Abstract
In recent years, liposome-based drug delivery systems have emerged as pivotal platforms in the pharmaceutical landscape, offering a myriad of opportunities for targeted and controlled release of therapeutic agents. This exhaustive review aims to provide an extensive exploration of the multifaceted domain of liposome technology. Encompassing a wide array of topics, including formulation methodologies, application domains, and cutting-edge advancements, this review offers a comprehensive analysis of the field. We delve into the intricate principles governing liposome design and synthesis, elucidating the diverse strategies employed to enhance drug encapsulation efficiency, stability, and pharmacokinetic profiles. Additionally, we meticulously scrutinize the therapeutic potential of liposomes across a spectrum of medical conditions, ranging from oncology and infectious diseases to inflammatory disorders. By synthesizing and critically evaluating the current state of research, this review equips healthcare professionals with a nuanced understanding of liposome-based drug delivery, facilitating informed decision-making and catalyzing future innovations in clinical practice.
Objective:
To provide a comprehensive overview of liposome-based drug delivery systems.
Purpose:
To equip healthcare professionals with a nuanced understanding of liposome technology for informed decision-making in clinical practice.
Conclusions:
Liposomes offer promising solutions for targeted drug delivery. Continued research promises to enhance their efficacy, potentially revolutionizing patient care.
Keywords
liposome-based drug delivery systems, targeted therapy, controlled release, application domains, advancements, liposome design, drug encapsulation efficiency, stability, pharmacokinetic profiles, therapeutic potential, innovation
Introduction
In recent years, liposome-based drug delivery systems have garnered significant attention and acclaim within the pharmaceutical realm. These versatile platforms offer unparalleled opportunities for precise and controlled release of therapeutic agents, revolutionizing the landscape of targeted therapy. This comprehensive review aims to navigate the intricate domain of liposome technology, providing a detailed exploration of its formulation methodologies, diverse application areas, and cutting-edge advancements. At the heart of liposome technology lies the principle of encapsulating drugs within lipid-based vesicles, offering protection and targeted delivery to specific tissues or cells. By modulating the composition and structure of liposomes, researchers can tailor their properties to optimize drug encapsulation efficiency, enhance stability, and improve pharmacokinetic profiles. Such versatility has propelled liposomes to the forefront of drug delivery research, promising breakthroughs in treating a myriad of medical conditions. This review delves into the underlying principles governing liposome design and synthesis, elucidating the diverse strategies employed to overcome challenges and maximize therapeutic efficacy. From innovative formulation methodologies to novel encapsulation techniques, each aspect contributes to the evolution of liposome-based drug delivery systems. Furthermore, we explore the vast application domains of liposomes, ranging from oncology and infectious diseases to inflammatory disorders. Through meticulous scrutiny of preclinical and clinical studies, we highlight the therapeutic potential of liposomes across diverse medical conditions. This comprehensive analysis equips healthcare professionals with a nuanced understanding of liposome-based drug delivery, empowering them to make informed decisions and optimize treatment strategies in clinical practice.
As we embark on this journey through the landscape of liposome-based drug delivery systems, we aim to provide valuable insights that not only enhance current therapeutic approaches but also pave the way for future innovations in patient care and pharmaceutical science.
LIPOSOMES
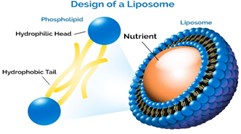
Structure:
At the core of liposome structure is the lipid bilayer. Lipids are molecules that have both hydrophilic (water-attracting) and hydrophobic (water-repelling) regions. When lipids are dispersed in an aqueous solution, they spontaneously form bilayers, with the hydrophilic "heads" oriented towards the surrounding water and the hydrophobic "tails" facing inward, shielded from water.
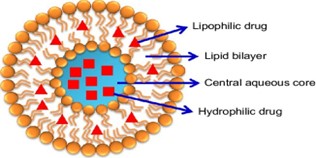
Within this lipid bilayer structure lies an aqueous core. This core is essentially a watery compartment enclosed by the lipid bilayer. It provides an environment suitable for encapsulating hydrophilic molecules such as drugs, enzymes, or nucleic acids.
