Abstract
The methodology was set up for synchronous estimation of a Nebivolol by RP-HPLC system. The chromatographic conditions were viably created for the unit of Nebivolol by using Inertsil - ODS C18 (250 x 4.6 mm, 5µ), stream is 1.0 ml/min, convenient stage extent was Methanol: Water (55:45), recognizable proof wave length was 274nm.
Keywords
Nebivolol, RP-HPLC, Acetonitrile, Methanol and Water.
Introduction
High Performance Liquid Chromatography (HPLC) was derived from the classical column chromatography and is one of the most important tools of analytical chemistry today. In the modern pharmaceutical industry, high-performance liquid chromatography (HPLC) is the major and integral analytical tool applied in all stages of drug discovery, development, and production. HPLC is the method of choice for checking peak purity of new chemical entities, monitoring reaction changes is in synthetic procedures or scale up, evaluating new formulations and carrying out quality control / assurance of the final drug products. The Goal of HPLC method is to try & separate, quantify the main drug, any reaction impurities, all available synthetic intermediates and any degradants. High Performance Liquid Chromatography is now one of the most powerful tools in analytical chemistry. It has the ability to separate, identify, and quantify the compounds that are present in any sample that can be dissolved in a liquid. HPLC is the most accurate analytical methods widely used for the quantitative as well as qualitative analysis of drug product and used for determining drug product stability. HPLC principle is the solution of sample is injected into a column of porous material (stationary phase) and liquid phase (mobile phase) is pumped at higher pressure through the column. The principle of separation followed is the adsorption of solute on stationary phase based on its affinity towards stationary phase.
The technique of HPLC has following features.
- High resolution
- Small diameter, Stainless steel, Glass column
- Rapid analysis
- Relatively higher mobile phase pressure
- Controlled flow rate of mobile phase
HPLC Method Development
Methods are developed for new products when no official methods are available. Alternate methods for existing (Non-Pharmacopoeial) products are to reduce the cost and time for better precision and ruggedness. When alternate method proposed is intended to replace the existing procedure comparative laboratory data including merit/demerits are made available. The goal of the HPLC-method is to try & separate, quantify the main active drug, any reaction impurities, all available synthetic inter-mediates and any degradants.
Steps involved in Method development are
- Understanding the Physicochemical properties of drug molecule
- Selection of chromatographic conditions
- Developing the approach of analysis
- Sample preparation
- Method optimization
- Method validation
Materials and Methods
Instruments used:
- HPLC – Waters Model NO.2690/5 series Compact System Consisting of Inertsil-C18 ODS column
- UV spectrophotometer (Systronics)
- Electronic balance (SARTORIOUS)
- Sonicator (FAST CLEAN)
Chemicals used:
- Methanol HPLC Grade
- Buffer (KH2PO4) HPLC Grade
Drug Profile:
NEBIVOLOL
Synonym:
Nebivolol, Nebivololum
Chemical Structure:
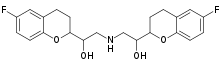
Fig.1 Chemical Structure of Nebivolol
IUPAC:
(1R)-1-[(2R)-6-fluoro-3,4-dihydro-2H-chromen-2-yl]-2-[[(2R)-2-[(2S)-6-fluoro-3,4-dihydro-2H-chromen-2-yl]-2-hydroxyethyl]amino]ethanol
Molecular Framework:
Aromatic heteromonocyclic compounds
Molecular formula:
C22H25F2NO4
Molecular weight:
405.4 g/mol
Monoisotopic:
405.17516460 g/mol
Physico Chemical Properties
Appearance:
Solid in nature
Solubility:
0.091g/100mL in water
Melting Point:
223.0-228.00C
pKa :
Strongest Acidic - 8.13
Strongest Basic - 8.22
log P: 4.183
EXPERIMENTAL WORK:
Stock and standard Working solution:
Nebivolol is used as working standard in this method development
Stock Solution Preparation:
Take 100 mg Nebivolol working standard in 100 ml Volumetric flask, add methanol up to the mark sonicate for 30 minutes (i.e. 1000 ppm solution).
