Abstract
An abnormally developing tumour that starts out as a localised illness that may expand and affect other organs or critical functions is what causes cancer. Cancer is the result of unchecked cell proliferation. Cancer remains, causing many deaths every year. continues to be among the world's worst illnesses. The purpose of this research was to provide a summary of previously released papers about current developments in the discovery of anticancer drugs. Chemotherapy involves delivering drugs to the tumor and is very effective in treating cancer. Anti-inflammatory drugs such as camptothecin (CPT) are important for liver and tumor treatment. This study successfully developed novel HA-coated CPT nanocrystals to specifically deliver hydrophobic CPT to tumors using a precipitation method which was inspired by the unique CD44 binding potential of hyaluronic acid (HA). These nanocrystals of CPT coated with HA.HA may be used as a ligand to create tailored nanovehicles for potential use in cancer treatment in the future. Our goal in this review is to give a summary of several in vitro and in vivo research that address this possibility.
Keywords
Hyaluronic acid, Tumor targeting, Camptothecin
Introduction
Uncontrolled cell proliferation, which results in the development of aberrant tumours, is the hallmark of the condition known as cancer. These tumours have the ability to infect vital organs or systems, impairing their regular operations [1]. Cancer is considered a multifactorial disease because it is caused by the interaction of many factors, including pollutants, dietary poisons, infections, medications, ionizing radiation, and genetic alterations. [2]. Multiple systems that have been preserved throughout evolution strictly control cell division, ensuring that two genetically identical cells are produced [3].Every year, many people lose their lives to cancer, one of the worst diseases in modern times. Global differences, the accessibility of healthcare, and other socioeconomic considerations all affect how this condition is managed [4].
Global cancer figures indicate that 20 million additional instances of cancer will occur. and by 2023, cancer will kill 10 million people worldwide. Cancer rates are expected to rise by more than 60% over the next two years, putting pressure on individuals, communities and healthcare systems. A study in Ethiopia between 2000 and 2016 found that cancer killed 50,913.5 people of all ages and genders (95% confidence interval), with the standard death rate with age being 93.5 per 100,000 people and the crude death rate being 93.5 per 100,000 people. He found that it was. Most patients are women. The increase in cancer cases, especially due to increasing lifestyles, has caused the pharmaceutical industry to invest heavily in this field. Cancer drug research is still very difficult, and the desired therapeutic results have not yet been attained, despite these efforts [5].Driven by an increased understanding of the physiopathology of the disease, drug makers Despite their high cost-benefit ratio, have persisted in producing therapies. since the first half of the 20th century [6,7].
Many classes of anticancer medications have been created by pharmaceutical corporations, including immunomodulators, hormones and hormone antagonists, and cytotoxic pharmaceuticals (such as botanical extracts, antibiotics, alkylating agents, antimetabolites, and other cytotoxic agents) [8,9].Nevertheless, these chemotherapies have drawbacks such cytotoxicity, lack of selectivity, multidrug resistance induction, and stimulation of stem-like cell proliferation[10].
The possibility of creating more potent medicines has expanded with the discovery of new molecular targets. The primary obstacles in the search for new cancer drugs Monoclonal antibodies and antibody-small molecule conjugates have been used to combat will reduce side effects and increase selectivity. These advances solve the problems caused by antibiotics such as toxicity, lack of selectivity, and side effects., producers and researchers have thoroughly documented therapeutic targets and novel approaches for drug development. The objective of this review is to gather and synthesise research on new chemical compounds that show cytotoxic action against cancer cells in vitro, with a focus on novel target proteins and biomarkers that may have therapeutic applications.
An Overview of the Discovery of Anticancer Drugs
Anticancer medications are complicated, expensive, time-consuming, and demanding, which has presented researchers and pharmaceutical corporations with many difficulties in their design and development. [11]. In addition to being extremely dangerous and complicated, first-line medications do not directly target cancer cells. [12,13]. Manufacturers are still working hard to create anticancer medications, but there is still a great deal of interest in creating unique, targeted small-molecule medications. Using in silico techniques has proved very beneficial in this endeavour in the past several years [14].
AI, or artificial intelligence, has recently emerged as a promising tool for the development of anticancer drugs, enabling faster, more efficient, and more effective methods than traditional methods. AI has the capacity to quicken the finding of new drugs and the development of better treatment candidates. When searching for antibodies, AI can help identify targets and then screen potential products structure-based, ligand-based, and fragment-based.AI also makes it easier to repurpose drugs, accurately forecast drug reactions unique to anticancer treatments, and create big compounds from scratch for anticancer objectives [15,16].New developments in anticancer treatments come from synthetic or natural sources, taking into account the therapies' effectiveness and toxicity.
Drug design and discovery heavily relies on both conventional computer-aided drug design (CADD) and artificial intelligence-based technologies. Furthermore, the practice of repurposing drugs based on prospective targets has become more widespread [17-19]. Cancer immunotherapy does face certain challenges, though , including immune response evasion by cancer cells, resistance, and problems with delivery systems [20].Recent developments indicate that these problems might be resolved by using nanoparticles with nanocarriers as their vehicles [21].Because of their special properties, which include reduced Nanoparticles' impacts include toxicity, greater permeability, improved stability, precise targeting, and prolonged retention are a significant tool in the fight against cancer [22].
Targets and Biomarkers for Anticancer Drugs: Current Progress
In order to increase overall survival rates and decrease side effects from cancer treatment, targeted therapy is essential. In contrast to patients who received no matching targeted therapy, those who did saw significant improvements in overall survival and progression free endurance [23]. Because of problems with toxicity and efficacy that have made many molecularly targeted drugs unsuccessful in treatment, recent advances in molecular biology have forced researchers to concentrate on therapeutic targets that may successfully destroy cancer [24].
Kinases as targets
One family of anticancer medications called kinase inhibitors works by physically interfering with target enzymes' active sites to prevent kinase activation. An estimated 2000 kinases, classified as tyrosine-specific or serine/threonine-specific, are thought to be present in the human genome. There is a connection between these kinases [24]. Imatinib was the initial drug to be launched in the field of clinical oncology.; bosutinib, sorafenib, and sunitinib came next. All of these medications target catalytic binding sites of tyrosine kinases by competitive ATP inhibition; however, the range of kinases they target, their pharmacokinetic properties, and their substance-specific adverse effects differ [25].