Liposomes can vary in size from tens to hundreds of nanometers in diameter. They can adopt different morphologies, including spherical (unilamellar vesicles) or multilamellar structures (multiple concentric bilayers).
Liposomes can be unilamellar (single bilayer) or multilamellar (multiple concentric bilayers). Unilamellar liposomes are generally preferred for drug delivery applications due to their homogeneity and controlled release properties.
The outer surface of liposomes can be modified to alter their properties, such as stability, circulation time in the bloodstream, and targeting specificity. Surface modifications can involve attaching targeting ligands, polymers (e.g., PEGylation for enhanced circulation), or other functional molecules.
Liposomes possess a degree of flexibility due to the fluid nature of lipid bilayers. This flexibility allows them to deform and adapt to their environment, facilitating interactions with biological membranes and cellular uptake.
History of Liposomes:
The history of liposomes dates back to the 1960s when Alec D. Bangham, a British hematologist, first discovered them while studying blood clotting. In 1961, Bangham and his colleagues observed that lipids extracted from egg yolks formed closed bilayer structures when suspended in water. These structures resembled the phospholipid bilayer of biological membranes and were later termed "liposomes." In 1964, Bangham published a seminal paper describing the properties and potential applications of liposomes. His work laid the foundation for further research into liposome technology, particularly in the field of drug delivery. Throughout the 1970s and 1980s, scientists explored various methods for preparing and characterizing liposomes. This period saw significant advancements in liposome formulation techniques, including the development of sonication, extrusion, and reverse-phase evaporation methods. In 1981, liposomal doxorubicin (Doxil®) became the first liposome-based drug to receive regulatory approval from the United States Food and Drug Administration (FDA) for the treatment of Kaposi's sarcoma, a type of cancer commonly associated with AIDS.
Since then, liposomes have been extensively studied for their potential applications in drug delivery, diagnostics, gene therapy, and vaccines. Researchers have continued to refine liposome formulations, exploring novel lipid compositions, surface modifications, and targeting strategies to enhance their efficacy and safety profiles. Today, liposomes represent one of the most widely investigated drug delivery systems, with numerous liposomal formulations approved for clinical use and many more in various stages of preclinical and clinical development. Their versatility and biocompatibility make them promising tools for addressing diverse therapeutic challenges and advancing medical treatment options.
Types of Liposomes:
- Based on their Structure
- Multilamellar Vesicles (MLVs):
These are large, onion-like structures composed of multiple lipid bilayers separated by aqueous compartments. MLVs are formed by the hydration of dry lipid films and are characterized by their concentric layers of lipid bilayers.
- Small Unilamellar Vesicles (SUVs):
SUVs are small, spherical vesicles consisting of a single lipid bilayer encapsulating an aqueous core. They are typically formed by sonication or extrusion techniques and have diameters ranging from 20 to 100 nanometers.
- Large Unilamellar Vesicles (LUVs):
LUVs are similar to SUVs but have larger diameters, typically ranging from 100 to 1000 nanometers. They are also formed by sonication or extrusion methods and are commonly used in drug delivery and research applications.
- Giant Unilamellar Vesicles (GUVs):
GUVs are very large vesicles with diameters exceeding 1 micrometer. They are formed by various techniques such as electroformation or gentle hydration of lipid films. GUVs are often used in biophysical studies and cell membrane mimicking experiments.
- Oligolamellar Vesicles (OLVs):
OLVs are intermediate structures between MLVs and SUVs, consisting of a few concentric lipid bilayers with relatively larger diameters compared to SUVs. They are formed by controlled hydration of lipid films and offer a compromise between the stability of MLVs and the size uniformity of SUVs.
- Based on their Method of preparation
- Conventional Liposomes:
These liposomes are typically prepared by the thin-film hydration method. In this process, lipid components are dissolved in an organic solvent, and then the solvent is evaporated to form a thin lipid film. Subsequent hydration of the lipid film with an aqueous solution results in the formation of multilamellar vesicles (MLVs), which can then be sonicated or extruded to obtain smaller unilamellar vesicles (SUVs) or large unilamellar vesicles (LUVs), respectively.
