Acetylcholine (ACh), a key neuromodulator, plays a critical role in cognitive function and has garnered significant interest for its involvement in memory processes, particularly in the context of Alzheimer's disease (AD). This review explores the intricate influence of cholinergic modulation on hippocampal activity and memory abilities, shedding light on the role of ACh in AD pathophysiology and memory function. We discuss the atrophy of the cholinergic system in early AD, alterations in ACh receptor function, and the impact of amyloid ? peptide (A?) accumulation on cholinergic dysfunction. Additionally, we examine the modulation of neural pathways within the medial temporal lobe by ACh, focusing on its effects on synaptic plasticity and network dynamics. Furthermore, we explore the complex interplay of ACh in memory processes, highlighting its selective modulation of memory function and the diverse effects of cholinergic enhancement on memory encoding and consolidation. Understanding these complexities is crucial for developing effective therapeutic interventions for memory-related disorders like AD.
Acetylcholine, Alzheimer's disease, Memory function, Cholinergic modulation, Hippocampal activity, Synaptic plasticity, Neural pathways.
Understanding the Role of Acetylcholine in Alzheimer's disease and Memory Function
Acetylcholine (ACh), identified initially as a neurotransmitter at the neuromuscular junction, has garnered substantial interest for its role as a vital modulator of cognitive functions [1]. Particularly, attention has focused on the cholinergic system due to its implication in dementia, notably Alzheimer’s disease (AD). AD, a progressive neurodegenerative condition primarily affecting mid- to late-age adults, prominently exhibits episodic memory decline from its early stages. Studies have consistently demonstrated cholinergic system atrophy in the basal forebrain of early AD patients and those at high risk of AD development [2]. This atrophy encompasses a decrease in both cholinergic neuron count and choline acetyltransferase levels, crucial for ACh synthesis [3]. Additionally, alterations in muscarinic and nicotinic ACh receptor function are linked to AD pathophysiology [4]. The administration of nicotine, enhancing ACh levels, has shown to improve cognitive functions in elderly individuals prone to memory issues [4, 5]. Conversely, anticholinergic medications, commonly prescribed for gastrointestinal and dizziness disorders, may contribute to dementia, underscoring the cholinergic system's relevance in memory [6]. While the precise pathophysiology of AD remains elusive, extensive human and animal studies suggest a strong correlation between amyloid ? peptide (A?) accumulation and neurofibrillary tangle formation with AD development. Notably, A? accumulation in cholinergic neurons of the basal forebrain correlates with aging and AD progression. A?'s interference with ACh synthesis, release, and receptor signalling further underscores its role in cholinergic dysfunction. Cholinergic dysfunction significantly impacts the hippocampus, a brain region crucial for memory function [7, 8, 9, 10]. Reduced cholinergic projections from the medial septum to the hippocampus, coupled with decreased cholinergic receptor levels and ACh binding, characterize AD patients and mouse models of AD [11, 12, 13] . These findings underscore the critical role of the cholinergic system in hippocampal function and its implication in AD pathology.
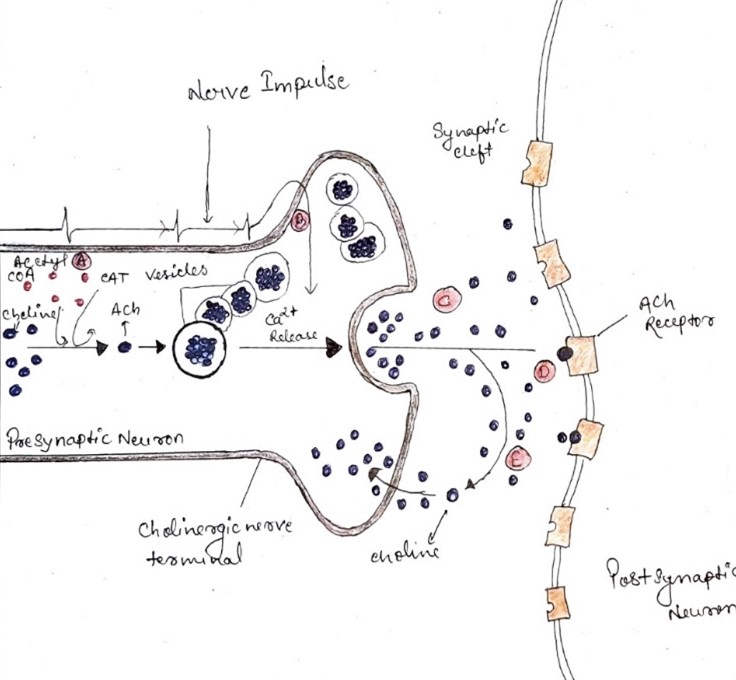