Structure 1.1
Tubulin/ microtubule as target
A 52 kD globular protein called tubulin polymerizes to create microtubules, which are an essential part of the eukaryotic cytoskeleton. Throughout the whole cell cycle, microtubules expand and shrink. The development of cancer cells depends on the microtubules' strength., which divide and multiply more quickly than normal cells. As a result, since microtubule-targeting drugs impede cell proliferation and growth, their development has drawn attention in the fight against cancer. Thus, tubulin has emerged as a top target for the development of anticancer medications. To find safer and more potent therapeutic options, researchers have synthesised a variety of tubulin-targeting compounds and performed structure-activity relationship studies [24,26].

Structure 1.2
Vascular targeting agents
Trained to particularly target the vasculature of tumours, vascular targeting agents (VTAs) impede the growth and development of tumours. These drugs take advantage of the fact that tumour cells divide quickly, making them dependent on a constant supply of nutrients and oxygen. Tumour metastasis and progression depend on the creation and upkeep of blood vascular networks. Because Vascular disruptors, or VDAs, have the ability to halt blood flow to tumours, they are a prospective adjunct to systemic chemotherapy in the treatment of cancer [27,28].

Structure 1.3
Inhibitors of Angiogenesis
Angiogenesis a new family of medications called inhibitors aims to stop the vascularization process of tumours. VEGF-A overexpression is often linked to the development, invasion, and metastasis of tumours. Currently, inhibitors of VEGFA and VEGFR2 are used to target VEGF-A [28].When treating bevacizumab and additional inhibitors of angiogenesis for non-small-cell lung cancer (NSCLC)and ramucirumab are utilised to target VEGFs in an effort to block their effect [29].
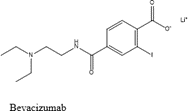
Structure 1.4
Graphical abstract:

Figure 1: Graphical abstract
HA Micelle
Due to their potential to solubilize anticancer medicines that are water-insoluble and their efficient tumor-targeting properties, polymeric micelles (PMs), which are self-assembled structures, have been thoroughly explored as targeted drug carriers [30–31] via interactions that are chemical, physical, and electrostatic, tiny hydrophobic medicines The hydrophobic core of polymeric micelles (PMs), which are amphiphilic in nature, can contain it [32–34]. You have the option to keep the medication submerged in water, extend blood circulation, and make it more soluble in water tumour through the improved permeability and retention (EPR) impact at locations, they help deliver hydrophobic drugs to their intended targets [35], thereby improving the efficacy of anticancer treatments [36–38].
After systemic delivery, PMs can aggregate selectively in angiogenic tumour tissues through fenestrated vasculature because of their nanosized structure (20 ~ 100 nm) [36, 39]. Combining hydrophobic polymers, tiny pharmaceuticals, or pharmaceuticals with hyaluronic acid (HA) can result in effective medications. Effective nanocarriers (Figure 2) that release medications in response to certain stimuli, including redox processes or exposure to acidic pH, can also be coated on HA to increase its anticancer impact.

Figure 2: Hyaluronic Acid (HA) Polymeric Micelles Schematic Representation
Amphiphilic HA-ligands can conjugate or encapsulate medications to form micelles in aqueous solutions. Polymers can graft onto Block copolymer, tiny molecules, and the HA backbone like dendrimers and medications or positively charged molecules through electrostatic interactions (shown by green circles) can modify HA. These changes improve the antitumor efficaciousness of HA polymeric micelles by allowing them to respond to stimuli like acidic pH or redox processes.
1.HA Polymeric Micelles (PMs)
It has been extensively studied to conjugate hydrophobic polymers with HA is used to form amphiphilic polymers that can be combined into micelles with graft polymers [40], block copolymers [41], and ring-opening polymers [42]. These hydrophobic polymers contain caprolactone, methacrylate’s, and ethylene glycol, among other ingredients. Parker et al. [42-44] studied cross-linked HA polymer micelles (PM) formed from HA graft polymers HA-poly (pyridine) disulfide methacrylate (HA)-b-P(PDSMA) and HA-b-poly (caprolactone) (HA-b-PCL) are two examples. The disulphide bond was used in this crosslinking technique (Figure 3) 2-To create a block copolymer containing HA, 2-(Pyridyldithio)-ethyl was joined to the methacrylate chain. The core-crosslinked HA polymeric micelle (PM), with its solid structure, reduces medication loss during circulation. But in the glutathione-rich environment characteristic to tumours, which is found in large amounts in the cytoplasm of cancer cells, the crosslinked disulfide bond can break, releasing medication quickly.
These bio-reducible PMs are intended to increase the effectiveness of treatment by encouraging the release of the drug in response to particular stimuli. PEGylation, or poly(ethylene glycol) conjugation, has been devised to further improve selective accumulation at tumour locations [45, 46].Reduced liver absorption and an extended bloodstream circulation period are two benefits of PEGylation. It may, nevertheless, also obstruct the way in which cancer cells' receptors interact with it [32].To improve anticancer therapy, Camptothecin (CPT), an anticancer medication, was encapsulated in PEGylated HA micelles. Camptothecin-loaded HA nanoparticles (CPT-P-HA-NP) are hydrolyzed by the enzyme HAdase, which releases camptothecin into tumor tissues. Furthermore, the Costa group investigated cancer chemophotothermal treatment (PTT) using Dox and IR780-loaded HA-functionalized micelles, two widely used phototherapy probes. [47]. To add a hydrophobic moiety to HA and promote Grafted rau HA with Poly(maleic anhydride-alt-1-octadecene) (PMAO), causing it to self-assemble into HPN (HA-g-PMAO) nanoparticles. These HPNs can be loaded with Dox and IR780 to create formulations like IR/Dox-HPN and IR-HPN for cancer chemo-phototherapy.

Figure 3 Hydroxyapatic acid (HA) polymer-grafted redox-sensitive hydrophobic polymer micelles
(A) HA-b-P methacrylate HA-polypyridyl disulfür (PDSMA)chemical structure.