- Sonication Method:
This method involves the direct dispersion of lipids in an aqueous medium followed by sonication using high-frequency sound waves. The mechanical agitation leads to the formation of small unilamellar vesicles (SUVs) or large unilamellar vesicles (LUVs) depending on the sonication parameters.
- Extrusion Method:
Liposomes prepared by the extrusion method are produced by forcing a lipid suspension through a series of polycarbonate membranes with defined pore sizes. This process results in the formation of liposomes with uniform size distribution, typically small unilamellar vesicles (SUVs).
- Reverse-Phase Evaporation Method:
In this method, lipids and an aqueous phase containing the drug are mixed in an organic solvent. The solvent is then evaporated under reduced pressure, resulting in the formation of a lipid film. Subsequent hydration of the lipid film leads to the formation of multilamellar vesicles (MLVs), which are then sonicated to obtain small unilamellar vesicles (SUVs) or large unilamellar vesicles (LUVs).
- Freeze-Thawing Method:
Liposomes can also be prepared by the freeze-thawing method, which involves freezing and thawing lipid dispersions multiple times. This process promotes the formation of multilamellar vesicles (MLVs) with a high encapsulation efficiency for hydrophilic drugs.
- Hydration of Lipid Films:
Liposomes can be formed by hydrating pre-formed lipid films with an aqueous solution containing the drug of interest. This method is often used for the preparation of large unilamellar vesicles (LUVs) and allows for precise control over the lipid composition and drug encapsulation.
- Based on their Application:
- Drug Delivery:
Liposomes are extensively used as carriers for delivering drugs, including chemotherapy agents, antibiotics, and antifungals. They can encapsulate both hydrophilic and hydrophobic drugs, protecting them from degradation and enhancing their bioavailability.
- Vaccine Delivery
Liposomes can encapsulate antigens and adjuvants, improving the immune response to vaccines. They facilitate antigen presentation to immune cells and can be tailored to target specific cells or tissues, enhancing vaccine efficacy.
- Gene Delivery:
Cationic liposomes are employed for delivering nucleic acids, such as DNA, siRNA, and mRNA, into cells for gene therapy, gene silencing, or genetic vaccination. They can form stable complexes with nucleic acids, promoting their cellular uptake and intracellular delivery.
- Cosmetics and Personal Care:
Liposomes are used in skincare products for delivering active ingredients like vitamins, antioxidants, and moisturizers. They enhance the penetration of these ingredients into the skin, improving their efficacy.
- Diagnostic Imaging:
Liposomes can be loaded with imaging agents, such as fluorescent dyes or contrast agents, for diagnostic purposes. They enable the visualization of specific tissues or organs in imaging techniques like fluorescence microscopy or magnetic resonance imaging (MRI).
D. Based upon specialty characteristics of liposomes:
- Targeting Liposomes:
Immunoliposomes:
Modified with targeting ligands like antibodies for specific cell or tissue targeting.
Receptor-Targeted Liposomes:
Engineered to bind to specific receptors overexpressed in diseased tissues, enhancing targeted drug delivery.
- Stimuli-Responsive Liposomes:
pH-Sensitive Liposomes:
Release drugs in response to changes in pH, useful for targeted delivery to acidic environments like tumor tissues.
Temperature-Sensitive Liposomes:
Release drugs upon changes in temperature, enabling controlled drug release at specific sites.
- Functional Liposomes:
Theranostic Liposomes:
Combine therapeutic and diagnostic capabilities for simultaneous imaging and treatment.
Radiolabeled Liposomes: Incorporate radioactive isotopes for imaging or targeted radionuclide therapy.
- Modified Liposomes:
Stealth Liposomes:
Coated with polymers like PEG to evade immune recognition and prolong circulation time.
Long-Circulating Liposomes:
Engineered for extended circulation to enhance drug delivery efficiency.
- Specialized Liposomes:
Nanovaccines:
Liposomal formulations designed for vaccination purposes, often tailored to enhance immune responses.