Acetylcholine serves as both a neurotransmitter and a neuromodulator in the nervous system, attracting considerable interest for its impact on cognitive functions. Cholinergic neurons, responsible for producing ACh, are distributed throughout the brain, with notable concentrations in regions like the brainstem, basal forebrain, stratum, and medial habenular nucleus. These neurons project to various brain areas, including the thalamus, basal ganglia, tectum, and basal forebrain, influencing diverse neural functions [14, 15, 16, 17, 18]. In the basal forebrain, cholinergic neurons located in regions like the medial septum and diagonal band of Broca provide essential inputs to the hippocampus, crucial for memory formation [19, 20]. Selective lesions of these neurons impair hippocampus-dependent memory function, indicating the significance of septohippocampal cholinergic projections [21]. Similarly, disruptions in the vesicular acetylcholine transporter (VAChT), vital for ACh release, lead to synaptic plasticity impairments in the hippocampus and affect RNA processing in the prefrontal cortex, resulting in memory and attention deficits [22, 23, 24, 25]. Cholinergic receptors comprise two main classes: nicotinic and muscarinic receptors, expressed throughout the central and peripheral nervous systems [26]. Nicotinic receptors are excitatory ion channels, while muscarinic receptors are G protein-coupled receptors. In the hippocampus, nicotinic receptor subtypes like a7 homomeric and a4b2 heteromeric receptors play essential roles in synaptic plasticity and modulate neurotransmitter release. Muscarinic receptors, particularly M1 subtype, regulate neuronal excitability, while M2 and M4 subtypes modulate neurotransmitter release at synaptic terminals [27, 28, 29, 30]. Understanding the distribution and function of these receptors sheds light on the intricate mechanisms underlying cholinergic modulation of neural processes, particularly in cognitive functions.
Modulating neural pathways within the medial temporal lobe through the influence of acetylcholine
Acetylcholine plays a significant role in shaping neuronal circuits, impacting neurogenesis, spine formation, and synapse formation [31]. These lasting alterations necessitate protein synthesis, as evidenced by studies [32]. Furthermore, acetylcholine exerts immediate effects on synaptic plasticity by regulating neuronal spiking activity and neurotransmitter release. This section will delve into the rapid effects of acetylcholine, particularly its correlation with changes in extracellular acetylcholine levels in the medial temporal lobe during the learning process [33].
Inside the Hippocampal region
Research has revealed that cholinergic neurons located in the medial septum play a crucial role in regulating hippocampal circuits. By utilizing optogenetic techniques, scientists have demonstrated that stimulating these cholinergic neurons not only alters the firing patterns of hippocampal neurons but also influences theta-band oscillations within the hippocampus in live subjects [34]. Both experimental and computational studies have shed light on acetylcholine's role in modulating hippocampal pathways involved in memory consolidation and encoding. Acetylcholine selectively inhibits intrinsic pathways, which contribute to memory consolidation circuits, while enhancing afferent projections, which are part of the encoding pathway [35]. Activation of muscarinic acetylcholine receptors in interneurons within the CA3 region suppresses the recurrent pathway, ensuring preferential activation of circuits carrying extrinsic information over intrinsic projections. In the CA1 region of the hippocampus, acetylcholine has been observed to potentiate the Schaffer collateral pathway by activating either a7 or non-a7 nicotinic acetylcholine receptors in pyramidal neurons and GABAergic interneurons. However, there are conflicting findings regarding this effect, with some studies suggesting inhibition of the Schaffer collateral pathway by acetylcholine [36, 37]. This inconsistency may stem from the timing-dependent nature of acetylcholine's impact on synaptic plasticity and the activation of different cholinergic receptor subtypes under varying conditions. Moreover, the frequency of cholinergic neuron stimulation can evoke different responses in hippocampal interneurons, ranging from depolarization to hyperpolarization, mediated by distinct ACh receptor subtypes. In the dentate gyrus, acetylcholine has been found to enhance long-term potentiation through the activation of both nicotinic and muscarinic receptors. Additionally, septal cholinergic projections activate astrocytes to modulate dentate granule cells [38, 39, 40].
