Abstract
Drug repurposing, the identification of new anticancer properties in existing non-cancer drugs, repurposing offers a faster and more cost-effective alternative to traditional drug development. This study has made to the discovery of novel anticancer agents, including repurposed drugs like tamoxifen, sulindac, and celecoxib. Drug repurposing in cancer has the potential to expand treatment options, improve patient result, and enhance quality of life for those affected by cancer.
Keywords
Drug Repurposing, Drug Discovery, Cancer Therapy, Anticancer Properties, Genomics, Drug Combination.
Introduction
Repurposing of drugs indicates use of drugs in diseases other than those prescribed for. Cancer is a genetic disorder that results from genetic alterations in the somatic cells and has abnormal cell growth which may spread to any other body part. Cancer is the abnormal proliferation of any of the different kind of cells in the body. There are more than hundred different type of cancer. It was predicted by Global demographic, characteristics that about 420 million new cases of cancer by 2025 annually, which means increasing cancer incidence in years. The uncontrolled cell. Therapy, with potentially lower overall costs and shorter time. Growth results from large no.of of risk factors. These include gender, lifestyle, ethnicity, age, professional hazard. Three usual methods exist: surgery (surgical removal of primary tumor), radiotherapy and pharmacotherapy. The choice of the right anticancer agents, as well as the right doses, administration and management of toxicities is not a simple process. Repurposing non-oncology small-molecule drugs has been increasingly becoming an attractive approach to improve cancer. Drug repurposing or repositioning most used to the therapeutic applications of a drug for that its use is extending beyond its original scope. Repurposing non-oncology small-molecule drugs has been increasingly becoming an effective approach to improve cancer therapy, by lowering overall costs and shorter time. Several non-oncology drugs approved by FDA have been recently reported to treat different types of human cancers, Therefore, in this review, we focus on summarizing the therapeutic potential of non-oncology drugs, including cardiovascular drugs, microbiological drugs, small-molecule antibiotics, anti-viral drugs, anti-inflammatory drugs, anti-neurodegenerative drugs, antipsychotic drugs, antidepressants, and other drugs in human cancers.
1. Aspirin (Anti-Inflammatory) - shows promise in preventing certain types of cancer, such as colorectal cancer.
2. Metformin (Anti-Diabetic) - has been investigated for its potential anti-tumor effects in various cancers, including breast and lung cancer.
3. Statins (Cholesterol-Lowering) - have been studied for their potential anti-cancer properties, particularly in reducing the risk of certain types of cancer, such as colorectal and prostate cancer.
Non-Small Cell Lung Cancer:
Global Cancer Statistics (2018): According to estimates, 17 million new cancer cases and 9.5 million cancer-related deaths occurred in 2018. These figures highlight the global burden of the disease, affecting a significant portion of the population, making it more widespread than many other infectious diseases combined (like malaria, HIV/AIDS, and tuberculosis). Lung Cancer: As you mentioned, lung cancer is both the second most frequently diagnosed cancer and the leading cause of cancer death worldwide. The 2020 estimates project 2.2 million new cases and 1.8 million deaths due to lung cancer. This is a significant portion of global cancer cases, representing about 11.4% of all cancer diagnoses and 18.0% of all cancer-related deaths. The high fatality rate is partly due to the fact that lung cancer is often diagnosed at a later stage, when treatment options may be more limited Lung cancer is indeed a complex and varied disease, with two main categories: non-small cell lung cancer (NSCLC) and small cell lung cancer (SCLC). As you mentioned, NSCLC is far more common, making up around 80-85% of cases, and includes three primary subtypes: adenocarcinoma, squamous cell carcinoma, and large cell carcinoma. Adenocarcinoma: The most prevalent subtype, accounting for 40% of all lung cancers, is primarily found in non-smokers, women, and younger individuals. It originates in the glands of the lung's outer regions and tends to be more prevalent in those who have never smoked. Squamous Cell Carcinoma: This subtype, comprising 25-30% of lung cancers, is most commonly linked to smoking. It often begins centrally, in the larger airways of the lungs, and is more common in men. Large Cell Carcinoma: Making up 10-15% of cases, this is the rarest and often more aggressive form of lung cancer, with a tendency to grow and spread quickly. On the other hand, SCLC is a more aggressive form but represents only 15-20% of cases. It is often linked to heavy smoking and has a poorer prognosis due to rapid spread.
