Abstract
Hypoglycemic jelly formulations are more suitable for diabeties patients which offer rapid dissolution and absorption of drug there by early onset of action.The aim of this presented study is to introduce sugar free jelly as hypoglycemic agents. Preformulation organoleptic, weight variation, diameter & wideness drug content uniformity, disintegration, dissolution and stability study were conducted. The uv absorption studies were shown that drug absorption and drug content uniformity with respect to ug/ml. The concentration of gelling agent influence spreability gelling capacity. Jelly with gelatin and tragacanth having better stability during storage (gelling capability) The formulation F-1, F2, F3 & F4 were formulated & F1 formulation satisfy sepcification limits of the evaluation parameters. F1 formulation show more than 80?sorption & bioavailability and stability more than 90 days. As per ICH guidelines (stability protocol). The F1 formulation satisfied all the parameters and could be empolyed as hypoglycemic in diabeties patients.
Keywords
Custurd Apple leaves, gelling capacity, bioavailability, turbidity, stability study.
Introduction
Lozenges are flavoured medicinal dosage forms that are intended to be sucked and kept in the mouth or throat and contain one or more medications, generally in a sweetened foundation. Lozenges are used to treat oropharyngeal symptoms induced by local infection, as well as for systemic medication absorption. Medicated lozenges are intended to maximise dosage form retention in the oral cavity, which increases bioavailability, minimises gastrointestinal discomfort, and skips first pass metabolism.[1] Today's culture is concerned with living a healthy lifestyle by ingesting natural components. Because plants are all around us, they are the best source for traditional medicine. The World Health Organisation estimates that 80% of the world's population has used herbal substances as medication for health-related purposes. A. squamosa, commonly known as custard apple, is one of the herbal plants that may be used as medicine, and prior research investigations have shown that it has a variety of pharmacological properties that are beneficial to the body.[2-5]Annona squamosa L.'s other names include sitaphal, custard apple, sugar apple, and sweet sops. It is a member of the Annonaceae family, which has around 135 genera and 2300 species. It is now grown in tropical and subtropical climates all over the world. Annona squamosa grows to a height of 3-8 m. Different leaf shapes include oblong lanceolate or lanceolate, 6-17 cm long and 3-5 cm in diameter, alternately arranged on short petioles; bark is thin and grey in colour; greenish flowers and shoot elongates openly; and different fruit shapes include round, heart-shaped, ovate or conical, 5-10 cm in diameter, with a lot of round protuberance. .[6-9]

Figure 1: Leaves of Custard Apple Leaves
Tribal males in and around the villages of Aligarh district in the state of Uttar Pradesh, India and Chota Nagpur district in the state of Bihar, India consume the young leaves of Annona squamosa extensively for its anti-diabetic effect. Several studies have been conducted to investigate the anti-diabetic potential of ethanolic and aqueous extracts of Annona squamosa leaf against various type 1 and type 2 diabetic models. [10-13] Numerous active compounds found in Annona squamosa, such as acetogenins and flavonoids, have been shown in phytochemical studies to have cytotoxic, antimalarial, antidiabetic, and immunosuppressive properties. ASLs extract helps maintain plasma insulin and lipid balances while also considerably lowering blood glucose and lipid peroxidation.[14]
History [15]
Herbal remedies have a long history that dates back to human evolution. Plant records from the ancient past suggest that plants were historically employed for medical purposes in India, China, Egypt, and Greece. The fruit's common name is Sitaphal. According to legendary accounts, Lord Rama and Sita consumed this fruit during her vanvaas. Another tradition is that when Ravana kidnapped Sita during the Vanvaas period, the droplets of her tears from her eyes fell on the spot where Stephan trees first appeared.

Synonyms [16]
MATERIAL AND COLLECTION.[17]
The leaves of Custard Apple were collected and kept for shade dry for 15 days. Stevia, gelatine, tragacanth, glycerine, lactose, etc were collected from the local market of the city and college labs.
Extraction Process [18]
The powder of Custard Apple leaves were weighed accurately and mixed with 120ml of water before being slowly boiled to produce 30ml of extract. The extract was filtered and then chilled. Filtrate was taken to formulate lozenges.
Identification process of flavonoids.[17]
Shinoda test
Magnesium turnings are added to the sample, which is then treated with strong hydrochloric acid. The colour red is produced.
Lead acetate test
When the extracts were treated with a few drops of lead acetate solution, yellow precipitate formed, indicating the presence of flavonoids.
Alkaline reagent test
Separate drops of sodium hydroxide were added to the extracts. The presence of flavonoids is indicated by the formation of a bright yellow colour that turns colourless when a few drops of dilute acid are added.
Preparation of Candy Lozenges:
The required amount of stevia syrup was made by combining stevia and water. Lactose was dissolved in a little amount of water and heated to 110? until entirely dissolved, resulting in a clear viscous syrup. The lactose solution was then poured into the stevia syrup, which was then heated to 160? until the colour changed to golden yellow. The temperature was reduced to 90? before the API and other chemicals were introduced. The mould was filled with the solution. To avoid moisture absorption, the produced lozenges were wrapped in aluminium foil and placed in desiccators. .[19]