(B) Diagram showing the doxorubicin (Dox)-loaded cross-linked HA polymer micelles. When doxorubicin combines with glutathione (GSH), it is liberated from core cross-linked polymeric micelles based on HA (CC-HAM).
Source: Used by permission; reproduced from [43]. Copyright Elsevier Ltd., 2015.
Using photothermal treatment (PTT), IR780 demonstrated strong cytotoxicity under near-infrared (NIR) irradiation. Additionally, loading Dox gave breast cancer cells chemotherapy and created a synergistic impact. These tactics so reveal that when drugs are loaded into polymer-grafted HA micelles, they exhibit significant cytotoxicity., augmenting anticancer therapy in vivo as well as in vitro.
2. HA Coating Micelle
Positively charged nanocarriers and hyaluronic acid (HA), a polyanionic biomacromolecule, interact electrostatically. Drug leakage is stopped and active targeting to CD44 receptors is made possible by coating HA on the surface of these carriers [48].The Ryu group presented findings on amphiphilic HA micelles that incorporate peptide-drug conjugates and small compounds like NIR cyanine that target mitochondria, demonstrating selective cancer therapy [49, 50].They created a positively charged pyridinium moiety in a water-soluble, mitochondria-targeting indocyanine dye (IR-Pyr), and through electrostatic interactions, they created HA micelles (HA-IR-Pyr). By using photodynamic therapy (PDT) to target the mitochondria of cancer cells, these indocyanine dyes function as sensitizers, allowing HA-IR-Pyr micelles to improve anticancer therapy (Figure 4).
In the SCC-7 tumor model, HA-IR-Pyr worked better than other PDT dyes, highlighting the significance of HA in cancer. Nanostructures are created by combining well-charged tripeptide (KCK-CPT) with CPT in aqueous solutions, via electrostatic interactions, change into micelles (HA-KCK-CPT). Compared to KCK-CPT alone, HA-KCK-CPT was more specific for cancer cells, particularly SCC-7 cells. Furthermore, Liu and colleagues [51] The HA-Fe-C14/Dox micelles dissolve and release Dox as they transition from hydrophilic to hydrophobic ferrocene in the GSH-rich tumor microenvironment. Studies conducted in vivo demonstrate that HA-Fe-C14/Dox can stop tumor development. Wu et al. created a two-layer method to encapsulate chiral (?)-gossypol for cancer treatment utilizing HA-coated PEI-g-stearic acid (PgS). [52].
An amphiphilic branching polymer (PgS) is produced when stearic acid is grafted onto PEI. PgS has the ability to form micelles in water and enclose (-)-gossypol ((-)-G) through electrostatic interactions. Positively charged PgS micelles are encapsulated in negatively charged HA. The anticancer drug activity and in vitro and in vivo stability of chiral medicines are enhanced by bilayer (?)-G-PgS/HA. Luo's group employed PCL-PDEAMPC', an amphiphilic copolymer made of poly(caprolactone), to create HA-coated polymeric micelles (PM).N,N-di Ethylaminomethylmethacrylate -b-poly2 (methacryloyloxy)ethylphosphorylcholine, or -r-poly(2- [53].
The size of Mcelle was 76.3 nm instead of 72.6 nm. after HA coating. A doxorubicin (Dox) load was applied to PCL-PDEAMPC micelles. Throughout the 48-hour incubation period, Dox-loaded PCL-PDEAMPC-HA micelles demonstrated toxicity towards 4T1 cells, despite the fact that the micelles themselves were innocuous to the cells. Consequently, HA-coated micelles provide a number of benefits, such as the safe administration of hydrophobic medications, improved cellular absorption, and strong anticancer activity.

Figure 4: Schematic Illustration of HA-Coated Carriers for the Management of Cancer
(A Diagram illustrating the The procedure of producing HA-indocyanine dye (IR-Pyr) micelles.
(B) How HA contributes to the buildup of HA-IR-Pyr tumors ; in comparison to IR-Pyr and IR-780 dyes, HA-IR-Pyr shows improved localization.
Source: Used by permission; reproduced from [49]. The Royal Society of Chemistry, All rights reserved.
Physicochemical and structural properties
Hyaluronic acid, often known as A glycosaminoglycan present in the extracellular matrix is hyaluronic acid (HA). Composed of glucuronic acid disaccharide units and N-acetylglucosamine that repeat. The fact that this basic chemical structure is preserved in all species shows how important it is to biology (Chen and Abatangelo, 1999). Hyaluronate, a salt present in significant quantities in a variety of Skin, blood vessels, synovial fluid, and vitreous humor are among the tissues that contain hyaluronic acid (HA). It is also present in significant concentrations in tissues such as the kidney, brain, muscles, and lungs.
1.Chemical structure
The disaccharide unit of hyaluronic corrosive is composed of the d-glucuronic corrosive and d-N-acetylglucosamine connections between Î?2;-1,4 and Î?2;-1,3 glycosidic linkages (allude to Figure 1). These sugars have the ability to adopt a beta configuration in which the majority of bunches (the anomeric carbon of the adjoining sugar, the carboxylate moiety, and the hydroxyl bunch) are present but the hydrogen particles are varied are positioned similarly because of their structural resemblance to glucose. Axial position is met. The disaccharide units are more robust and stable in this configuration.

Structure 1.5
2. Resolution framework
Hyaluronan's backbone takes on a stiffer the composition of a physiological solution as a result of interactions with the solvent, internal hydrogen bonding, and the chemical arrangement of its disaccharide units. By virtue of the disaccharide structure While axial hydrogen atoms may form a nonpolar, hydrophobic face, recitational side chains provide a polar, hydrophilic face akin to a twisted ribbon. It has a water basis and offers effective lubrication. Hyaluronan polymer chains expand into a random coil shape while in solution. Their unique rheological behaviour is partly due to the entanglement and intermingling of these chains even at low concentrations. Hyaluronan solutions exhibit a very high viscosity that is dependent on shear pressures at increasing concentrations.
Solutions containing hyaluronan have exceptional rheological qualities that make them excellent lubricants. Hyaluronan in 1% solution looks like jelly, but when applied pressure, it flows smoothly and with a small-bore needle for injection, which is why it's called a "pseudo-plastic" material. These treatments are incredibly lubricating, effectively separating tissue surfaces that glide past one another. Research has indicated that the lubricating characteristics of hyaluronan significantly reduce the formation of adhesions during surgery. orthopaedic and abdominal procedures.