Mitogen-Activated Protein Kinase (MAPK) Pathway:
The MAPK (Mitogen-Activated Protein Kinase) signaling pathway is crucial in regulating numerous cellular processes such as cell survival, differentiation, proliferation, apoptosis, and metastasis, all of which are highly relevant to cancer biology. In cancer, oncogene overexpression or mutations in components of the MAPK pathway often lead to uncontrolled cell growth and resistance to cell death, contributing to tumor genesis. These elements MAPKKK (MAPK kinase kinase) – This is the upstream kinase that responds to external and internal signals. Once activated, it phosphorylates and activates the middle kinase. MAPKK (MAPK kinase) – The middle kinase that, in turn, phosphorylates and activates the downstream MAPK. MAPK – The terminal kinase that transmits the signal to the nucleus, affecting gene expression and cellular functions. Role of MAPK in NSCLC: In the context of non-small cell lung cancer (NSCLC), several mechanisms contribute to the activation of the MAPK pathway: Overexpression of MAPK components: For example, mutations or overexpression of EGFR (epidermal growth factor receptor) can activate downstream MAPK signaling. These mutations often lead to the sustained activation of the MAPK pathway, promoting tumor cell proliferation and survival. KRAS mutations: Mutations in the KRAS gene are common in NSCLC, especially in smokers. KRAS is an important regulator of the MAPK pathway. Mutations in KRAS can lead to continuous activation of the MAPK pathway, promoting malignant growth and resistance to apoptosis. BRAF mutations: Mutations in the BRAF gene, another component of the MAPK pathway, are less common in NSCLC but can also contribute to tumor genesis by enhancing MAPK signaling. Loss of tumor suppressors: The loss of negative regulators like PTEN (phosphatase and tensin homolog) or DUSP (dual specificity phosphatases) can also lead to aberrant activation of the MAPK pathway, contributing to tumor progression.
Receptor Tyrosine Kinases (RTKS):
Once the RTK is activated and the intracellular signal is propagated, the Ras/Raf/MEK/ERK pathway is one of the major signaling pathways that transduce the signal further into the cell, especially influencing cell growth and survival.
Ras Activation:
The phosphotyrosine residues on the activated RTK provide docking sites for adaptor proteins like Grb2, which then recruit SOS (Son of Seven less), a guanine nucleotide exchange factor (GEF). SOS facilitates the exchange of GDP for GTP on the small GTPase Ras (a key molecular switch). Activated Ras (Ras-GTP) then triggers the next step in the pathway by activating the RAF kinase (a MAPKKK, or MAPK kinase kinase).
Raf Activation:
RAF, once activated by Ras-GTP, initiates the phosphorylation and activation of the next kinase in the pathway, MEK (MAPK/ERK kinase, a MAPKK). Raf phosphorylates MEK on specific serine/threonine residues, causing its activation.
MEK Activation:
Activated MEK then phosphorylates the final kinase in the cascade, ERK (Extracellular Signal-Regulated Kinase, a MAPK). This phosphorylation event activates ERK, leading to its translocation into the nucleus.
ERK and Transcriptional Regulation:
Once in the nucleus, ERK phosphorylates various transcription factors and other effector proteins that regulate gene expression. ERK targets include transcription factors such as Elk-1, c-Fos, c-Myc, and AP-1, which control the expression of genes involved in cell cycle progression, cell survival, differentiation, and growth
RAS: Ras is a small ATPase that regulates upstream and downstream protein interactions, and ATPase hydrolyses GTP into GDP. RAS proteins are bound to the plasma membrane’s internal surface and function as binary switch kinases. This results in a conformational shift that allows downstream effectors to bind to and activate Ras, which triggers signaling cascades.
