Abstract
Objectives.
The study was designed to evaluate the hepatoprotective activity of aqueous-methanolic extract of stem bark of Gmelina arborea Roxb against carbon tetrachloride (CCl4), Paracetamol (PCM) and Thioacetamide (TAA) induced hepatotoxicity in albino rats. Methods. In each model, rat of either sex were pre-treated with Silymarin (100 mg/kg), low and high dose of Gmelina arborea bark extract (100 and 500 mg/kg, orally) and subsequently subjected to CCl4 (0.5 ml/kg), PCM (2 g/kg) and TAA (100 mg/kg) administration to induce hepatotoxicity. The influence of prophylactic treatment was analyzed by quantification of biomarkers such as Aspartate transaminase, Alanine transaminase, Alkaline phosphatase and serum bilirubin (Total and Direct) along with histopathological studies of liver tissues. Results. The activities of all marker enzymes registered an extremely significant elevation in CCl4, PCM and TAA treated rats which were significantly recovered towards an almost normal level in rats co-administered with extracts. The results were also supported by histopathological finding. Conclusion. The results of the present study indicate that stem bark of Gmelina arborea possess hepatoprotective effect and probably it is due to antioxidants.
Keywords
Gmelina arborea Roxb, Aqueous-methanolic extract, Silymarin, CCl4, PCM and TAA induced toxicity, Biochemical parameters, Histopathological studies.
Introduction
The liver diseases are some of the fatal diseases in the world today. They pose a serious challenge to international public health. In spite of tremendous strides in the modern medicine, there are not much drugs available for the treatment of liver disorders and may cause serious adverse effect so there is a worldwide trend to go back to traditional medicinal plants. Herb and herbal remedies are very promising for the treatment of liver ailments due to its availability and apparent safety profile.1,2 A large number of plants and herbal formulations have been claimed to have hepatoprotective activity. Nearly 160 phytoconstituents from 101 plants have been claimed to possess liver protecting activity.3
Gmelina arborea Roxb belongs to family Verbenaceae, is a fast-growing deciduous tree. G.arborea is one of the herbs mentioned in all ancient scriptures of Ayurveda and it has great medicinal value.4 There are reports which showed that bark is stomachic, used in anthelmintic, improve appetite, abdominal pain, fever. Leaf is used to relieve headache and wash for ulcers. Flowers sweet, cooling and astringent, useful in leprosy and blood disease. Fruits acrid, bitter and tonic, useful in anaemia, vaginal discharge, to promote growth of hairs. Root is used to treats flatulence and reliever of menstrual irregularities. The phytochemical studies revealed the presence of flavonoids, glycosides, tannins, carbohydrates, alkaloids and saponins. The plants of this genus were reported to contain lignans, gmelinol, n-hexacosnol, n-octanol, luteonil, arboreal, isoarboreol, etc.5-10 G.arborea bark extracts were reported to have antioxidant,11 anthelmintic,12 antimicrobial,13 antidiuretic,14 cardioprotective activity,15 antidiabetic,16 anti-inflammatory17 and antiulcer.18 The reactive oxygen species are free radicals plays an important role in the etiology of various diseases such as inflammation, atherosclerosis, rheumatism, arthritis, ischemia reperfusion injury including liver disorders.19 Therefore, in the present study the hepatoprotective effect of aqueous-methanolic extract of G.arborea have been evaluated in CCl4, PCM and TAA induced acute liver damage in the rats.
MATERIAL AND METHODS
Chemicals
The following chemicals were obtained from the indicated commercial Carbon tetrachloride (Qualigens, India), Thioacetamide (Sigma-Alorich, USA), Paracetamol (National Chemicals, India), Silymarin (Levanol, Micro B&B). Serum enzymes reagents were obtained from Robonik (Prietest), India. The stem bark of Gmelina arborea was collected from local area and was authenticated by Ramnarain Ruia College, Mumbai. The solvent and chemical used were of analytical grade.