Hyaluronan's molecular structure is responsible for its distinct rheological behaviour. The reason hyaluronan has a stronger helical structure in solution is because hydrogen bonds occur in-betiend the hydroxyl groups throughout the polymer chain. With this arrangement, the polymer is able to coil into a structure that can trap molecules of water, holding about 1000 times its own weight in water [54].
Polymer structure
Large linear polymers of hyaluronic acid are formed by hyaluronic acid synthase from substrates like the monomeric UDP-glucuronic acid and the N-acetylglucosamine. Conversion enzymes add glucuronic acid and N-acetylglucosamine to the growing chain of hyaluronic acid to generate a repeating structure. [55]. Hyaluronic acid molecules can contain more than 10,000 repeating disaccharides, giving them a molecular weight of more than 4 million daltons. Each repeating disaccharide has a weight of around 400 daltons. A disaccharide's average length is one nanometer. Put another way, a hyaluronic acid molecule stretched end to end may measure 10 microns in length. This is achieved by repetition 10,000 times. This length is approximately equal to a human erythrocyte's diameter [54].
Technique of action
Although the primary mechanism of action of exogenous hyaluronic acid (HA) is still unknown, investigations conducted in vivo, in vitro, and in clinical settings support its range of physiological benefits. The physiochemical actions of HA are protective, which could perhaps account for its long-term impacts on articular cartilage and contribute to its chondroprotective properties in vivo. Research suggests that HA may reduce the sensitivity and nerve impulses associated with pain. This glycosaminoglycan has proven to be protective to cartilage properties in experimental forms of osteoarthritis [56].Studies further reveal that cartilage incorporates exogenous hyaluronic acid [57].Exogenous hyaluronic acid (HA) decreases the synthesis and activity of matrix metalloproteinases and proinflammatory mediators while increasing the synthesis of HA and proteoglycans by chondrocytes. By stopping the migration and aggregation of leukocytes and macrophages, scavenging free radicals produced by reactive oxygen, and inhibiting the attachment of immune complexes to polymorphonuclear cells, it modifies the behaviour of immune cells [58].Furthermore, HA controls the proliferation of fibroblasts. Depending on its molecular weight, these physiological consequences of exogenous HA may differ [59-62].
Deckert et al. (2006) have suggested that the remarkable hygroscopicity of hyaluronic acid is important for maintaining tissue hydration and osmotic equilibrium. Hyaluronic acid, a dissociated basic particle, works in tandem with cell surface receptors to regulate cell motility, expansion, and separation, also functions as a signaling molecule. Carcinogenesis and embryogenesis stand to gain [63]. The extracellular matrix and body's hydration are significantly impacted by its hygroscopic qualities. Hyaluronic acid also causes signals linked to genetics, cell migration, and proliferation through its interactions with various receptors. [64, 65]
Making of CPT Nanocrystals with HA Coating
Using a bottom-up approach, uncovered and HA-coated CPT nanocrystals were created by ultrasonic treatment in order to increase the precipitation resistance [66, 67]. Ten milliliters of HA solution (pH = 4) were added to the DMSO mixture at several concentrations (0.25â??1.00%, w/v). To encourage HA adsorption, the mixture was sonicated forcefully at 200 W for 20 minutes. After that, it was constantly stirred at room temperature, first at 500 rpm for 10 minutes, then at 300 rpm for thirty minutes, and lastly at 100 rpm for many hours. Finally, the wrapped question—which may be CPT nanocrystals coated with HA or left uncovered—is channeled via a 50 nm polycarbonate film channel. At that point, the nanocrystals were resuspended in pH = 4 deionized water after being rinsed three times with 5 mL of course of action. Three distinct quantities of HA-coated camptothecin nanocrystals (0.25%, 0.5%, and 1%) as well as white camptothecin nanocrystal powder were obtained by using a cement drier (FO-IC-50, Boyikang Exploratory Defiant Organization).
Characterization of Physical and Chemical Properties
The following procedures were used to create aqueous dispersions of HA-coated and naked CPT nanocrystals with different concentrations of HA: How the CPT nanocrystals, both bare and covered with HA crystallize the powder is a major factor in verifying the saturation of the nanocrystals during extraction. For a minimum of 48 hours, add 5 mL of deionized water to the mixture and gently shake it at room temperature. Dynamic light scattering (DLS) technology was used to evaluate the zeta potential, PDI value, and size (Z-mean) of different samples. Spectrophotometric measurements of the camptothecin content in the supernatant were made at 370 nm using the calibration curve as a reference. First, at room temperature, we thoroughly dissolved the powders of our CPT nanocrystals, both bare and covered with HA in DMSO. The CPT was then calculated by extracting the CPTs. Using UV spectrophotometry, the content of camptothecin was ascertained by comparing the absorbance of camptothecin Finally, the wrapped question—which may be CPT nanocrystals coated with HA or left uncovered—is channeled via a 50 nm polycarbonate film channel. At that point, the nanocrystals were resuspended in pH = 4 deionized water after being rinsed three times with 5 mL of course of action. Three distinct quantities of HA-coated camptothecin nanocrystals (0.25%, 0.5%, and 1%) as well as white camptothecin nanocrystal powder were obtained by using a cement drier (FO-IC-50, Boyikang Exploratory Defiant Organization).
Drug Loading Efficiency (Wt%)
Amount Of Cpt In Drug Nanocrystals / Total Amount Of Cpt In Feed X 100 (1)
To confirm the completeness of the HA layer on the CPT nanocrystal surface, add up to reflectance (ATR) Fourier change infrared (FTIR) analysis was carried out using an ATR-FTIR spectrometer (Nicolet Nexus 670, Thermo Electron Enterprise). The CPT nanocrystal powders, both uncovered and coated with HA, were mixed with coarse HA and KBr to create a cloth suitable for 400â??4000 cmâ??1 ATRâ??FTIR spectroscopy analyses. The Creation software was used to gather and examine the data. We selected our HA-coated CPT nanocrystals for further investigation because they had the best watery scattering, molecule estimation, zeta potential, and sedate stacking productivity.