<a href="https://www.ijpsjournal.com/uploads/createUrl/createUrl-20250428131755-2.png" target="_blank">
<img alt="A Pie Chart Representing the Classification Of Lung Cancer.png" height="150" src="https://www.ijpsjournal.com/uploads/createUrl/createUrl-20250428131755-2.png" width="150">
</a>
Figure 2 | A Pie Chart Representing the Classification Of Lung Cancer
Drug Repurposing Strategies:
1. Hypothesis Generation: Identifying the core targets of a disease and potential drugs that could target them.
2. In Vitro And In Vivo Testing: Determining the efficacy of the drug in laboratory and animal models.
3. Clinical Trials: Conducting Phase II clinical trials if Phase I trials show promising results
Drug repurposing in oncology has largely been driven by either understanding disease pathways or serendipitous findings. To increase success, innovative strategies are needed to match existing drugs with new applications. Computational and experimental methods can be used to identify potential repurposed drugs. Recent technological advances have led to the development of cutting-edge strategies that can be consciously paired with novel indications, making drug repurposing a promising approach to accelerate drug development and improve patient outcome.
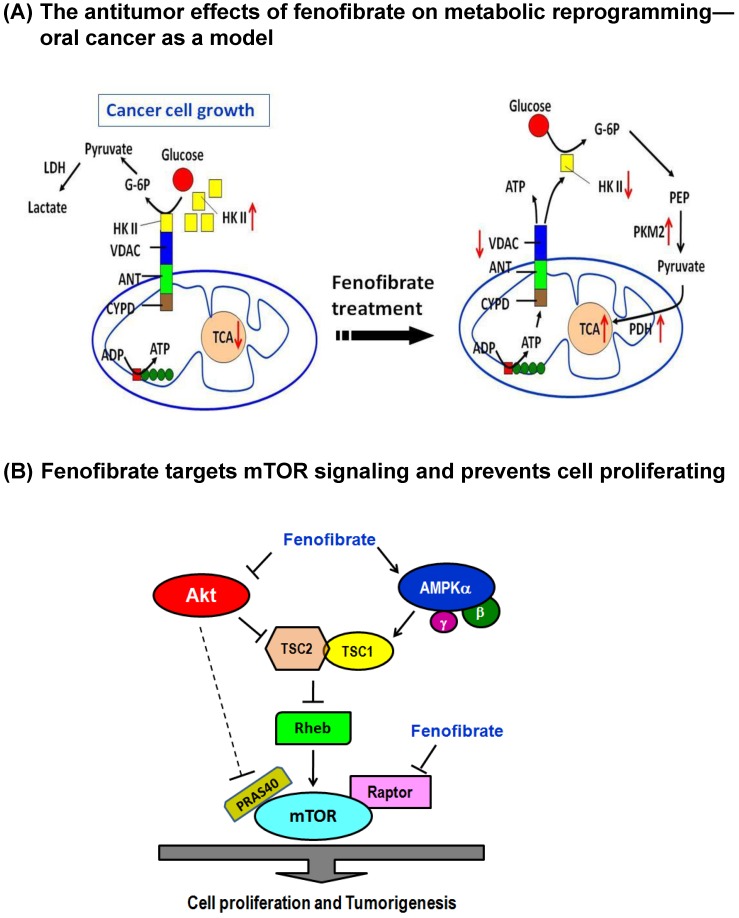
Fenofibrate Suppresses Oral Tumor genesis, Potential Drug Repurposing for Oral Cancer:
The Warburg effect is a phenomenon where cancer cells exhibit increased glucose metabolism and lactate production, even in the presence of oxygen. Targeting mitochondrial metabolism to induce cell death is a promising anticancer strategy. Fenofibrate, a lipid-lowering agent, has shown potential as an anticancer drug. Your study demonstrates that Fenofibrate can delay oral tumor development by reprogramming metabolic processes. This is a novel mechanism of action for Fenofibrate, and it's exciting to see how it can be used to combat cancer. By targeting mitochondrial metabolism, Fenofibrate may be able to:
1. Reduce energy production in cancer cells
2. Inhibit the Warburg effect
3. Induce cell death in cancer cells
Fenofibrate is inducing cytotoxicity in cancer cells by targeting their metabolic pathways. Here's a breakdown of the results:
1. Decreased OCR (Oxygen Consumption Rate): Fenofibrate reduces the amount of oxygen being consumed by the cells, which indicates a decrease in cellular respiration.
2. Increased ECAR (Extracellular Acidification Rate): As a result, the cells are producing more lactic acid and other metabolic byproducts, leading to an increase in extracellular acidification.
3. Reduced ATP Content: Fenofibrate is decreasing the amount of ATP (adenosine triphosphate) available to the cells, which is essential for their survival and proliferation.