Figure 2: Preparation of jellies in moulds
Preparation of Phosphate Buffer [PH 6.8] [20]
To prepare 3600ml of phosphate buffer add 103.68 gm of Disodium hydrogen phosphate with 41.22 gm of Potassium dihydrogen phosphate and stir untill it get dissolved.

Table 1: Formulation [For 5 Lozenges]
Evaluation Parameter
Weight variation test [20]
Individual weights of ten lozenges were recorded, and the average weight of lozenges was obtained by dividing the total weight by ten. It was then compared to typical monographs.
Diameter and Thickness [21]
The diameter, size, and shape of lozenges are determined by the moulds used. Lozenges of various sizes and forms can be prepared, although they are typically circular with flat or biconvex faces. Lozenges were drawn at random from each batch and their thickness was assessed.
Drug Content Uniformity [22-23]
The content uniformity test ensures that each lozenge form in the batch includes an original quantum of drug factors, i.e., active pharmaceutical chemicals. To make a 100 ug/ ml result, one treated jelly from each expression was dissolved in 50 ml of phosphate buffer pH6.8. 1,1.5, and 2 ml of the forenamed results were taken and made up to 10 ml with pH6.8 phosphate buffer to get 10, 15, and 20 ug/ ml results, independently. Each result's absorbance will be measured at 254 nm with a UV-visible spectrophotometer.
Disintegration Time Evaluation [24]
The disintegration time is the time necessary for a lozenge or its particles to completely vanish from the tester net. The produced lozenges were disintegrated, using a disintegration tester and a disintegration medium of phosphate buffer with pH 6.8 maintained at 37 ± 0.5°C.
Evaluation Of Lozenges By Dissolution Apparatus [25]
The USP apparatus II (paddle type) was employed for the in vitro dissolution investigations. Loratadine lozenge formulations were accurately weighed and deposited in 900 mL of pH 6.8 phosphate buffer. The temperature was held constant at 37°C, and the mixture was mixed at a speed of 100 rpm. At 5, 10, 15, etc minute intervals, a 5 ml aliquot of the material was removed and replaced with an equivalent volume of plain buffer held at 37°C. The samples collected were filtered (#0.45 m) and analysed at 254 nm with a UV-visible spectrophotometer.
Stability Test. [26]
According to International Conference on Harmonisation guidelines, optimised jelly formulations were packed in aluminium foils, transferred to high-density polyethylene containers, tightly closed, and stored at room temperature (25°C ± 5°C), 8°C ± 1°C and 40°C ± 2°C/75 ± 5% relative humidity for 90 days. At the end of 90 days, the samples were evaluated for changes in appearance, pH, stiffness, and medication content.
RESULTS AND DISCUSSION
Prepared jellies were evaluated through various parameters. All the results obsereved under specifications as below.
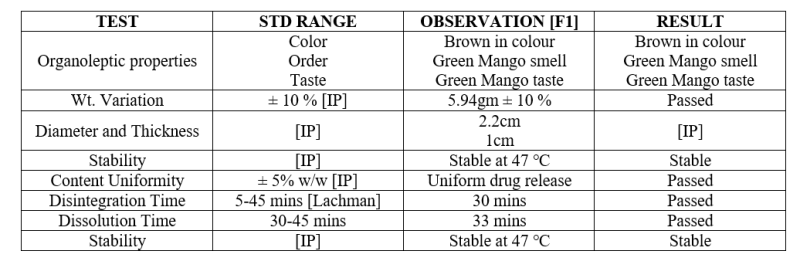
Table 2: Evaluation Parameter