Animals
Rats of either sex weighing 175-250 g were housed at 25° ± 5°C, RH 50 ± 5% in a well-ventilated animal house under 12:12 h light dark cycle. Institutional Animal Ethics Committee approved the experimental protocol. The animals were maintained under standard conditions in an animal house as per the guidelines of Committee for the Purpose of Control and Supervision on Experiments on Animals (CPCSEA). The Institutional ethical committee approved the experimental protocol (SDCP/IAEC-11/2010-11).
Plant extract
The stem bark was chopped into small pieces and dried in the shade. The dried stem bark was ground to a coarse powder and extracted (100 g) successively with 80% aqueous-methanol in a Soxhlet extractor at 50oC for 48 hrs. The extract was concentrated by using rotary evaporator to yield a light brown solid (12.5% w/w) and kept in dark place. The extract obtained was then subjected to phytochemical analysis to determine the chemical constituents present in the extract. The G.arborea bark extract (GABE) was suspended in 5 % gum acacia as suspending agent for oral administration.
Acute toxicity and dose selection
The dose selection of Gmelina arborea bark extract (GABE) were based on acute toxicity studies, carried out according to OPPTS (Office of Prevention, Pesticide and Toxic Substance) following the limit test procedure.20 The animals were fasted overnight prior to the studies. Mice were divided into two groups of three each. Test dose of 2 g/kg body weight and 5 g/kg body weight were given orally to either group of mice. Mice were observed for 72 hours for mortality. 1/10th and 1/50th of the maximum safe dose corresponding to 500 and 100 mg/kg body weight were selected as high and low doses respectively.
Experimental design
- Carbon tetrachloride induced acute hepatitis in rats21
Carbon tetrachloride (CCl4) induced liver injury. The animals were divided into 5 groups consisting of six animals. The animals were then subjected to either one of the following treatments for 9 days. The CCl4 was administered after dilution with liquid paraffin the ratio of 1:1. Food was withdrawn 12 hrs before CCl4 administration to enhance liver damage in animals of groups II, III, IV and V. The animals were sacrificed 24 hrs after the administration of CCl4..
Rats of either sex were divided into 5 treatment groups of six animals each.
- Group-I- Vehicle; 1ml/250 g, p.o. (Normal control)
- Group-II- Distilled water for 9 days + CCl4 (0.5 ml /kg, p.o.) on ninth day. (Toxic control)
- Group-III- Silymarin (100 mg/kg/day, p.o.) for 9 days CCl4 (0.5 ml /kg, p.o.) on ninth day. (Standard)
- Group-IV- Low dose of GABE (100 mg/kg/day, p.o.) for 9 days + CCl4 (0.5 ml /kg, p.o.) on ninth day.
- Group-V- High dose of GABE (500 mg/kg/day, p.o.) for 9 days + CCl4 (0.5 ml /kg, p.o.) on ninth day.
- Paracetamol induced liver toxicity in rats22
The same procedure as mentioned above was followed except that the liver was damaged using PCM (2 g/kg, p.o.) diluted with sucrose solution (40% w/v). PCM was administered in 3 divided dose on day 9 and animals were sacrificed 48 hrs after administration of PCM.
- Thioacetamide induced liver necrosis in rats23
The same procedure as mentioned above was followed. Damage was induced by using TAA (100 mg/kg, s.c.), which was prepared in distilled water (2% solution) and animals were sacrificed 48 hrs after administration of TAA.
Assessment of liver damage
Blood samples were collected by retro-orbital puncture method from each model after completion of the treatment and blood sample were kept at room temperature for 30 min and centrifuged at 4000 rpm for 10 min to obtain , which was kept at -20 0 C until further assay. serum was used for assay of marker enzymes such as aspartate aminotransferase (AST), alanine aminotransferase (ALT), alkaline phosphatase (ALP) and serum bilirubin (Total and Direct). The liver was isolated and washed with normal saline, dried using filtered paper and weighed immediately. The liver samples collected in 10% formalin were used for histopathological examination. Paraffin sections were stained with haemotoxylin and eosin examined microscopically at a magnification of x400.