Inside the Para hippocampal region
Acetylcholine plays a significant role in modulating neuronal activity within the entorhinal cortex (EC), which serves as a crucial link between the neocortex and hippocampus. Its effects vary depending on the specific circuits within the EC [41]. In the superficial layers (layer I, II, and III), where cortical neurons receiving external signals project, acetylcholine has been observed to increase neuronal spiking and promote synchronized oscillatory activity. These superficial layers are involved in memory encoding and are active during wakefulness. Conversely, the deep layers of the EC (layer V and VI in rodents) receive inputs from the hippocampus and project back to the neocortex for memory consolidation [42, 43]. Deep-layer EC neuron activity peaks during sharp-wave sleep, coinciding with heightened memory consolidation [44]. Studies also indicate that rhythmic activity in these deep layers correlates with hippocampal activity during slow-wave sleep but not wakefulness. Recent unpublished research suggests that acetylcholine activates hippocampal interneurons, which in turn inhibit hippocampal outputs to the deep EC layers. Exploring how cholinergic inputs affect hippocampal area activity poses an intriguing question for further investigation [44].
The intricate interplay of acetylcholine in modulating memory processes
Understanding the intricate effects of medications altering extracellular acetylcholine (ACh) levels or its receptor activity on memory functions is crucial, particularly in the context of Alzheimer's disease treatment. Drugs like donepezil, rivastigmine, and galantamine are commonly prescribed for Alzheimer's patients due to their ability to inhibit acetylcholinesterase, thereby increasing ACh levels. However, the impact of these drugs on memory enhancement can vary significantly among individuals, highlighting the complexity and unpredictability of ACh's role in memory regulation [45]. One fundamental aspect contributing to the complexity of ACh's impact on memory is its selective modulation of memory function. ACh predominantly influences hippocampus-dependent learning, particularly declarative memory such as episodic and semantic memory, while exerting less influence on procedural or implicit memory [46, 47]. Activation of cholinergic receptors is crucial for episodic and spatial memory but less so for procedural memory. Moreover, the manipulation of ACh release through various techniques leads to diverse findings, further complicating the understanding of its role in memory. Cholinergic neurons often release neurotransmitters like GABA and glutamate alongside ACh, complicating the interpretation of experimental results [48, 49, 50]. Studies targeting ACh release selectively or using specific ACh receptor antagonists have provided unique insights compared to studies ablating cholinergic neurons. Another layer of complexity in ACh's modulation of memory lies in its differential effects at various stages of memory processing. While ACh tends to facilitate memory encoding, it may suppress memory consolidation. Memory formation involves two primary stages: encoding, where sensory inputs are relayed from the cortex to the hippocampus, and consolidation, where temporary memories are transferred back to the cortex for long-term storage [51, 52, 53]. ACh seems to play a pivotal role in mediating the transition between these two stages of memory formation. The diversity of ACh receptor subtypes, each exhibiting distinct desensitization characteristics, further adds to the complexity of its effects on memory. Nicotinic ACh receptors, for instance, display various conformations, with a7-containing receptors being particularly prone to rapid desensitization. The kinetics of receptor desensitization can significantly influence the overall effects of ACh on memory function [54,55, 56]. Furthermore, the efficacy of cholinergic enhancement of memory function is closely intertwined with basal cholinergic activity [57]. Individuals with lower baseline cognitive performance may experience more substantial cognitive improvements in response to pharmacological increases in ACh levels compared to those with higher baseline performance [58, 59]. This suggests a nonlinear relationship, resembling an inverted U-shaped curve, between ACh concentration and memory function, where an optimal effect occurs within a specific range of ACh levels. In summary, the intricate interplay of ACh in memory processes involves multiple signaling pathways with opposing effects on memory function [60, 61]. The delicate balance of ACh levels and receptor activity, influenced by factors such as receptor subtype, release modulation, and basal cholinergic activity, ultimately determines its overall impact on memory. A comprehensive understanding of these complexities is indispensable for the development of effective therapeutic interventions for memory-related disorders like Alzheimer's disease [62, 63].

Fig. 2. Influence of acetylcholine (ACh) on cognitive function is nuanced and intricate


 Arnab Roy* 4
Arnab Roy* 4
 Ankita Singh 1
Ankita Singh 1
 Mahesh Kumar Yadav 1
Mahesh Kumar Yadav 1
 Indrajeet Kumar Mahto 2
Indrajeet Kumar Mahto 2
 Chandan Bauri 3
Chandan Bauri 3
 Rajan Kumar Mahto 3
Rajan Kumar Mahto 3
 Shiny Kumari 3
Shiny Kumari 3
 Pratik Kumar 3
Pratik Kumar 3
 Arti Kumari 3
Arti Kumari 3
 Anish Kumar Manoj 3
Anish Kumar Manoj 3
 Vivek Kumar Gupta 3
Vivek Kumar Gupta 3

 10.5281/zenodo.11060164
10.5281/zenodo.11060164