Bioactive Liposomes:
Loaded with bioactive compounds for therapeutic purposes like wound healing or tissue regeneration.
PREPARATION OF LIPOSOMES:
Material :
- Lipids:
Lipids are the primary building blocks of liposomes and play a crucial role in their formation. Common lipids used in liposomal formulations include phospholipids such as phosphatidylcholine (PC), phosphatidylglycerol (PG), phosphatidylethanolamine (PE), and cholesterol. These lipids self-assemble into bilayer structures, forming the membrane of the liposomes.
- Drugs or Active Ingredients:
Liposomes can encapsulate a wide range of drugs or active ingredients, including hydrophobic and hydrophilic compounds. Examples include anticancer drugs, antibiotics, antifungal agents, vitamins, antioxidants, and nucleic acids (e.g., siRNA, mRNA). Encapsulation within liposomes can improve the solubility, stability, and bioavailability of these compounds.
- Stabilizers:
Stabilizers are often added to liposomal formulations to enhance their stability and prevent aggregation or fusion of liposomes. Common stabilizers include sugars (e.g., sucrose, trehalose), polymers (e.g., polyethylene glycol, PEG), and proteins (e.g., serum albumin). These stabilizers can also help prevent premature release of encapsulated drugs and improve circulation time in vivo.
- Targeting Ligands:
In targeted drug delivery applications, targeting ligands such as antibodies, peptides, or aptamers may be incorporated into liposomal formulations. These ligands can selectively bind to receptors or antigens expressed on target cells or tissues, enabling specific delivery of drugs to desired sites while minimizing off-target effects.
- Buffering Agents:
Buffering agents such as phosphate-buffered saline (PBS) or HEPES may be used to maintain the pH and osmolarity of the liposomal formulation, ensuring stability and compatibility with biological systems.
- Chelating Agents:
Chelating agents like ethylenediaminetetraacetic acid (EDTA) may be included to chelate metal ions, which can promote lipid oxidation and destabilize liposomal formulations.
- Antioxidants:
Antioxidants such as ?-tocopherol (vitamin E) or butylated hydroxytoluene (BHT) may be added to liposomal formulations to protect lipids from oxidation and improve stability during storage.
- Solvents:
Organic solvents like chloroform or ethanol may be used during the preparation of liposomal formulations to dissolve lipids and drugs, although efforts are typically made to remove these solvents before administration.
Method:
- Thin-Film Hydration (Film Dispersion) Method:
In the Thin-Film Hydration method, lipids are dissolved in an organic solvent, typically chloroform or methanol, to form a thin lipid film on the walls of a round-bottom flask or a rotary evaporator. The solvent is then removed under reduced pressure, leaving behind a lipid film. Subsequently, an aqueous phase containing the drug of interest is added to the flask, and the mixture is agitated or subjected to sonication. This process rehydrates the lipid film, leading to the formation of liposomes or lipid nanoparticles with the drug encapsulated inside.
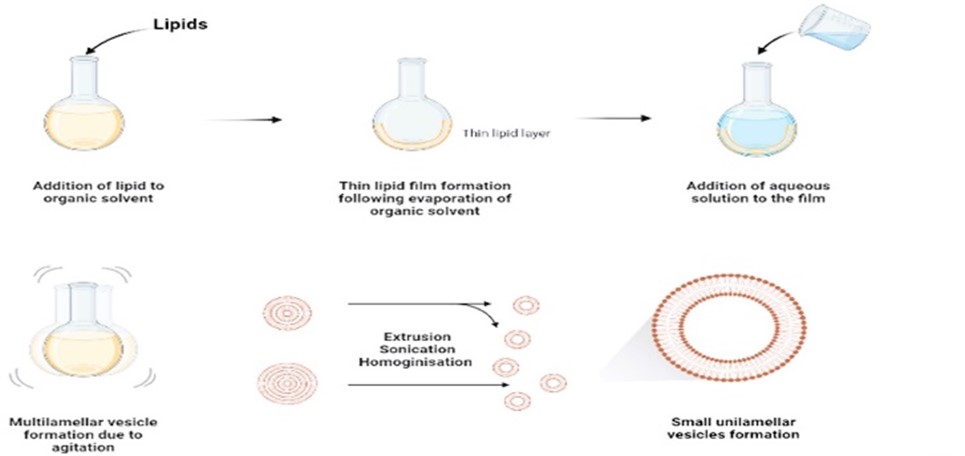
Lipids are dissolved in an organic solvent to form a thin lipid film by evaporation under reduced pressure. The film is then hydrated with an aqueous solution containing the desired drug or payload, followed by sonication or extrusion to form liposomes.