Statistical analysis
The data were expressed as mean ± SEM and the difference between experimental groups compared by one-way ANOVA followed by Tukey’s test at 5% significance level.
RESULTS
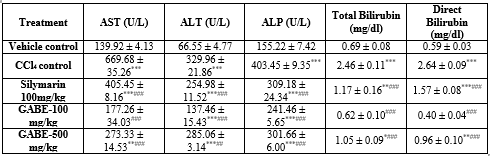
- Carbon tetrachloride induced acute hepatitis (Table 1)
Plasma AST, ALT, ALP and D-bilirubin increased extremely significant (P<0>
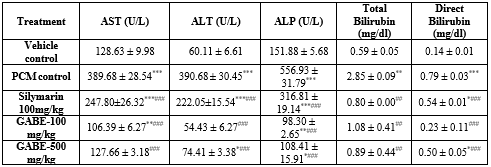
- Paracetamol induced liver toxicity in rats (Table 2)
By executing the experimental protocol it was documented that the prophylactic treatment with the groups like toxic control (PCM), standard (Silymarin) exhibited extremely significant (P<0>
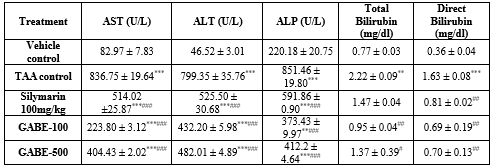
- Thioacetamide induced liver necrosis in rats (Table 3)
By performing the experimental protocol it was recorded that the prophylactic treatment with the groups like toxic control (TAA), standard (Silymarin) demonstrated extremely significant (P <0>
The pre-treatment of Standard (Silymarin) and both low dose and high dose of Gmelina arborea (GABE-100 and GABE-500) exhibited an extremely significant (P<0>
DISCUSSION
CCl4, PCM and TAA induced hepatic injuries are commonly used models for the screening of hepatoprotective drugs and the extent of hepatic damage is assessed by the level of released cytoplasmic AST, ALP, ALT and Bilirubin in circulation.24 CCl4 is one of the most commonly used hepatotoxins in the experimental study of liver diseases. The CCl4 toxicity begins with the in endoplasmic reticulum, which results in the loss of metabolic enzymes located in the intracellular structures. The toxic metabolite CCl3 (carbon trichloride) radical produced by microsomal oxidase system and bind covalently to macromolecule and causes per oxidative degradation of lipid membranes of the adipose tissues. The elevated levels of serum enzymes are indicative of cellular leakage and loss of functional integrity of cell membrane in liver. The recorded results suggested that the Gmelina arborea bark extract (GABE) demonstrating the hepatoprotective activity by reducing the marker enzyme such as AST, ALT, ALP and Bilirubin level in the serum against the CCl4 induced hepatotoxicity. This may be due to the protective activity of hepatic cells by scavenging oxidative free radicals and thereby diminishing the permeability of these endogenous biomarkers into extra hepatic regions.25,26 The analgesic paracetamol (PCM) causes a potentially fatal, hepatic centrilobular necrosis when taken in overdose. These findings indicated that PCM was metabolically activated by cytochrome P450 enzymes to a reactive metabolite that depleted glutathione (GSH) and covalently bound to protein. It was shown that repletion of GSH prevented the toxicity. The reactive metabolite was subsequently identified to be N-acetyl-p-benzoquinone imine (NAPQI). Although covalent binding has been shown to be an excellent correlate of toxicity. Paracetamol also leads to macrophage activation in kupffer cells which leads to release a number of inflammatory cytokines, including IL-1, IL-6, and TNF-? and multiple cytokines are released in PCM toxicity which leads to cell death.25,26 Damaged induced by liver is accompanied by the increase in the activity of some serum enzymes. The anti-hepatotoxic actions of the GABE (100 and 500 mg/kg) was substantiated by significant attenuation of the increased levels of serum enzymes in rats intoxicated with PCM, this may be due to antioxidant which present is bark extract of G.arborea. Thioacetamide (TAA) is a potent hepatotoxic that is metabolized by Cyt-p450 enzymes present in the liver microsomes and is converted to a toxic reactive intermediate called thioacetamide S-oxide due to oxidation process. It is responsible for the changes in cell permeability, increase intracellular concentration of Ca++, increase in nuclear volume and enlargement of nucleoli and also inhibits mitochondrial activity which leads to cell death. Pre-treatment with GABE (100 and 500 mg/kg) significantly reversed the elevated serum enzymes markers in animals treated with TAA. This effect may also be due to anti0xidant effect of GABE, which may neutralize the reactive metabolite of TAA.27 So, the hepatoprotective activity of GABE (at dose of 100mg/kg and 500mg/kg) exhibited significant reduction of serum enzymes such as AST, ALT, ALP and Bilirubin against the CCl4, PCM and TAA induced liver damage.
