potent antiviral properties, shedding light on their binding mechanisms to elucidate their antiviral activity further. Various stages of the HIV replication cycle serve as viable drug targets, with 25 anti-HIV drugs identified across four principal targets: reverse transcriptase, integrase, protease, and entry processes. Recent studies indicate the presence of the 1,3,4-oxadiazole scaffold in numerous HIV-1 inhibitors, spanning three key targets (reverse transcriptase, integrase, and protease), highlighting its significance as a privileged structure in the quest for novel anti-HIV agents.[22]

R1=H, F, Ome,2-furyl,2-thienyl,4-pyridyl R2=H, Et, Ph
Fig 19; 1,3,4-oxadiazole derivatives showing Anti-HIV activity.
ANTI- ALLERGIC ACTIVITY
H. Khalilullah et al carried out the various biological activities of 1,3,4-oxadiazole derivatives. A new group of compounds was created and tested for their ability to block histamine release and allergic reactions in rats. One compound, 3-chloro-2-(2,3dihydro-2-oxo-1,3,4-oxadiazol5yl)benzo[b]thiophene, was particularly effective, showing 15 times more potency than disodium cromoglycate in blocking histamine release.[23]

Fig 20; Chemical structure of the 1,3,4-oxadiazole derivatives was investigated for their antiallergic activity.
ANALGESIC ACTIVITY
Suman Bala et al carried out the diverse biological activities of heterocyclic 1,3,4-oxodiazole compounds. A new group of compounds, derivatives of 1-(4-phenoxyphenyl)-3-[5(substituted aryl)1,3,4oxadiazol-2-yl]propan-1-ones, showed strong pain-relieving effects in tests using acetic acid to induce pain. One specific compound in this group, the 2-acetoxy phenyl derivative, provided 76% pain protection, which was higher than the effectiveness of the standard drug indomethacin.[10]

Fig 21; 2-acetoxy phenyl derivative
ANTI- INFLAMMATORY ACTIVITY
Harish Rajak et al carried out anti-inflammatory activity of substituted 1,3,4oxadiazoles. Compounds containing an oxadiazole which shows anti-inflammatory activity and here substituted oxadiazoles from substituted thiosemicarbazides were used to develop new nonsteroidal anti-inflammatory agents. These compounds were tested for their ability to reduce carrageenan-induced edema in rat paws and to inhibit bovine serum albumin denaturation to understand their cellular mechanism. Some compounds were also evaluated for toxicity using LD50 values.[24,25]