4. Changes In Protein Expressions:
- Hexokinase II (HK II): Fenofibrate is reducing the expression of HK II, which is a key enzyme in the Warburg effect.
- Pyruvate kinase: Fenofibrate is also reducing the expression of pyruvate kinase, another important enzyme in the Warburg effect.
- Pyruvate dehydrogenase: The expression of pyruvate dehydrogenase is being reduced, which is involved in the regulation of metabolic pathways.
- Voltage-dependent anion channel (VDAC): Fenofibrate is affecting the expression of VDAC, which is involved in the regulation of cellular metabolism and energy production
Ug repurposing: a retrospective revolution in breast cancer medicine
Breast cancer is the most common type of cancer affecting women, and it's also one of the leading causes of cancer-related deaths. The HER (Human Epidermal Growth Factor Receptor) family of tyrosine kinase receptors plays a significant role in the development and progression of various human malignancies, including:
<a href="https://www.ijpsjournal.com/uploads/createUrl/createUrl-20250428131755-1.png" target="_blank"><imgalt="2.png"height="150" src="https://www.ijpsjournal.com/uploads/createUrl/createUrl-20250428131755-1.png" width="150">
</a>
1. Non-small cell lung cancer (NSCLC)
2. Colon cancer
3. Breast cancer
Antimicrobial drugs repurposed for breast cancer:
Amprenavir, a protease inhibitor, has shown potential as an anticancer agent in human MCF-7 breast cancer cells and xenografts. The study's results indicate that Amprenavir:
1. Suppresses tumor proliferation
2. Induces apoptosis (programmed cell death)
3. Inhibits ERK2 kinase activity
Some potential advantages of Amprenavir as an anticancer agent include:
1. Targeted therapy: Amprenavir specifically targets ERK2 kinase activity, reducing harm to healthy cells.
2. Apoptosis induction: Amprenavir induces programmed cell death in cancer cells, reducing tumor growth.
3. Anti-tumor effects: Amprenavir demonstrates anti-tumor activity in both in vitro and in vivo models.
ERK2 (Extracellular Signal-Regulated Kinase 2) is a protein involved in cell signaling pathways that regulate cell growth, differentiation, and survival. By inhibiting ERK2 kinase activity, Amprenavir disrupts cancer cell signaling, leading to apoptosis and reduced tumor growth. Artemether (ART) and other artemisinin derivatives have shown promising results in clinical studies for breast cancer therapy. Their benefits include:
1. Apoptosis induction: ART initiates programmed cell death in cancer cells.
2. Anti-proliferation: ART reduces cancer cell growth.
3. Anti-migration: ART disturbs cell movement, reducing metastatic potential.
4. Chemo sensitization: ART improves the effectiveness of chemotherapy.
5. Anti-angiogenesis: ART inhibits tumor blood vessel formation.
6. Tumor selectivity: ART targets cancer cells while sparing healthy cells.
7. Inhibition of drug efflux transporters: ART reduces the development of drug resistance.
Chloroquine, a drug commonly used to treat malaria, has shown significant potential in treating breast cancer. The results are promising across various cell lines and in early-stage clinical trials, both as a standalone treatment and in combination with other medications.
The mouse studies are particularly encouraging, demonstrating that Chloroquine treatment:
1. Increases survival time
2. Decreases the size and number of lung metastases
3. Reduces the volume of the primary tumor
Chloroquine mechanism of action in breast cancer is not fully understood but is thought to involve:
1. Inhibiting autophagy (a process by which cancer cells recycle damaged or dysfunctional components)
2. Disrupting lysosomal function (lysosomes are cell organelles responsible for breaking down and recycling cellular waste)
3. Inducing apoptosis (programmed cell death).
Anti?Inflammatory Drugs:
Anti-inflammatory drugs, more commonly known as non-steroidal anti-inflammatory drugs (NSAIDs), are a class of medications widely used to treat conditions involving pain, inflammation, and fever. These drugs work by inhibiting certain enzymes (primarily COX-1 and COX-2) involved in the production of prostaglandins, which are chemicals that promote inflammation, pain, and Fever.