Figure 3: Weight variation test of Jellies

Figure 4: Diameter and Thickness
Figure 5: Disintegration Time
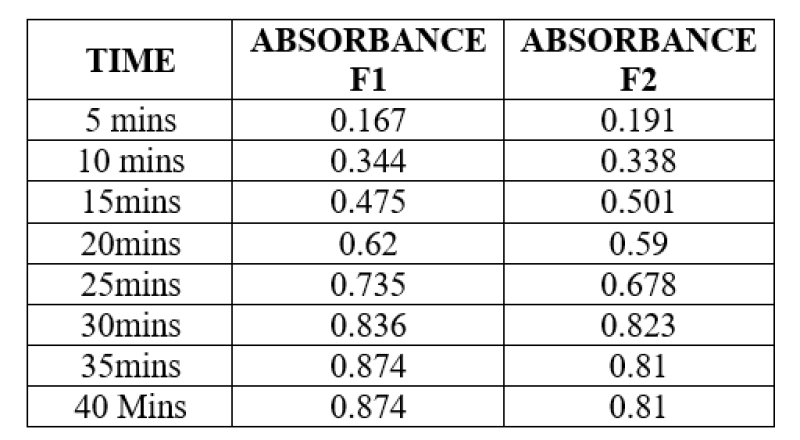
Table 3: Content Uniformity
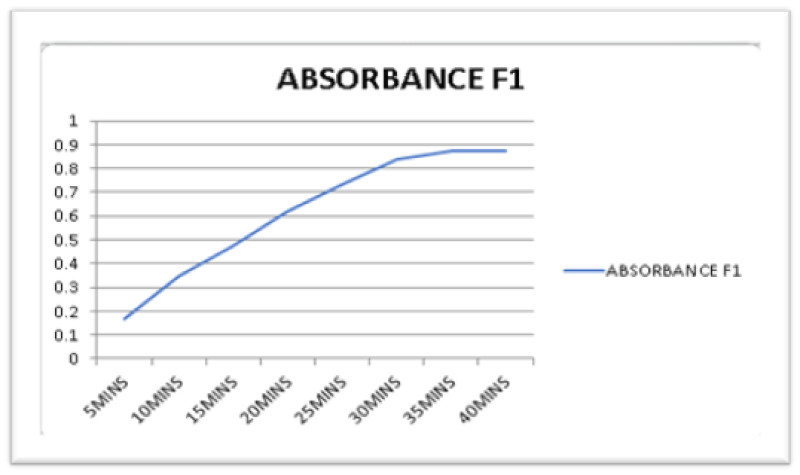
Figure 6: UV Absorbance of F1 product
Stability Testing:


Figure 7: Three months stability of jelly.
The prepared Jellies were optimized which show desired Physico-chemical properties and stability. Jellies on administration via Sublingually which show greater bioavailability and fast onset of action of drug as hypoglycemic than oral dosage form.
CONCLUSION
Custurd Apple leaves jellies were prepared by using gelatin, Tragacanth, stevia, lactose and preservative with leaves extract. The prepared Jellies were optimized which show desired Physico-chemical properties and stability. Jellies on administration via Sublingually which show greater bioavailability and fast onset of action of drug as hypoglycemic than oral dosage form.
REFERENCES
- Chandrawanshi MJ, Sakhare RS, Dr. Nagoba SN, et al., A Review On Medicated Lozenges, World Journal Of Pharmaceutical Research. 2019; 8(2): 396-412.
- Solikhah TI, Solikhah GP, Susilo RJK, Aloe vera and Virgin Coconut Oil (VCO) accelerate healing process in domestic cat (Felis domesticus) suffering from scabies, Iraqi J Vet Sci. 2021;35(4):699-704.
- Safira A, Savitri SL, Putri ARB. Review on the pharmacological and health aspects of Hylocereus or Pitaya: An update. J Drug Deliv Ther. 2021;11(6):297-303.
- Khairullah AR, Solikhah TI, Ansori ANM. Review on the Pharmacological and Health Aspects of Apium Graveolens or Celery : An Update. Syst Rev Pharm. 2021;12(1):606-612.
- Kalidindi N, Thimmaiah NV, Jagadeesh NV, et al., Antifungal and antioxidant activities of organic and aqueous extracts of Annona squamosa Linn. leaves. J Food Drug Anal. 2015;23(4):795-802.
- Raj DS, Vennila JJ, Ajyavu C, et al., The hepatoprotective effect of alcoholic extract of annona squaosa leaves on experimentally induced liver injury in swiss albino mice, Int. J. Biol., 2009; 5: 182-186.
- Srivastava S., V.K Lal and K.K pant, Medicinal potential of annona sqamosa; At a glance, J. Pharm Res. 2011; 4: 4596-4598.
- Ngiefu CK, Paquot C and Vieux A, Oil-bearing plants of Zaire ??? Botanical families providing oils of relatively high unsaturation, Oleagineus. 1977; 32: 535-537.
- Yang HJ, Xiang Li, Tang YN, et al., Supercritical fluid CO2 extraction and simultaneous determination of eight annonsceous acetogenins in Annona genus plant seeds by HPLC-DAD method, J. Pharm. Biomed Anal. 2009; 49: 140-144.
- Atique A, Iqbal M, Ghouse, A K M. Use of Annona squamosa and Piper nigrum against diabetes. Fitoterapia. 1985: 56 (3): 190-192.
- Topno KK, Plants used by tribals of Chotanagpur against diabetes, Botanica. 1997; 47: 99–101.
- Gupta RK, Kesari AN, Murthy PS, et al., Hypoglycemic and antidiabetic effect of ethanolic extract of leaves of Annona squamosa L. in experimental animals, Journal of Ethnopharmacology. 2005; 99: 75–81.
- Kaleem M, Medha P, Bano B, et al., Beneficial effects of Annona squamosa extract in streptozotocin-induced diabetic rats, Singapore Medical Journal. 2009; 49: 800-804.
- Kumar Manoj, Changan Sushil, Tomar Maharishi, et al., Custard Apple (Annona squamosa L.) Leaves: Nutritional Composition, Phytochemical Profile, and Health-Promoting Biological Activities, Biomolecules. 2021; 11: 614.
- Narwade, D and Aher, A Review on:Phytochemical and pharmacological Study of Annona Squamosa, Pharmatutor. 2019; 7(1): 5-10.
- Saha R, Pharmacognosy And Pharmacology Of Annona Squamosal: A Review, Int. J. of pharm. & Life sci.(IJPLS). 2011; 2(10): 1183-1189.
- Billa Vamsi, et al., Anti-ulcer and Analgesic activity of Ethanolic extract of Annona squamosa leaves. Department of Pharmacology, J.K.K. Nattraja College of Pharmacy, Komarapalayam. 2012; 1-85.
- Telange Patil PV, et. al., Formulation and Evaluation of Herbal Cough Syrup of Clove by using Jaggery Base, International Journal of Creative Research Thoughts. 2022; 10(12): 2320 – 2882.
- Moulika Lakshmi B, Brahma K, G Swathi, et al., Formulation and evaluation of domperidone candy lozenges, World Journal of Pharmacy and Pharmaceutical Sciences. 2017; 6(12): 1167-1175.
- Anand Binu, Thomas Irene, P Beena, et al., Formulation and Evaluation of Herbal Lozenges Containing Eucalyptus Oil and Coleus Aromaticus Oil. Am. J. Pharm Tech Res. 2018; 8(1): 317-322.
- Chanda Rupali, Nallaguntla Lavanya, Formulation And Evaluation Of Medicated Lozenges For Sore Throat. Asian J Pharm Clin Res. 2020; 13(10): 62-67.
- Jadhav SB and Shinde MK, Development and evaluation of oral medicated jelly of ondansetron hydrochloride, World J of Pharmacy and Pharm Sci. 2017; 6(9): 1537-1554.
- Ahire TV, Formulation, Development and Evaluation of Albendazole oral jellies, Indo American Journal of Pharmaceutical Sciences. 2016; 3(7): 707-709.
- Kumar Anshul, Mishra Manish Kumar, et al., Development And Evaluation Of Polyherbal Lozenges For Cold And Flu, Indian Journal of Pharmaceutical Education and Research. 2019; 53(2): 159-163.
- Chaudhari PD, Chaudhari SP, Lanke SD, et al., Formulation and in vitro evaluation of taste masked or dispersible dosage form of levocetirizine dihydrochloride, Indian J Pharm Educ. 2007; 41: 319-328.
- Katakam Prakash, Varanasi M. Satyanarayana, Hwisa T. Nagiat, et al., “Formulation development and evaluation of novel oral jellies of carbamazepine using pectin, guar gum, and gellan gum”, Asian Journal of Pharmaceutics. 2014; 8: 241-9.


 Vaishnavi V. Gampawar*
Vaishnavi V. Gampawar*
 Shubham B. Dongare
Shubham B. Dongare













 10.5281/zenodo.10777603
10.5281/zenodo.10777603