To confirm the completeness of the HA layer on the CPT nanocrystal surface, add up to reflectance (ATR) Fourier change infrared (FTIR) analysis was carried out using an ATR-FTIR spectrometer (Nicolet Nexus 670, Thermo Electron Organization). The CPT nanocrystal powders, both uncovered and coated with HA, were mixed with coarse HA and KBr to create a cloth suitable for 400â??4000 cmâ??1 ATRâ??FTIR spectroscopy analyses. The Creation software was used to gather and examine the data. We selected our HA-coated CPT nanocrystals for further investigation because they had the best fluid scattering, molecular measure, zeta potential, and sedate stacking productivity. SEM synopsis.
With the use of filtering electron microscopy (SEM), the area and estimation of HA-coated, crude, and exposed CPT nanocrystals were measured. In order to conduct SEM experiments, a silicon wafer was sputter-coated with an Au/Pd conductive layer for a length of 1.0 millimeter after being coated with around 20 μL of the sample solution and dried using nitrogen. SEM images were captured using the Auriga measured pillar workstation. At a speed of 0.03°/min. 2.°. 40°, the X-ray diffraction (XRD) designs of HA-coated CPT nanocrystals, uncovered CPT nanocrystals, and crude CPT were examined using a checking X-ray diffractometer (Rigaku).
In Vitro Drug Release Study.
The in vitro release of camptothecin from both uncovered and HA-coated camptothecin nanocrystals was investigated using the sifting process in phosphate buffer solution (PBS) at two distinct pHs (7.4 and 5.5). To put it briefly: Using PBS (pH 7.4 or pH 5.5), the CPT-free solution and exposed or HA-coated CPT nanocrystals were weakened to a concentration of 100 μg/mL in DMSO arrangement. Each of the 6000–8000 dialysis tubes was filled with one milliliter of the suspension. The tubes were then fixed and stored in 500 milliliters of PBS (pH = 5.5 or 7.4) at 37 °C with constant shaking. The in vitro discharge of camptothecin from exposed and HA-coated camptothecin nanocrystals was examined at two different pHs (7.4 and 5.5) using the sifting preparation in phosphate buffer arrangement (PBS). In a nutshell: The CPT-free solution and exposed or HA-coated CPT nanocrystals were weakened with PBS (pH 7.4 or pH 5.5) to a concentration of 100 μg/mL in DMSO arrangement. One milliliter of the suspension was recently added to each of the 6000–8000 dialysis tubes, which were then fixed and stored in 500 milliliters of PBS (pH = 7.4 or pH = 5.5) at 37 °C while being gently shaken.
Biocompatibility Evaluation: Hemolysis Assay
The initial technique employed to isolate red blood cells (RBCs) from whole blood from Kunming (KM) rats was spinning in heparinized tubes for 10 minutes at 4 °C and 1000 rpm. Following that, 2% solution was used to dissolve the red blood cells, and they were then rinsed three times with physiological saline. Following the brooding period, the mixture was centrifuged for ten minutes at 1000 rpm. Thermo Logical Enterprise supplied a microplate reader that was used to quantify the density of hemin released in the supernatants of each group at 540 nm. Utilizing the following formula, determine the % hemolysis:
To calculate the % hemolysis, divide the sample absorbance by the negative control absorbance and multiply the result by 100.
Evaluation of Cell Migration:
Wound Healing Assay
The anti-migratory impact of A wound test was used to examine HA-coated CPT nanocrystals on mesenchymal-like MDA-MB-231 cells. MDA-MB-231 cells were seeded onto 24-well plates at a thickness of 2 ý? 104 cells/well, and they were then brooded for a whole day. Following that, PBS was added to the medium used for cell culture, and sterile 10 ?L pipette tips were used to physically scrape the confluent monolayer, creating wounds in the middle of each well. Use PBS to wash cells and detritus three times. Scratched cell monolayers in the process. The cells are then withdrawn for a whole day. Using a light source, pictures of the injured region were obtained at 0 and 24 hours following application. Using ImageJ software, the migration of several cell types in relation to the wound area was assessed.
FINAL REPORT AND DISCUSSION
Applying HA coating to CPT nanocrystals:
Optimisation and Characterization: A traditional bottom-up method was used to create CPT nanocrystals that were both uncoated and coated with HA [67]. Re-forming nanoscale CPT crystals in pH 4 DI water containing different HA concentrations (0.00, 0.25, 0.50, and 1.00%) was accomplished with success. Their physical characteristics were evaluated, such as their aqueous dispersion, zeta potentials, medication loading efficiency, particle sizes, and values of the polydispersity index (PDI). Over 90% drug loading efficiencies were shown by all drug nanocrystals, indicating the efficacy of the antisolvent precipitation technique and the particular recrystallization circumstances.

Figure 5. (a) The CPT discharge profile from HA-coated CPT nanocrystals (NC) over time in vitro under physiological conditions (pH 7.4) as compared to free CPT and uncovered CPT nanocrystals (NC). (b) A comparison of the release of HA-coated camptothecin nanocrystals at varying pH (7.4 versus 5.5) and time intervals. In physiological medium (PBS, pH 7.4), zeta potential and measure variations of uncovered and HA-coated CPT nanocrystals over time are shown in (c). Opportunities for producing efficient nanocrystals. The fluid scatterings of the uncovered and HA-coated CPT nanocrystals were more prominent than those of the crude CPT (almost 1.2 μg/mL).
The concentration of CPT in the supernatant of CPT nanocrystals provided in 0.25% HA arrangement is 21.4 times more notable than that of rough camptothecin, and the maximum fluid dispersion may reach 26.9 μg/mL. Because of its watery nature and nanoscale size, CPT's zone has a bigger volume than its endless fluid scattering, making it more wettable. Furthermore, because of the negative charge of the HA polymer, the zeta potential of the HA-coated CPT nanocrystals was, respectively, 22.8, 26.9, and 28.5 mV lower than that of naked CPT.