<a href="https://www.ijpsjournal.com/uploads/createUrl/createUrl-20250428131755-0.png" target="_blank">
<img alt="3.png" height="150" src="https://www.ijpsjournal.com/uploads/createUrl/createUrl-20250428131755-0.png" width="150">
</a>
Fig. 2 Schematic representation of various actions of NSAIDs through different targets and its consequences in various conditions and cancer types. NSAIDS have been known to inhibit NF-KB, COX-1, COX-2, 5 LOX, PAF, and PP2A respectively, and suppress EGFR in different cancer types
Traditional NSAIDs (like aspirin, ibuprofen, and naproxen) inhibit both COX-1 and COX-2. This inhibition can lead to side effects such as gastrointestinal irritation, ulcers, and bleeding (due to COX-1 inhibition), as well as kidney problems. Selective COX-2 inhibitors (like celecoxib) were developed to reduce the inflammatory effects of COX-2 without interfering with COX-1, thereby potentially reducing gastrointestinal side effects. COX-1 is involved in routine, protective functions in various tissues, while COX-2 is more involved in inflammatory responses and pathological conditions. The balance between these two isoforms is crucial for maintaining health, and drugs that modulate COX activity need to be carefully managed to minimize side effect The link between inflammation and the development of colorectal cancer (CRC) is a complex process, influenced by multiple factors including cytokines, chemokine, growth factors, and proteolysis enzymes, along with the role of the gut micro biota, environmental influences, and host genetics. Inflammation and Tumor Development: Chronic inflammation plays a crucial role in cancer development. Tumor-promoting inflammation can be seen as a double-edged sword: while inflammation is essential for immune defense and tissue repair, chronic, unresolved inflammation can create an environment conducive to cancer initiation and progression.
ADVANTAGES:
- Faster time-to-market: Repurposed drugs have already completed initial safety testing, reducing the time and cost to bring them to cancer patients.
- Known safety profile: Repurposed drugs have a established safety profile, reducing the risk of unexpected toxicities.
- Combination therapies: Repurposed drugs can be combined with existing cancer treatments to enhance efficacy.
- Improved patient outcomes: Repurposed drugs can lead to better response rates, progression-free survival, and overall survival.
DISADVANTAGES:
- Lack of understanding of mechanism of action: The exact mechanism by which a repurposed drug exerts its anti-cancer effects may not be fully understood.
- Limited commercial interest: Pharmaceutical companies may be less invested in repurposed drugs due to lower profit margins.
- Potential for unexpected toxicities: Repurposed drugs may exhibit unforeseen side effects in cancer patients.
- Patient variability: Repurposed drugs may not be effective in all patient populations
CONCLUSION:
Drug repurposing in cancer is a promising strategy for treating cancer that can save time and money, and may help fight drug resistance: In this review, we presented an overview of drug repositioning for anti-cancer applications, with a focus on target-based non- cancer drug repurposing. Targeting the Ras/Raf/MEK/ERK is a promising and alternative method in NSCLC treatment, according to the information presented in this review. MAPK signaling pathways are a vital aspect of NSCLC and have aided in the advancement of therapies for this carcinoma. Learning more about this signaling is critical because of its significant functions in carcinogenesis and wide spectrum of crosstalk with major tumor-promoting signaling pathways. Drug repurposing has the potential to alleviate the present drug shortage.
REFERENCES
- V. Andresen, B.T. Gerstein, Drug repurposing for the treatment of acute myeloid leukemia, Front. Med. 4 (2017) [17].
- C. Verbaanderd, L. Meheus, et al., Repurposing drugs in oncology: next steps, Trends in Cancer 3 (2017) 543–546 [62].
- Wong RSY. Role of nonsteroidal anti-inflammatory drugs (NSAIDs) in cancer prevention and cancer promotion. Adv. Pharmacol Sci. 2019; 2019:3418975. https://doi.org/10.1155/2019/3418975 [43].
- Fokunang C. Overview of non-steroidal anti-inflammatory drugs (nsaids) in resource limited countries. MOJ Toxicol. 2018;4(1):5–13. https://doi.org/10.15406/mojt.2018.04.00081 [44].
- Kirtonia, A. et al. Repurposing of drugs: an attractive pharmacological strategy for cancer therapeutics. Semin. Cancer Biol. 68, 258–278 (2021) [16].
- Jiang, W., Hu, J. W., He, X. R., Jin, W. L. & He, X. Y. Statins: a repurposed drug to fight cancer. J. Exp. Clin. Cancer Res. 40, 241 (2021) [130].