Further Dilution (or) Trials Solution:
Take 10 ml of above solution in 100 ml Volumetric flask, add Methanol up to the mark sonicate for 10 minutes (i.e. 100 ppm solution)
Selection of Wave Length:
Scan standard solution in UV spectrophotometer between 200 nm to 400 nm on spectrum mode, using diluents as a blank. Nebivolol shows ?max at 274nm.
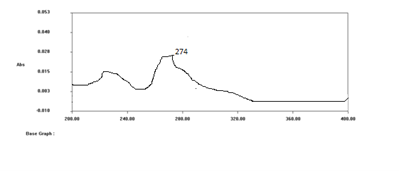
Fig.2 UV Spectrum of Nebivolol at 274nm
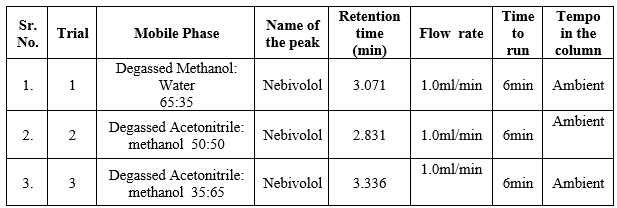
Development of HPLC Method:
The goal of this study was to improve the assay technique for simultaneous quantification of Nebivolol on literature surveys. As a result, the trials detailed below show how the optimization was accomplished.
Table.1 Chromatographic Conditions
Method Validation:
System Suitability:
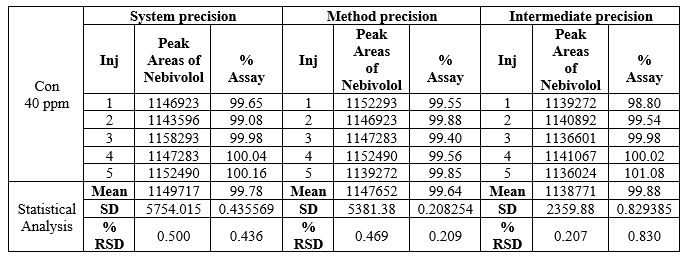
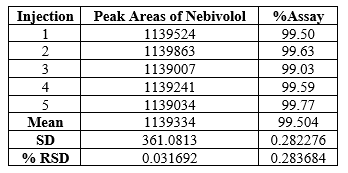
A Standard solution was prepared by using Nebivolol working standard as per test method and was injected five times into the HPLC system. The system suitability parameters were evaluated from standard chromatograms by calculating the % RSD from five replicate injections for Nebivolol retention times and peak areas.
Specificity:
Solutions of standard and sample were prepared as per the test method are injected into chromatographic system.
Precision:
Repeatability:
System precision: Standard solution prepared as per test method and injected five times.
Method precision:
Prepared five sample preparations individually using single as per test method and injected each solution.% relative standard deviation of individual Nebivolol, from the five units should be not more than 2.0%.The assay of Nebivolol should be not less than 98% and not more than 102.0%.
Intermediate precision:
The study was conducted by as per test method. The individual assays of Nebivolol should not be less than 98% and not more than 102% and %RSD of assay should be NMT 2.0%.
Repeatability:
Table.2 Data of Repeatability
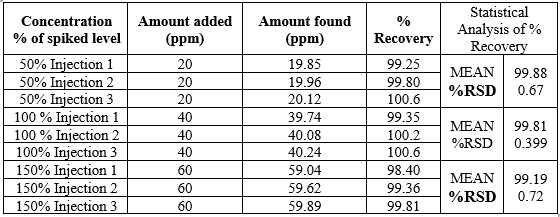
In this validation study the accuracy can be determined by calculating the following concentration such as 50%, 100% and 150%. The assay of Nebivolol should not be less than 98% and not more than 102.0%. The obtained results are shown in table.
Table.3 Data of Accuracy
Linearity:
The limit of detection (LOD) is the smallest concentration that can be detected but not necessarily quantified as an exact value. The limit of quantification (LOQ) is the lowest amount of analyte in the sample that can be quantitatively determined with suitable precision and accuracy.