R=C6H5,p-CH3C6H4,p-OCH3C6H4, p-BrC6H4
Fig 22; 1,3,4-oxadiazole derivatives investigated for their Anti-inflammatory activity.
CONCLUSION
1,3,4-oxadiazoles are a class of heterocyclic compounds displayed a wide range of biological activities therefor; this nucleus appears in the drug discovery and development processes. The biological profiles of the above derivatives of 1,3,4oxadiazole. 1,3,4-oxadiazole derivatives showed good biological activities such as anti-cancer, ant-inflammatory, anti-allergic, anti-tuberculosis, anti-convulsant, analgesic etc. The present review is about the 1,3,4- oxadiazole derivatives and focused on its biological activities such as anti-cancer, anti-convulsant, antiinflammatory, anti-microbial, analgesic, anti-microbial, anti-tuberculosis, thyrosinase inhibitor, anti-HIV activity.
ACKNOWLEDGEMENT
We own our heartful gratitude to GOD ALMIGHTY for all the blessings showered on us during the course of this project. At first, we express deep sense of gratitude indebtedness to Mar Dioscorus College Of Pharmacy, for helping in the completion of our review article. We extremely grateful to Prof Dr. Preeja G Pillai Principal Mar Dioscorus College Of Pharmacy, for her guidance, constant encouragement and valuable suggestions which made to complete our work.
We also grateful to Prof. Rachel Mathew Vice Principal And Mrs. Anjana V S Assistant Professor Department Of Pharmaceutical Chemistry, Mar Dioscorus College Of Pharmacy, , for there guidance in completing our work.
REFERENCES
- Asif Husain et al. Synthesis of novel 1,3,4-oxadiazole derivatives and their biological Properties. Acta Pharm.2009;59:223-233.
- B. Chandrakantha et al. Synthesis, characterization and biological activity of some new 1,3,4-oxadiazole bearing 2-flouro-4-methoxy phenyl moiety. European Journal of Medicinal Chemistry 2010;(45):1206-1210.
- Franski, R et al. Biological activities of the compounds bearing 1,3,4oxa(thia)diazole ring Asian J. Chem. 2005;17:2063-2075.
- Rakesh et al. Oxadiazole: a biologically important heterocycle Der Pharm Chemica.2009;1:130-140.
- K. Ajay Kumar et al. Comprehensive Review On The Chemistry Of 1,3,4oxadiazoles And Their Applications. International Journal of ChemTech Research.2012;4(4):17821791.
- Suman Bala et al. Heterocyclic 1,3,4-oxadiazole compounds with diverse biological activities: A comprehensive review. Journal of Pharmacy Research. 2010;3(12):29932997.
- Keshari Kishore Jha et al. Design, synthesis and biological evaluation of 1,3,4-oxadiazole derivatives. The European Journal of Medicinal Chemistry.2010;45(11):4963-4967.
- H. Khalilullah et al. 1,3,4-oxadiazole: A Biologically Active Scaffold H.Mini-Reviews in Medicinal Chemistry. 2012;(12):789-801.
- N. Yadav et al. Development of 1,3,4-oxadiazole thione based novel anticancer agents: Design, synthesis and in-vitro studies. Biomedicine & Pharmacotherapy.2017;(95):721730.
- Yin Luo et al. 1,3,4-oxadiazole derivatives as potential antitumor agents: discovery, optimization and biological activity valuation. Med. Chem.Commun.2016;7(2):263271
- Akshay R. Yadav et al. Synthesis, Characterization and Biological Evaluation of some Novel 1,3,4-oxadiazole Derivatives as Potential Anticancer Agents. International Journal of Scientific Research in Science and Technology. 2020;7(2):275-282.
- Satvir Singh et al. A Review on Anticancer Activity of 1, 3, 4oxadiazole. Int J. Pharm. Drug. Anal.2018;6(5):499-508.
- Shafiee et al. Synthesis, Anticonvulsant and Muscle Relaxant Activities of Substitute 1,3,4-oxadiazole, 1,3,4-thiadiazole and 1,2,4triazole. Acta Chim. Slov. 2007;317(54):317- 324.
- Banylla Felicity Dkhar Gatphoh et al. Synthesis, in silico Studies, and Anticonvulsant Activity of 1,3,4-oxadiazole Derivatives. Trends Sci.2021;18(23):701.
- G.Sahin et al. Synthesis and antimicrobial activity of some 1,3,4oxadiazole derivatives. Il Farmaco .2002;(57):539-542.
- Bhardwaj N et al. Synthesis, evaluation and characterization of some1 3, 4-oxadiazoles as antimicrobial agents, E-journal of Chemistry, 2009, 6(4), 1133-1138.
- Rakesh Chawla et al. Synthesis of novel 1,3,4-oxadiazole derivatives as potentialmantimicrobial agents. Acta Poloniae Pharmaceutica Drug Research. 2010;67(3):247-253.
- Sambhaji T. Dhumal et al. Synthesis and antitubercular activity of new 1,3,4-oxadiazoles bearing pyridyl and thiazolyl scaffolds. Bioorganic & Medicinal Chemistry Letters. 2016;15(26):3646-3651.
- Rikta Saha et al. Recent Updates on Biological Activities of Oxadiazoles. Mini-Reviews in Medicinal Chemistry, 2013;(13):1027-1046.
- H. Khalilullah et al. 1,3,4-oxadiazole: A Biologically Active Scaffold H. Mini-Reviews in Medicinal Chemistry. 2012;(12):789-801.
- Salahuddin et al. Updates on synthesis and biological activities of 1,3,4-oxadiazole: A review. An International Journal for Rapid Communication of Synthetic Organic Chemistry. 2017;47(20):1805-1847.
- Z. Li, P. Zhan et al. 1,3,4-oxadiazole: A Privileged Structure in Antiviral Agents MiniReviews in Medicinal Chemistry. 2011;(11):1130-1142.
- Guda Dinneswara Reddy et al. Antiallergic Activity Profile in Vitro RBL-2H3 and in Vivo Passive Cutaneous Anaphylaxis Mouse Model of New Sila-Substituted 1,3,4-oxadiazoles. Journal of medicinal chemistry.2012;55(14):6438–6444.
- Harish Rajak et al. Synthesis of Some Novel Oxadiazole and Oxadiazoline Analogue for Their Anti-inflammatory Activity. The Pharmaceutical Society of Japan. 2007;127(10):17571764.
- Aravind kumar Singh et al. Synthesis, Characterization and AntiInflammatory Activity of Some 1,3,4-oxadiazole Derivatives. Iranian Journal of Pharmaceutical Research.2013;12(2):319-323.


 Rachel Mathew*
Rachel Mathew*
















 10.5281/zenodo.11264100
10.5281/zenodo.11264100