Advantages:
Simple and widely used method, suitable for small-scale preparations.
Disadvantages:
Variability in liposome size, low encapsulation efficiency for hydrophilic drugs.
- Reverse Phase Evaporation Method:
Lipids, including phospholipids and cholesterol, are dissolved in an organic solvent such as chloroform or ether. Additionally, the drug of interest, often hydrophobic, is added to the organic phase.

The organic phase is then mixed with an aqueous phase containing a buffer solution or distilled water. The mixture is typically sonicated or vortexed vigorously to form a water-in-oil emulsion. This emulsion consists of small droplets of the aqueous phase dispersed within the organic phase.
Description:
Lipids and an organic solvent are mixed to form a water-in-oil emulsion. The organic solvent is then evaporated under reduced pressure, leading to the formation of a lipid bilayer vesicle in the aqueous phase.
Advantages:
Suitable for encapsulating hydrophilic drugs, higher encapsulation efficiency compared to thin-film hydration.
Disadvantages:
More complex than thin-film hydration, requires specialized equipment.
- Extrusion Method:
The Extrusion Method is a widely used technique in pharmaceutical science for the preparation of liposomes or lipid nanoparticles, crucial vehicles for drug delivery systems. Initially, lipids, often phospholipids and cholesterol, are dissolved in an organic solvent along with the desired drug compound. This solution is then dried to form a lipid film, typically on the surface of a glass vessel or a solid support like a porous membrane. Following the formation of the lipid film, hydration occurs as an aqueous solution is introduced, usually a buffer or saline solution. Subsequently, the hydrated lipid suspension undergoes extrusion through a membrane or filter with defined pore sizes. This extrusion process, often facilitated by a syringe extruder or similar device, imparts disruptive forces on the lipid bilayers, prompting their rearrangement into liposomes.
Description:
Lipids are dissolved in an organic solvent, followed by hydration with an aqueous solution. The resulting multilamellar vesicles (MLVs) are then extruded through polycarbonate membranes with defined pore sizes to obtain uniformly sized liposomes.
Advantages:
Allows for precise control of liposome size, suitable for large-scale production.
Disadvantages:
Requires extrusion equipment, may not be suitable for fragile or heat-sensitive drugs.
- Sonication Method:
The Sonication Method is a common technique for making liposomes or lipid nanoparticles. Lipids are dissolved in a solvent, dried to form a film, then rehydrated in water. Ultrasonic waves are applied to break up the lipids into smaller vesicles. This method is quick, versatile, and suitable for encapsulating various types of drugs.

Description:
Lipids are dissolved in an organic solvent and hydrated with an aqueous solution to form MLVs. The MLVs are then subjected to ultrasonic irradiation, leading to the formation of smaller unilamellar vesicles (SUVs) or large unilamellar vesicles (LUVs).
Advantages:
Simple and rapid method, suitable for small-scale preparations.
Disadvantages:
Limited control over liposome size distribution, may lead to lipid degradation or drug loss.
- Detergent Removal Method (Dialysis):
The Detergent Removal Method, typically carried out through dialysis, is a technique for preparing liposomes or lipid nanoparticles. Initially, lipids and the drug are dissolved in an organic solvent along with a detergent, forming a lipid-detergent solution. This solution is then mixed with an aqueous phase, leading to the formation of mixed micelles. Dialysis is employed to remove the detergent, allowing lipid bilayers to spontaneously form and encapsulate the drug within liposomes. This method offers control over drug encapsulation, particularly for hydrophobic drugs, and is suitable for large-scale production.