By preventing steric obstruction and electrostatic repugnance, the negatively charged HA coating enhances the colloidal soundness of CPT nanocrystals by anticipating reticuloendothelial framework (RES) phagocytosis and Ostwald development. As the HA chains expanded, the typical hydrodynamic measure of CPT nanocrystals increased from 196.5 nm (uncovered) to 434.8 nm. The effective authoritative of HA to CPT nanocrystals was directly screened using zeta potential and molecular estimation changes.
CONCLUSION
Hyaluronic acid-based nanoparticles that are biomaterials targeted to specific tumours are seen as a viable and alluring approach to enhance cancer treatment, as seen by the many publications published in the last few years. The goal of this work was to By precipitating the hydrophobic anticancer medication CPT, HA-coated CPT nanocrystals were created that target cancer cells that overexpress the CD44 receptor. The HA-coated CPT nanocrystals exhibit remarkable physicochemical properties such as tall medication stacking, forward water dispersibility, extended solidity, enlarged circulation duration, and a pH-sensitive sedate discharge profile. Furthermore, all available data suggest that, in contrast to exposed and rough camptothecin nanocrystals, HA-coated nanocrystals are necessary for transportation in order to increase resistance and lessen adverse effects.
REFERENCE
- Otto T, Sicinski P. Cell cycle proteins as promising targets in cancer therapy. Nat Rev Cancer. 2017;17(2):93–115.
- Graziano G, Stefanachi A, Contino M, Prieto-Díaz R, Ligresti A, Kumar P, et al. Multicomponent Reaction-Assisted Drug Discovery: A Time-and Cost-Effective Green Approach Speeding Up Identification and Optimization of Anticancer Drugs. Int J Mol Sci. 2023;24(7):6581. doi:10.3390/ijms24076581.
- Matthews HK, Bertoli C, De Bruin R. Cell cycle control in cancer. Nat Rev Mol Cell Biol. 2022;23(1):74–88.
- Chhikara BS, Parang K. Global Cancer Statistics 2022: The trends projection analysis. Chem Biol Lett. 2023;10(1):1–16.
- Kim KW, Roh JK, Wee HJ, Kim C. Cancer Drug Discovery. 1st ed. Berlin/Heidelberg, Germany: Springer; 2016. p. 276.
- Magalhaes LG, Ferreira LL, Andricopulo AD. Recent advances and perspectives in cancer drug design. . Anais da Acad Brasileira de Ciências. 2018;90(1):1233–50.
- Lythgoe MP, Krell J, Mills MS, Vasudevan EC, Savage A. Development and economic trends in anticancer drugs licensed in the UK from 2015 to 2019. Drug Discov Today. 2021;26(2):301–8.
- Liu Z, Delavan B, Roberts R, Tong W. Lessons learned from two decades of anticancer drugs. Trends Pharmacol Sci. 2017;38:852–72.
- Ma X, Wang Z. Anticancer drug discovery in the future: an evolutionary perspective. Drug Discov Today. 2009;14:1136–78.
- Cheng Z, Li M, Dey R, Chen Y. Nanomaterials for cancer therapy: Current progress and perspectives. J Hematol Oncol. 2021;14(1):1– 27.
- Wang L, Song Y, Wang H, Zhang X, Wang M, He J. Advances of Artificial Intelligence in Anti-Cancer Drug Design: A Review of the Past Decade. Pharmaceuticals. 2023;16(2):253. doi:10.3390/ph16020253.
- Alcántar GM, Picchetti P, Casini A. Gold Complexes in Anticancer Therapy: From New Design Principles to Particle-Based Delivery Systems. Angewandte Chemie. 2023;62(22):e202218000. doi:10.1002/anie.202218000.
- Hirlekar BU, Nuthi A, Singh KD, Murty US, Dixit VA. An overview of compound properties, multiparameter optimization, and computational drug design methods for PARP-1 inhibitor drugs. Eur J Med Chem. 2023;252:115300. doi:10.1016/j.ejmech.2023.115300.
- Bojórquez NDCQ, Campos MR. Traditional and Novel ComputerAided Drug Design (CADD) Approaches in the Anticancer Drug Discovery Process. . Current Cancer Drug Targets. 2023;23(5):333– 78.
- Kumar R, Saha P. A review on artificial intelligence and machine learning to improve cancer management and drug discovery. Int J Res Appl Sci Biotechnol. 2022;9(3):149–56.
- You Y, Lai X, Pan Y, Zheng H, Vera J, Liu S, et al. Artificial intelligence in cancer target identification and drug discovery. Signal Transduct Targeted Ther. 2022;7(1):156.
- Shim JS, Liu JO. Recent advances in drug repositioning for the discovery of new anticancer drugs. Int J Biol Sci. 2014;10(7):654– 63.
- Siddiqui AJ, Jahan S, Singh R, Saxena J, Ashraf SA, Khan A, et al. Plants in anticancer drug discovery: from molecular mechanism to chemoprevention. BioMed Res Int. 2022;p. 5425485. doi:10.1155/2022/5425485.
- Rahman MA, Saikat AS, Rahman MS, Islam M, Parvez MA, Kim B. Recent Update and Drug Target in Molecular and Pharmacological Insights into Autophagy Modulation in Cancer Treatment and Future Progress. Cells. 2023;12(3):458. doi:10.3390/cells12030458.
- Hu CM, Aryal S, Zhang L. Nanoparticle-assisted combination therapies for effective cancer treatment. Therapeutic Deliv. 2010;1(2):323–57.
- Bajpai S, Tiwary SK, Sonker M, Joshi A, Gupta V, Kumar Y, et al. Recent advances in nanoparticle-based cancer treatment: a review. ACS Applied Nano Mater. 2021;4(7):6441–70.
- Brigger I, Dubernet C, Couvreur P. Nanoparticles in cancer therapy and diagnosis. . Adv Drug Deliv Rev. 2012;54(5):24–36.
- Zhou Z, Li M. Targeted therapies for cancer. BMC Med. 2022;20:90. doi:10.1186/s12916-022-02287-3.
- Kumar B, Singh S, Skvortsova I, Kumar V. Promising Targets in Anti-cancer Drug Development: Recent Updates. Curr Med Chem. 2017;24(42):4729–52.
- Hartmann JT, Haap M, Kopp HG, Lipp HP. Tyrosine kinase inhibitors - a review on pharmacology, metabolism and side effects. Metab Side Effects. 2009;10(5):470–81.