CONCLUSION
On the basis of the results obtained in the present study, it is concluded that an aqueous-methanolic extract of Gmelina arborea stem bark witnessed significant hepatoprotective activity of both low and high dose (100 mg/kg and 500 mg/kg) against CCl4, PCM and TAA induced liver damage. But low dose (100 mg/kg) found to be therapeutically more potential than high dose (500 mg/kg). Bark extract found to have hepatoprotective activity due to presence of large amounts of flavonoids, tannins and phenolic compounds, exhibits high antioxidant and free radical scavenging activities. The liver protective effect of bark extract may be attributed to the individual or combined action of phytoconstituents present in it.
Further investigations need to be carried out to isolate and identify the antioxidant compounds present in the plant extract. Furthermore, investigation is in progress to determine the exact phytoconstituent(s) responsible for hepatoprotective effect.
ACKNOWLEDGEMENT
Authors would like to extend their thanks to Mr. Sadanand Shetty, Chairman, Mr. Nidhish Shetty, Vice Chairman, Shree Devi Education Trust for providing essential requirement to complete this research study.
ABBREVIATION
ALP : Alkaline phosphatase
ALT : Alanine transaminase
AST : Aspartate transaminase
CCl3 Carbon trichloride
CCl4 : Carbon tetrachloride :
GABE : Gmelina arborea bark extract
Hrs : Hours
PCM : Paracetamol
TAA : Thioacetamide
REFERENCES
- Karan M, Vasist K, Handa SS. Antihepatotoxic activity of Swertia chirata on carbon tetrachloride induced hepatotoxicity in rats. Phytotheraoy Research 1999; 13:24-30.
- Raza M, Chuodary MI, Atta-ur-rahman. Anticonvulsant medicinal plants. Studies in natural product chemistry, Elsvier Science Publisher, Netherland 1999, p. 507-553.
- Thyagarajan SP, Jayaram S, Gopalakrishnan V, Hari R, Jeyakumar P, Ms Sripathi. Herbal medicines for liver disease in India. J Gastroen Hepatol 2002; 17:S370-S376.
- Available from: URL: http://en.wikipedia.org/wiki/Gmelina_arborea
- Available from: URL: http://www.herbalcureindia.com/herbs/gmelina-arborea.htm
- Anjaneyulu A S R, Jaganmohan Rao. The structures of lignans from Gmelina arborea Linn. - Elsevier. Tetrahedron 1975; 31(10):1277-1285.
- URL: http://www.himalayahealthcare.com/herbfinder/h_gmelina.htm. Fitoterapia 1992; 63: 295.
- Khare C.P. Encyclopedia of Indian Medicinal Plants. Springer-Verlag Berlin-Heidelberg, Germany. 2004: 236-237.
- Unnikrishnan KP, Raja S, Remashree AB, Balachandran I. Occurance of gmelinol in stem bark of Gmelina arborea Roxb. Aryavaidyan 2007; 21: 34-37.
- Satyanarayana P, Koteswara PR, Ward RS, Pelter A. Arborone and 7-oxo-dihydrogmelinol: two new keto-lignanas from Gmelina arborea. J. Nat.Prod 1986; 49(6):1061-1064.