Description:
Lipids and a detergent are mixed with an aqueous solution containing the payload. The detergent is then removed by dialysis against a buffer solution, leading to the spontaneous formation of liposomes.
Advantages:
Suitable for encapsulating hydrophilic drugs, gentle method that preserves drug integrity.
Disadvantages:
Requires careful optimization of detergent concentration and dialysis conditions, may result in lower encapsulation efficiency.
EVALUTION OF LIPOSOME

APPLICATION OF LIPOSOMES:
- Drug Delivery:
Liposomes are extensively used as drug delivery vehicles due to their ability to encapsulate both hydrophobic and hydrophilic drugs. They can improve the solubility, stability, and bioavailability of drugs, as well as enable targeted delivery to specific tissues or cells, reducing systemic side effects. Applications include cancer therapy, treatment of infectious diseases, and delivery of vaccines and gene therapies.
- Cosmetics and Personal Care:
Liposomes are utilized in skincare and cosmetic products for their ability to deliver active ingredients such as vitamins, antioxidants, and moisturizers to the skin. They enhance the penetration of these ingredients, leading to improved efficacy and skin hydration.
- Food and Nutraceuticals:
Liposomes are employed in the food and nutraceutical industries for encapsulating bioactive compounds such as vitamins, antioxidants, and omega-3 fatty acids. This enhances their stability and bioavailability, allowing for controlled release and improved absorption in the body.
- Diagnostic Imaging:
Liposomes can be loaded with imaging agents such as fluorescent dyes, magnetic nanoparticles, or radioactive isotopes for various imaging modalities including fluorescence imaging, magnetic resonance imaging (MRI), and nuclear imaging. They enable targeted imaging of specific tissues or organs for diagnostic purposes.
- Biotechnology and Research:
Liposomes are used in various biotechnological and research applications, including drug screening, cell culture studies, and protein delivery. They serve as versatile tools for studying cellular processes, drug interactions, and membrane biology.
- Therapeutic Delivery Systems:
Liposomes can be engineered to deliver therapeutic agents such as peptides, proteins, and nucleic acids, including siRNA and mRNA, for the treatment of various diseases. They offer protection against enzymatic degradation and immune clearance, facilitating the delivery of sensitive molecules to target cells or tissues.
- Industrial and Environmental Applications:
Liposomes find applications in industrial processes such as encapsulation of enzymes for biocatalysis, delivery of agricultural agents for crop protection, and remediation of environmental pollutants. They offer controlled release and targeted delivery of functional molecules in these applications.
Advantages of Liposomes
- Targeted Delivery:
Liposomes can be engineered to target specific tissues or cells, minimizing systemic side effects and enhancing therapeutic efficacy.
- Controlled Release:
Liposomes enable controlled and sustained release of drugs, allowing for prolonged therapeutic effects and reduced dosing frequency.
- Improved Drug Solubility:
Lipid bilayers of liposomes can encapsulate hydrophobic drugs, improving their solubility and bioavailability.
- Enhanced Stability:
Liposomes protect encapsulated drugs from degradation, enzymatic metabolism, and premature clearance, leading to improved stability and longer circulation times in the body.
- Versatility:
Liposomes can encapsulate various types of drugs, including small molecules, peptides, proteins, and nucleic acids, making them suitable for a wide range of therapeutic applications.
- Biocompatibility:
Liposomes are generally biocompatible and biodegradable, minimizing the risk of adverse reactions and promoting compatibility with biological systems.
- Combination Therapy:
Liposomes can encapsulate multiple drugs or therapeutic agents within the same vesicle, enabling synergistic effects and combination therapy for enhanced treatment outcomes.
- Tailored Properties:
Liposome characteristics such as size, surface charge, and lipid composition can be customized to optimize drug delivery efficiency and target-specific biological barriers.