- Khwaja S, Kumar K, Das R, Negi AS. Microtubule associated proteins as targets for anticancer drug development. Bioorg Chem. 2021;116:105320
- Thorpe PE. Vascular targeting agents as cancer therapeutics. Clin Cancer Res. 2004;10(2):415–42.
- Stevenson JP, Rosen M, Sun W, Gallagher M, Haller DG, Vaughn D, et al. Phase I trial of the antivascular agent combretastatin A4 phosphate on a 5-day schedule to patients with cancer: magnetic resonance imaging evidence for altered tumor blood flow. J Clin Oncol. 2003;21(23):442–66.
- Melincovici CS, Bo¸sca AB, ¸Su¸sman S. Vascular endothelial growth factor (VEGF)-key factor in normal and pathological angiogenesis. Rom J Morphol Embryol. 2018;59(2):455–67.
- Yang, S.; Zhu, F.; Wang, Q.; Liang, F.; Qu, X.; Gan, Z.; Yang, Z. Combinatorial targeting polymeric micelles for anti-tumor drug delivery. J. Mater. Chem. B 2015, 3, 4043–4051. [CrossRef]
- Ayre, A.; Kadam, V.; Dand, N.; Patel, P. Polymeric micelles as a drug carrier for tumor targeting. Chron. Young Sci. 2013, 4, 94. [CrossRef]
- Zhou, Q.; Zhang, L.; Yang, T.; Wu, H. Stimuli-responsive polymeric micelles for drug delivery and cancer therapy. Int. J. Nanomed. 2018, 13, 2921–2942. [CrossRef] [PubMed]
- Din, F.U.; Aman, W.; Ullah, I.; Qureshi, O.S.; Mustapha, O.; Shafique, S.; Zeb, A. Effective use of nanocarriers as drug delivery systems for the treatment of selected tumors. Int. J. Nanomed. 2017, 12, 7291–7309. [CrossRef] [PubMed]
- Yang, T.; Li, W.; Duan, X.; Zhu, L.; Fan, L.; Qiao, Y.; Wu, H. Preparation of two types of polymeric micelles based on poly(beta-L-malic acid) for antitumor drug delivery. PLoS ONE 2016, 11, e0162607.
- Chen, Z.G. Small-molecule delivery by nanoparticles for anticancer therapy. Trends Mol. Med. 2010, 16, 594–602. [CrossRef] [PubMed]
- Park, J.H.; Lee, S.; Kim, J.-H.; Park, K.; Kim, K.; Kwon, I.C. Polymeric nanomedicine for cancer therapy. Prog. Polym. Sci. 2008, 33, 113–137. [CrossRef]
- Varela-Moreira, A.; Shi, Y.; Fens, M.H.A.M.; Lammers, T.; Hennink, W.E.; Schiffelers, R.M. Clinical application of polymeric micelles for the treatment of cancer. Mater. Chem. Front. 2017, 1, 1485–1501. [CrossRef]
- Zhou, X.X.; Jin, L.; Qi, R.Q.; Ma, T. pH-responsive polymeric micelles self-assembled from amphiphilic copolymer modified with lipid used as doxorubicin delivery carriers. R. Soc. Open Sci. 2018, 5, 171654. [CrossRef]
- Shin, D.H.; Tam, Y.T.; Kwon, G.S. Polymeric micelle nanocarriers in cancer research. Front. Chem. Sci. Eng. 2016, 10, 348–359. [CrossRef]
- Zhang, Y.; Huang, Y.; Li, S. Polymeric micelles: Nanocarriers for cancer-targeted drug delivery. AAPS Pharmscitech 2014, 15, 862–871. [CrossRef] [PubMed]
- Lee, H.; Ahn, C.H.; Park, T.G. Poly[lactic-co-(glycolic acid)]-grafted hyaluronic acid copolymer micelle nanoparticles for target-specific delivery of doxorubicin. Macromol. Biosci. 2009, 9, 336–342. [CrossRef] [PubMed]
- Vasi, A.M.; Popa, M.I.; Butnaru, M.; Dodi, G.; Verestiuc, L. Chemical functionalization of hyaluronic acid for drug delivery applications. Mater. Sci. Eng. C Mater. Biol. Appl. 2014, 38, 177–185. [CrossRef] [PubMed]
- Wang, J.; Li, Y.; Wang, L.; Wang, X.; Tu, P. Comparison of hyaluronic acid-based micelles and polyethylene glycol-based micelles on reversal of multidrug resistance and enhanced anticancer efficacy in vitro and in vivo. Drug Deliv. 2018, 25, 330–340. [CrossRef] [PubMed]
- Lin, T.-C.; Chen, J.-H.; Chen, Y.-H.; Teng, T.-m.; Su, C.-H.; Hsu, S.-h. Biodegradable micelles from a hyaluronan-poly(?-caprolactone) graft copolymer as nanocarriers for fibroblast growth factor 1. J. Mater. Chem. B 2013, 1, 5977. [CrossRef]
- Han, H.S.; Choi, K.Y.; Ko, H.; Jeon, J.; Saravanakumar, G.; Suh, Y.D.; Lee, D.S.; Park, J.H. Bioreducible core-crosslinked hyaluronic acid micelle for targeted cancer therapy. J. Control. Release 2015, 200, 158–166. [CrossRef]
- Han, H.S.; Thambi, T.; Choi, K.Y.; Son, S.; Ko, H.; Lee, M.C.; Jo, D.G.; Chae, Y.S.; Kang, Y.M.; Lee, J.Y.; et al. Bioreducible shell-cross-linked hyaluronic acid nanoparticles for tumor-targeted drug delivery. Biomacromolecules 2015, 16, 447–456. [CrossRef]
- Choi, K.Y.; Yoon, H.Y.; Kim, J.-H.; Bae, S.M.; Park, R.-W.; Kang, Y.M.; Kim, I.-S.; Kwon, I.C.; Choi, K.; Jeong, S.Y. Smart nanoca
- Zhong, L.; Xu, L.; Liu, Y.; Li, Q.; Zhao, D.; Li, Z.; Zhang, H.; Zhang, H.; Kan, Q.; Wang, Y.; et al. Transformative hyaluronic acid-based active targeting supramolecular nanoplatform improves long circulation and enhances cellular uptake in cancer therapy. Acta Pharm. Sin. B 2019, 9, 397–409. [CrossRef]