Limit of Detection and Limit of Quantitation (LOD and LOQ):
From the linearity plot the SLOD and LOQ are calculated:
LOD = 3.3 ?/S
3.3×2431.578/31282 = 0.25
LOQ = 10 ?/S
10×2431.578/31282 = 0.77
Table.4 Data of Linearity
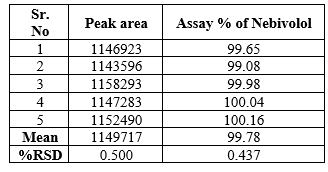
Fig.3 Linearity Plot (Concentration Vs Response)
Ruggedness:
System variability:
System variability study was conducted on HPLC system, under similar conditions at different times. Five samples were prepared and each was analyzed as per test method. The results obtained on HPLC system shows that the assay test method are rugged for System variability. The % relative standard deviation of Nebivolol from the five sample preparations should not be more than 2.0%. The % assay of Nebivolol should be between 98.0%-102.0%.
Table. 5 Data of system variability (sample)

Robustness:
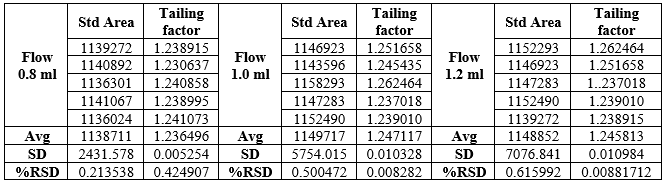
Effect of variation of flow rate:
A study was conducted to determine the effect of variation in flow rate. Standard solution prepared as per the test method was injected into the HPLC system using flow rates 1.0 ml/min and 1.2 ml/min. The system suitability parameters were evaluated and found to be within the limits for 1.0 ml/min and 1.2 ml/min flow. Nebivolol was resolved from all other peaks and the retention times were comparable with those obtained for mobile phase having flow rates 1.0ml/min. The Tailing Factor of Nebivolol standards should be NMT 2.0 for Variation in flow.
Table. 6 Data for Effect of variation in flow rate:
Marketed Sample Analysis:

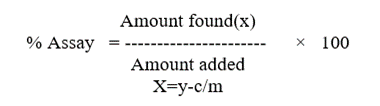
Table.6 Data for Marketed Sample
FTIR

Fig.4 FTIR Spectra for Nebivolol
SUMMARY AND CONCLUSION:
Different parameters were studied to create the analytical approach. For starters, the maximum absorbance of Nebivolol was discovered to be 274nm. The injection volume was set at 20µl, which resulted in a nice peak area. The Inertsil C18 column was employed in this work, and ODS picked a nice peak shape. The temperature of the ambient environment was determined to be adequate for the type of the medication solution. Because of the good peak area, adequate retention duration, and good resolution, the flow rate was set at 1.0ml/min. Different mobile phase ratios were investigated, however the mobile phase with a Methanol: Water (55:45) ratio was chosen because to its symmetrical peaks and high resolution. As a result, the planned research made use of this mobile phase. The accuracy of both the system and the procedure was determined to be precise and within the range. The correlation coefficient and curve fitting were discovered during the linearity investigation. The analytical approach of drug was shown to be linear throughout a range of 20-70ppm of the target concentration. The developed method was found to be rapid, accurate, precise, specific, robust and economical. Both robustness and ruggedness tests were passed by the analytical. The relative standard deviation in both circumstances was excellent.
REFERENCE:
- V. Gupta, A.D. K. Jain, N.S. Gill, K. Gupta, Development and validation of HPLC method - a review , Int. Res J Pharm. App Sci., (2012);2(4) 17-25
- Y. Kazakevich, R. Lobrutto, HPLC for Pharmaceutical Scientists, John Wiley & Sons, New Jersey, 2007.
- S. Ahuja, H. Rasmussen, Development for Pharmaceuticals, Separation Science and Technology, Elsevier, New York [2007] Vol.8
- M.S. Azim, M. Mitra, P.S. Bhasin, HPLC method development and validation: A review, Int. Res. J. Pharm. (2013);4(4):39-46.