FUTURE PROSPECTIVE:
Liposome-based drug delivery systems have garnered significant attention in the field of medicine due to their potential to enhance the therapeutic efficacy of drugs while reducing their side effects. Looking into the future, several promising prospects emerge for the continued development and utilization of liposome-based drug delivery systems:
- Targeted Drug Delivery:
One of the most exciting prospects is the refinement of targeting strategies. By modifying the surface properties of liposomes or attaching ligands specific to target cells or tissues, researchers can enhance the selective delivery of drugs to diseased sites while minimizing off-target effects. This targeted approach holds great promise for improving the efficacy of treatments for various diseases, including cancer, infectious diseases, and inflammatory disorders.
- Combination Therapy:
Liposomes offer a versatile platform for co-delivery of multiple drugs or therapeutic agents. Future research may focus on optimizing formulations for combination therapy, allowing for synergistic effects and enhanced therapeutic outcomes. This approach could revolutionize the treatment of complex diseases by simultaneously targeting multiple pathways or cellular processes involved in disease progression.
- Personalized Medicine:
Advances in technology, such as nanomedicine and precision medicine, pave the way for personalized drug delivery systems tailored to individual patient characteristics. Liposome-based platforms can be customized to accommodate different drug payloads, dosing regimens, and patient-specific factors, enabling more precise and effective treatments. This personalized approach holds tremendous potential for improving patient outcomes and reducing adverse reactions.
- Theranostic Applications:
Liposomes can be engineered to not only deliver drugs but also to serve as imaging agents or diagnostic tools. Theranostic liposomes offer the dual functionality of therapy and diagnosis, allowing for real-time monitoring of treatment response and disease progression. Integrating therapeutic and diagnostic capabilities into a single platform could revolutionize clinical practice by enabling early detection, accurate staging, and tailored treatment strategies.
- Biocompatibility and Safety:
Future research efforts may focus on further enhancing the biocompatibility and safety profile of liposome-based drug delivery systems. By optimizing formulation parameters, such as lipid composition, size, and surface properties, researchers can minimize adverse reactions and improve overall tolerability. This will be crucial for the translation of liposomal formulations from preclinical studies to clinical applications.
- Regulatory Approval and Commercialization:
As the field of liposome-based drug delivery continues to mature, overcoming regulatory hurdles and scaling up production will be key challenges. Streamlining the regulatory approval process and implementing cost-effective manufacturing strategies will facilitate the commercialization and widespread adoption of liposomal formulations in clinical practice.
CONCLUSION:
The review provides a thorough examination of the diverse applications, formulation strategies, and advancements in liposome-based drug delivery. The review underscores the versatility and potential of liposomes as versatile carriers for delivering various therapeutics, including small molecules, proteins, nucleic acids, and imaging agents. It highlights the importance of understanding the physicochemical properties of liposomes, such as size, surface charge, and lipid composition, in designing tailored drug delivery systems with enhanced efficacy and safety profiles. Furthermore, the review emphasizes the significance of addressing key challenges in liposome-based drug delivery, including stability, drug loading efficiency, and targeting specificity. It discusses innovative strategies such as surface modification with targeting ligands, stimuli-responsive liposomes, and co-delivery systems to overcome these challenges and improve therapeutic outcomes. Overall, the comprehensive review provides valuable insights into the current state-of-the-art and future directions of liposome-based drug delivery research. It serves as a valuable resource for scientists, researchers, and clinicians working in the field, facilitating the development of next-generation liposomal formulations for a wide range of therapeutic applications.
CONFLICT OF INTEREST:
The authors have no conflicts of interest regarding this investigation.
REFERENCES:
- Amarnath Sharma, Uma S. Sharma, “A Review on Liposomes in drug delivery: progress and limitations”, International Journal of Pharmaceutics 154 (1997) 123 140.
- Claudia Zylberberg and Sandro Matosevic, “Pharmaceutical liposomal drug delivery: a review of new delivery systems and a look at the regulatory landscape”, trading as Taylor & Francis Group. DOI: 10.1080/10717544.2016.1177136.
- Avi Schroedera.b.Joseph Kostb, Yechezkel Barenholza,“Review:Ultrasound,liposomes,and drug delivery : principles for using ultra sound to control the release of drugs from liposomes”, Chemistry and Physics of Lipids 162 (2009) 1–16.