- Alves, C.G.; de Melo-Diogo, D.; Lima-Sousa, R.; Costa, E.C.; Correia, I.J. Hyaluronic acid functionalized nanoparticles loaded with IR780 and DOX for cancer chemo-photothermal therapy. Eur. J. Pharm. Biopharm. 2019, 137, 86–94. [CrossRef] [PubMed]
- Lee, H.; Lee, K.; Park, T.G. Hyaluronic acid? paclitaxel conjugate micelles: Synthesis, characterization, and antitumor activity. Bioconj. Chem. 2008, 19, 1319–1325. [CrossRef]
- Thomas, A.P.; Palanikumar, L.; Jeena, M.T.; Kim, K.; Ryu, J.H. Cancer-mitochondria-targeted photodynamic therapy with supramolecular assembly of HA and a water soluble NIR cyanine dye. Chem. Sci. 2017, 8, 8351–8356. [CrossRef]
- Choi, H.; Jeena, M.T.; Palanikumar, L.; Jeong, Y.; Park, S.; Lee, E.; Ryu, J.H. The HA-incorporated nanostructure of a peptide-drug amphiphile for targeted anticancer drug delivery. Chem. Commun. 2016, 52, 5637–5640. [CrossRef]
- Mao, H.L.; Qian, F.; Li, S.; Shen, J.W.; Ye, C.K.; Hua, L.; Zhang, L.Z.; Wu, D.M.; Lu, J.; Yu, R.T.; et al. Delivery of doxorubicin from hyaluronic acid-modified glutathione-responsive ferrocene micelles for combination cancer therapy. Mol. Pharm. 2019, 16, 987–994. [CrossRef] [PubMed]
- Liu, H.; Li, K.; Lan, L.; Ma, J.; Zeng, Y.; Xu, L.; Wu, D. Double-layered hyaluronic acid/stearic acid-modified polyethyleneimine nanoparticles encapsulating (-)-gossypol: A nanocarrier for chiral anticancer drugs. J. Mater. Chem. B 2014, 2, 5238–5248. [CrossRef]
- Li, S.; Cai, Y.; Cao, J.; Cai, M.; Chen, Y.; Luo, X. Phosphorylcholine micelles decorated by hyaluronic acid for enhancing antitumor efficiency. Polym. Chem. 2017, 8, 2472–2483. [CrossRef]
- Cowman M.K., Matsuoka S. (2005): Experimental approaches to hyaluronan structure. Carbohydrate Research, 340, 791–809.
- Meyer K., Palmer J.W. (1934): The polysaccharide of the vitreous humor. Journal of Biology and Chemistry, 107, 629–634.
- Akmal M., Singh A., Anand A., Kesani A., Aslam N., Goodship A., Bentley G. (2005): The effects of hyaluronic acid on articular chondrocytes. Journal of Bone and Joint Surger y – British volume, 8, 1143–1149.
- Antonas K.N., Fraser J.R.E., Muirden K.D. (1973): Distribution of biologically labelled hyaluronic acid injected into joints. Annals of Rheumatic Diseases, 32, 103–111.
- Balazs E.A., Denlinger J.L. (1984): The role of hyaluronic acid in arthritis and its therapeutic use. In: Peyron J.G. (ed.): Osteoarthritis: Current Clinical and Fundamental Problems. Geigy, Basle Geigy. 165–174.
- Noble P.W. (2002): Hyaluronan and its catabolic products in tissue injury and repair. Matrix Biology, 21, 25–29.
- Uthman I., Raynauld J.P., Haraoui B. (2003): Intra-articular therapy in osteoarthritis. Postgradual Medicine Journal, 79, 449–453
- Hascall V.C., Majors A.K., de la Motte C.A., Evanko S.P., Wang A., Drazba J.A., Strong S.A., Wight T.N. (2004): Intracellular hyaluronan: a new frontier for inflammation? Biochimica and Biophysica Acta, 1673, 3–12.
- Medina J.M., Thomas A., Denegar C.R. (2006): Knee osteoarthritis: Should your patient opt for hyaluronic acid injection? Journal of Family Practice, 8, 667–675
- Kosaki R., Watanabe K., Yamaguchi Y. (1999): Overproduction of hyaluronan by expression of the hyaluronan synthase Has2 enhances anchorage-independent growth and tumorigenicity. Cancer Research, 59, 1141–1145.
- Turley E.A., Noble P.W., W. Bourguignon L.Y. (2002): Signaling properties of hyaluronan receptors. Journal of Biological Chemistry, 277, 4589–4592.
- Taylor K.R., Trowbridge J.M., Rudisill J.A., Termeer C.C., Simon J.C., Gallo R.L. (2004): Hyaluronan fragments stimulate endothelial recognition of injury through TLR4. Journal of Biological Chemistr y, 279, 17079–17084.
- Zhan, H.; Liang, J. F. Extreme activity of drug nanocrystals coated with a layer of non-covalent polymers from self-assembled boric acid. Sci. Rep. 2016, 6, 38668.
- Zhang, H.; Hollis, C. P.; Zhang, Q.; Li, T. Preparation and antitumor study of camptothecin nanocrystals. Int. J. Pharm. 2011, 415 (1?2), 293?300
- Sargazi, A.; Shiri, F.; Keikha, S.; Majd, M. H. Hyaluronan magnetic nanoparticle for mitoxantrone delivery toward CD44- positive cancer cells. Colloids Surf., B 2018, 171, 150?158
- Yuan, F.; Dellian, M.; Fukumura, D.; Leunig, M.; Berk, D. A.; Torchilin, V. P.; Jain, R. K. Vascular permeability in a human tumor xenograft: molecular size dependence and cutoff size. Cancer Res. 1995, 55 (17), 3752?3756


 Bhoye Krutika
Bhoye Krutika
 Deore Tanuja
Deore Tanuja
 Chavan Priyal
Chavan Priyal
 Chavan Sonali
Chavan Sonali










 10.5281/zenodo.12818449
10.5281/zenodo.12818449