- B.V. Rao, G.N. Sowjanya1, A. Ajitha, V.U.M. Rao, Review on stability indicating HPLC method development, World Journal of Pharmacy and Pharmaceutical Sciences, (2015);4(8)405-423.
- M.S. Charde, A.S. Welankiwar, J. Kumar, Method development by liquid chromatography with validation, International Journal of Pharmaceutical Chemistry, (2014);04(02): 57-61.
- S. Sood, R. Bala, N.S. Gill, Method development and validation using HPLC technique – A review, Journal of Drug Discovery and Therapeutics, 2014; 2(22): 18-24.
- M.W. Dong, Modern Hplc for practicing scientists, John Wiley & Sons, New Jersey, 2006.
- P.K. Singh, M. Pande, L.K. Singh , R.B. Tripathi, steps to be considered during method development and validation for analysis of residual solvents by gas chromatography, Int. Res J Pharm. App Sci., (2013); 3(5):74-80.
- Prathap, G.H.S. Rao, G. Devdass, A. Dey, N. Harikrishnan , Review on Stability Indicating HPLC Method Development, International Journal of Innovative Pharmaceutical Research, (2012); 3(3): 229-237.
- B. Sriguru, N.P. Nandha, A.S.Vairale, A.V. Sherikar, V. Nalamothu, Development and validation of stability indicating HPLC method for the estimation of 5-Fluorouracil and related substances in topical formulation, Int. J. Res. Pharm. Sci. (2010) ; 1(2): 78-85.
- C.K. Kaushal, B. Srivastava, A process of method development: A chromatographic approach, J. Chem. Pharm. Res. (2010) ; 2(2): 519-545.
- N.Toomula, A. Kumar, S.D.Kumar, V.S. Bheemidi, Development and Validation of Analytical Methods for Pharmaceuticals, J Anal Bioanal Techniques. (2011); 2(5): 1-4.
- K. Kardani, N. Gurav, B. Solanki, P. Patel, B. Patel, RP-HPLC Method Development and Validation of Gallic acid inPolyherbal Tablet Formulation, Journal of Applied Pharmaceutical Science. (2013); 3(5): 37-42.
- B. Nigovic, A. Mornar, M. Sertic, Chromatography – The Most Versatile Method of Chemical Analysis, Intech (2012) 385-425.
- T. Bhagyasree, N. Injeti, A. Azhakesan, U.M.V. Rao, A review on analytical method development and validation, International Journal of Pharmaceutical Research & Analysis, Vol (2014); 4(8): 444-448.
- Shrivastava, V.B. Gupta, HPLC: Isocratic or Gradient Elution and Assessment of Linearity in Analytical Methods, J Adv Scient Res, (212); 3(2); 12-20.
- V. Kumar, R. Bharadwaj, G.G., S. Kumar, An Overview on HPLC Method Development, Optimization and Validation process for drug analysis, The Pharmaceutical and Chemical Journal,(2015); 2(2) ; 30-40.
- Validation of Analytical Procedures: Text and Methodology, International Conferences on Harmonization, Draft Revised (2005), Q2 (R1).
- Validation of Compendial Procedures, United State Pharmacopeia, USP 36 NF, (2010) 27(2).
- https://ijpsr.com/bft-article/rp-hplc-method-development-and-validation-for-simultaneous-estimation-of-nebivolol-and-valsartan-in-bulk-and-dosage-form/
- https://www.primescholars.com/articles/method-development-and-validation-of-stability-indicating-rphplc-method-for-simultaneous-estimation-of-nebivolol-hcl-and-valsartan.pdf
- https://www.ijpsdr.com/index.php/ijpsdr/article/view/395
- https://en.wikipedia.org/wiki/Nebivolol
- https://go.drugbank.com/drugs/DB04861


 S. P. Kumbhar*
S. P. Kumbhar*










 10.5281/zenodo.11195367
10.5281/zenodo.11195367