- Parveen Goyal, Kumud Goyal, Sengodan Gurusamy Vijayakumar, Ajit singh, Omprakash Katare, Dinanath Mishra, “Liposomal drug delivery systems– Clinical applications”, Acta Pharm. 55 (2005) 1–25.
- Arkadiusz Kozubek, Jerzy Gubernator, Ewa Przeworska and Maria Stasiuk, “Review Liposomal drug delivery, a novel approach: PLARosomes”, Acta Biochemica Polonica, Vol. 47 No. 3/2000 639–649.
- Tarun Garg and Amit K. Goyal, “Liposomes: Targeted and Controlled Delivery System”, Drug Delivery Letters, 2014, 4, 62-71.
- Faisal Farooque, Mohd Wasi, Mohd Muaz Mughees, “Liposomes as Drug Delivery System: An Updated Review”, Journal of Drug Delivery & Therapeutics. 2021; 11(5-S):149-158.
- Abdus Samad, Y. Sultana and M. Aqil, “Liposomal Drug Delivery Systems: An Update Review”, Current Drug Delivery, 2007, 4, 297-305.
- Tianshun Lian, Rodney J. Y. Ho,“ Minireview: Trends and Developments in Liposome Drug Delivery Systems”, Jounral of Pharmaceutical Sciences, Vol. 90, no. 6, June 2001.
- Norbert Maurer, David B Fenske & Pieter R Cullis, “Developments in liposomal drug delivery systems”, Expert Opin. Biol. Ther. (2001) 1(6).
- Oula Penate Medina1, Ying Zhu1 and Kalevi Kairemo, “Targeted Liposomal Drug Delivery in Cancer”, Current Pharmaceutical Design, 2004, 10, 2981-2989.
- K. Egbaria and N. Weiner, “Liposomes as a topical drug delivery system”, Advanced Drug Delivery Reviews, 5 (1990) 287-300.
- Helene E. Schaeffer and David L. Krohn, “Liposomes in topical drug delivery”, Invest. Ophthalmol. Vis. Sci. February 1982.
- Wilczewska, A.Z.; Niemirowicz, K.; Markiewicz, K.H.; Car, H. Nanoparticles as drug delivery systems. Pharmacol. Rep., 2012, 64(5), 1020-1037.
- Swaminathan, J.; Ehrhardt, C. Liposomal delivery of proteins and peptides. Expert Opin. Drug Deliv., 2012, 9(12), 1489-1503.
- Garg, T.; Singh, O.; Arora, S.; Murthy, R., Scaffold: a novel carrier for cell and drug delivery. Crit. Rev. Ther. Drug Carrier Syst., 2012, 29(1), 1-63.
- Papisov, M.I. (1998) Theoretical consider ations of RES-avoiding liposomes: Molecular mechanics and chemistry of liposome interactions. Adv. Drug Deliv. Rev. 32, 119–138.
- Weiner, N., Williams, N. and Birch, G. et al. (1989) Topical delivery of liposomally encapsulated interferon evaluated in a cutaneous herpes guinea pig model, Antimicrob. Agents Chemother. 33, 1217-1221.
- Rodney, J.Y., Rae, L.B. and Thomas, C.M. (1989) Antigen-presenting liposomes are effective in treatment of recurrent herpes simplex virus genitalis in guinea pigs, J. Virol. 63(7), 2951-2958.
- Lichtenberg, D. and Barenholz, Y. (1988) Liposomes: preparation characterization and preser- vation. In: D. Glick (Ed.), Methods of Biological Analysis, Vol. 33, John Wiley, New York, pp.337- 461.
- Bangham, A.D. Liposomes in Biological Systems, John Wiley and Sons: Chichester, 1980.


 Bansode Krushna*
Bansode Krushna*
 Wankhede Jayshri
Wankhede Jayshri
 Vyawhare Pankaj
Vyawhare Pankaj
 Shewale Madhu
Shewale Madhu






 10.5281/zenodo.11235024
10.5281/zenodo.11235024