- Moffat, J. G., Vincent, F., Lee, J. A., Eder, J. & Prunotto, and M. Opportunities and challenges in phenotypic drug discovery: an industry perspective. Nat. Rev. Drug Discov. 16, 531–543 (2017){fig}.
- https://www.researchgate.net/figure/Drug-repurposing-in-NSCLC-Food-and-Drug-Administration-FDA-approved-drugs-identified_fig1_366893891 {fig.}.
- https://pubmed.ncbi.nlm.nih.gov/27313493/.
- Pradhan R, Singhvi G, Dubey SK, Gupta G, Dua K. MAPK Pathway: A Potential Target for the Treatment of Non-Small-Cell Lung Carcinoma. Future Med Chem (2019) 11:793–5. doi: 10.4155/fmc-2018-0468
- Pritchard AL, Hayward NK. Molecular Pathways: Mitogen-Activated Protein Kinase Pathway Mutations and Drug Resistance. Clin Cancer Res an Off J Am Assoc Cancer Res (2013) 19:2301–9. doi: 10.1158/1078-0432.CCR-12-0383
- Zappa C, Mousa SA. Non-Small Cell Lung Cancer: Current Treatment and Future Advances. Transl Lung Cancer Res (2016) 5:288–300. doi: 10.21037/ tlcr.2016.06.07
- Herbst RS, Morgenstern D, Bosh off C. The Biology and Management of Non-Small Cell Lung Cancer. Nature (2018) 553:446–54. doi: 10.1038/ nature25183
- Lee S, Rauch J, Kolch W. Targeting MAPK Signaling in Cancer: Mechanisms of Drug Resistance and Sensitivity. Int J Mol Sci (2020) 21(3):1102. Doi: 10.3390/ijms21031102
- Kanehisa M, Sato Y, Furumichi M, Morishima K, Tanabe M. New Approach for Understanding Genome Variations in KEGG. Nucleic Acids Res (2019) 47: D590–5. doi: 10.1093/nar/gky962
- Braicu C, Buse M, Busuioc C, Drula R, Gulei D, Raduly L, et al. A Comprehensive Review on MAPK: A Promising Therapeutic Target in Cancer. Cancers (Basel) (2019) 11(10):1618. doi: 10.3390/cancers11101618
- Lemjabbar-Alaoui H, Hassan OUI, Yang YW, Buchanan P. Lung Cancer: Biology and Treatment Options. Biochim Biophys Acta - Rev Cancer (2015) 1856:189–210. doi: 10.1016/j.bbcan.2015.08.002
- Pantziarka, P., Verbaanderd, C., Huys, I., Bouche, G. & Meheus, L. Repurposing drugs in oncology: from candidate selection to clinical adoption. Semin. Cancer Biol. 68, 186–191 (2021)
- Shim, J. S. & Liu, J. O. Recent advances in drug repositioning for the discovery of new anticancer drugs. Int. J. Biol. Sci. 10, 654–663 (2014).
- Xu, H., Jiao, D., Liu, A. & Wu, K. Tumor or ganoids: applications in cancer modeling and potentials in precision medicine. J. Hematol. Oncol. 15, 58 (2022)
- Wong RSY. Role of nonsteroidal anti-inflammatory drugs (NSAIDs) in cancer prevention and cancer promotion. Adv Pharmacol Sci. 2019; 2019:3418975.
- Doric J, Antonucci L, Karin M. Targeting inflammation in cancer prevention and therapy. Cancer Prev Res. 2016;9(12):895–90
- Misra S, Hassall VC, Mark Wald RR, O’Brien PE, Ghatak S. Inflammation and cancer. Wound Healing: Stem Cells Repair and Restorations, Basic and Clinical Aspects. Vol. 420, no. 6917. 2018. p. 239–74. H
- N. Cardio-renal safety of non-steroidal anti-inflammatory drugs. J Toxicol Sci. 2019;44(6):373–91.


 Pawar Shilpa*
Pawar Shilpa*
 Raut Aarti
Raut Aarti
 Ghule Shrutika
Ghule Shrutika
 Dorale Snehal
Dorale Snehal


 10.5281/zenodo.15294680
10.5281/zenodo.15294680