- Patil SM, Kadam VJ, Ghosh R. In vitro antioxidant activity of methanolic extract of stem bark of gmelina arborea Roxb. (verbenaceae). Int.J. PharmTech 2009; 1(4):1480-1484.
- Ambujakshi HR, Thakkar H, Shyamnanda. Anthelmintic activity of Gmelina arborea Roxb. Leaves extract. Indian Journal of Pharmaceutical Research and Development 2009 Nov; 1(9):1-5.
- El-Mahmood AM, Doughari JH, Kiman HS. In vitro antimicrobial activity of crude leaf and stem bark extracts of Gmelina arborea (Roxb) against some pathogenic species of Enterobacteriaceae. Afr. J. Pharm. Pharmacol 2010 June; 4(6):355-361.
- Sravani P, Murali CM, Syed S, Sadik BS, Soubia SN, et al. Evaluation of Diuretic Activity of Gmelina arborea Roxb. International Journal of Advances in Pharmaceutical Research 2011 Apr; 2(4):157-161.
- Vijay T, Dhana MS, Sarumathy K, Palani S, Sakthivel K. Cardioprotective, antioxidant activities and Phytochemical analysis by GC-MS of Gmelina arborea (GA) in Doxorubicin-induced myocardial necrosis in Albino rats. Journal of Applied Pharmaceutical Science 2011;1(05):198-204.
- Pattanayak P, Parhi PK, Mishra SK, Khandei PK. Screening Of Anti-Diabetic Activity Of Bark Extracts Of Gmelina Arborea In Streptozotacin Induced Diabetic Rats. International Journal of Pharmaceutical Sciences Review and Research 2011 May-June; 8(2):130-132.
- Pravat KP, Priyabrata P, Paresh M, Manoj KP. An in vivo study on analgesic and antipyretic activity of bark extract of Gmelina arborea. International Journal of Pharmaceutical Sciences Review and Research 2011 Sep-Oct; 10(2):78-81.
- Murali CM, Sravani P, Nizamuddin BS, Chitta SK, Syed S, Sadik S, et al. Evaluation of anti-ulcer activity of methanolic extract of Gmelina arbora in experimental rats. IJAPR 2011 Mar; 2(3):81-86.
- Roy HB. Free radical damage and its control. Elsevier Science 1994:125.
- Available from: URL: http://www.epa.gov/opptsfrs . 2010 Nov 15.
- Matsuda H, Samukawa K, Kubo M. Anti-hepatotoxic activity of Ginsenoside Ro. Planta med. 1991;57:523-526.
- Jyothi Y, Kamath JV, Asad M. Effect of Hexane extract of Boswellia serrata oleo-gum resin on chemically induced liver damage. Pak. J. Pharm. Sci. 2006;19(2):129-133.
- Roy CK, Kamath JV, Asad M. Hepatoprotective effect activity of Psidium gujava Linn. leaf extract. Indian J. Exp Biol. 2006;44:305-312.
- Baheti JR, Goyal RK, Shah GB. Hepatoprotective activity of Hemidesmus indicus R. Br. in rats. Indian J Exp Biol 2006 May; 44:399-402.
- Eberhardt M, Lee C, Liu RH. Antioxidant activity of fresh apples. Nature 2000;405:903-904.
- Liu RH, Eberhardt M, Lee C: Antioxidant and antiproliferative activities of selected New York apple cultivars. New York Fruit Quarterly 2001;9:15-17.
- Mohammed AA, Mahmood AA, Salmah I, Zahra AA. Hepatoprotective effect of Orthosiphon stamineus extract on Thioacetamide-induced liver cirrhosis in rats. Hindawi Publishing Corporation 2011:1-









 Rohit K. Kaswala*
Rohit K. Kaswala*
 Jagdish V. Kamath
Jagdish V. Kamath








 10.5281/zenodo.12737812
10.5281/zenodo.12737812