Abstract
Lipid-based formulations (LBFs) have emerged as a promising strategy for improving the solubility and bioavailability of hydrophobic drugs, which face absorption challenges due to poor water solubility. By utilizing lipid carriers such as emulsions, solid lipid nanoparticles, and liposomes, these formulations enhance drug dissolution and permeability in the gastrointestinal tract. LBFs can bypass first-pass metabolism through lymphatic transport, increasing systemic availability. Additionally, they offer protective encapsulation and sustained drug release, making them highly versatile in therapeutic applications. This approach has shown significant success in improving clinical outcomes, particularly in cancer therapies and antiviral treatments. Continuous advancements in lipid-based drug delivery systems are expected to further optimize drug performance and patient compliance.
Keywords
Lipid-based formulations, hydrophobic drugs, bioavailability, emulsions, liposomes.
Introduction
Hydrophobic drugs, characterized by their poor solubility in water, present significant challenges in the field of pharmaceutical development and drug delivery. These drugs often possess high lipophilicity, leading to limited absorption in biological systems, especially in the gastrointestinal tract where aqueous environments prevail. Consequently, their hydrophobic nature affects their pharmacokinetics, bioavailability, and therapeutic efficacy, making it imperative for researchers and formulators to devise strategies to enhance their solubility. Hydrophobicity refers to the tendency of a molecule to repel water and avoid interactions with aqueous environments. This property is typically observed in nonpolar compounds, which lack sufficient hydrogen bonding or polar interactions that facilitate solubility in water. Many drug candidates in the pharmaceutical industry exhibit hydrophobic characteristics due to their chemical structure, which often includes large aromatic rings, aliphatic chains, or functional groups that hinder solubility. The result is that these drugs do not dissolve readily in the fluids of the gastrointestinal tract, leading to challenges in achieving therapeutic concentrations in systemic circulation. The solubility of a drug is a critical determinant of its bioavailability—the fraction of the administered drug that reaches systemic circulation in an active form. For hydrophobic drugs, poor solubility can lead to incomplete absorption and variability in plasma drug concentrations. This inconsistency may result in therapeutic failure or increased toxicity, as the concentration of the drug in the bloodstream may fluctuate significantly between individuals or even within the same patient, depending on various factors such as the presence of food in the stomach, pH variations, and individual physiological differences. Moreover, the poor solubility of hydrophobic drugs can complicate the formulation process. Formulators may struggle to create stable and effective dosage forms that provide consistent and reliable drug release. Conventional oral dosage forms, such as tablets and capsules, may not be suitable for hydrophobic drugs, leading researchers to explore alternative strategies and formulations that can improve solubility and bioavailability.Several intrinsic and extrinsic factors influence the solubility of hydrophobic drugs. Intrinsically, the chemical structure of the drug plays a significant role. Molecular weight, polarity, and functional groups are key determinants of a compound's solubility. For instance, increasing molecular weight generally decreases solubility due to the increased energy required to dissolve larger molecules. Similarly, the presence of polar or ionizable functional groups can enhance solubility by promoting interactions with water molecules.Extrinsic factors include environmental conditions such as pH, temperature, and the presence of surfactants or co-solvents. The pH of the gastrointestinal environment can significantly affect the solubility of weakly acidic or basic drugs. For example, weakly acidic drugs may exhibit increased solubility in alkaline conditions, while weakly basic drugs may benefit from acidic environments. Temperature also impacts solubility; typically, higher temperatures increase the solubility of solids in liquids. To address the challenges associated with hydrophobic drugs, various strategies have been developed to enhance solubility and bioavailability. These strategies including Utilizing lipid formulations, such as self-emulsifying drug delivery systems (SEDDS) and lipid nanoparticles, has emerged as a promising approach to enhance the solubility of hydrophobic drugs. These formulations can improve drug solubilization and facilitate absorption through the lymphatic system, bypassing first-pass metabolism.This technique involves dispersing the hydrophobic drug in a polymer matrix, increasing the surface area available for dissolution. The use of amorphous forms can also enhance solubility.Surfactants can significantly improve the solubility of hydrophobic drugs by reducing surface tension and enhancing wettability. They can form micelles, encapsulating the drug and improving its solubility in aqueous media.Converting a hydrophobic drug into its salt form can enhance its solubility. Salts are generally more soluble in water than their parent compounds, providing a more effective means of drug delivery.Designing prodrugs chemically modified versions of the drug that enhance solubility can be an effective strategy. Prodrugs can convert to the active drug form after administration, improving absorption.In conclusion, hydrophobic drugs present significant solubility challenges that impact their therapeutic effectiveness and development. Understanding the intrinsic and extrinsic factors influencing solubility is crucial for devising effective strategies to improve bioavailability. The pharmaceutical industry continues to explore innovative formulation techniques, including lipid-based formulations, solid dispersions, and surfactants, to overcome the limitations posed by hydrophobicity. As research advances, the development of new strategies and technologies will be essential to enhance the solubility of hydrophobic drugs, ultimately improving patient outcomes and expanding the therapeutic potential of these compounds.
Understanding Lipid-based Formulations: Types and Mechanisms
Lipid-based formulations have emerged as a crucial approach in the pharmaceutical industry for enhancing the solubility and bioavailability of poorly water-soluble drugs. These formulations leverage the unique properties of lipids, which are hydrophobic in nature, to solubilize hydrophobic drugs and facilitate their absorption across biological membranes. Given the increasing number of hydrophobic compounds in drug development pipelines, understanding the types of lipid-based formulations and their mechanisms of action is essential for optimizing drug delivery and improving therapeutic outcomes.Lipid-based formulations have gained significant attention in the pharmaceutical industry as effective delivery systems for poorly water-soluble drugs. These formulations utilize lipids to enhance drug solubility, improve bioavailability, and facilitate absorption across biological membranes. As the number of hydrophobic compounds in drug development continues to grow, understanding the different types of lipid-based formulations becomes essential for optimizing therapeutic outcomes. This article will explore the various types of lipid-based formulations, their characteristics, mechanisms, and applications.
Lipid solutions represent the simplest form of lipid-based formulations, wherein the drug is dissolved in a lipid medium. Typically, medium-chain triglycerides (MCTs) or long-chain triglycerides (LCTs) are employed as the oil phase. Lipid solutions provide a straightforward method for solubilizing hydrophobic drugs, making them suitable for oral administration. The main advantage of lipid solutions is their ability to accommodate high concentrations of lipophilic drugs, which can lead to improved solubility in gastrointestinal fluids. However, one significant limitation is that these formulations may require high lipid concentrations to achieve adequate solubilization, which can result in gastrointestinal discomfort or adverse effects in patients. Despite this drawback, lipid solutions are often used in clinical practice, particularly for drugs with moderate hydrophobicity.
Emulsions are heterogeneous mixtures of two immiscible liquids, typically oil and water, stabilized by surfactants. In pharmaceutical applications, oil-in-water (O/W) emulsions are most commonly utilized for delivering lipophilic drugs. Emulsions can effectively solubilize substantial amounts of hydrophobic drugs, enhancing their bioavailability. The stabilizing agents, or emulsifiers, play a critical role in preventing the coalescence of droplets and maintaining the stability of the emulsion. The choice of surfactants, along with their concentration, influences the physicochemical properties of the emulsion, including droplet size, viscosity, and release characteristics.One of the advantages of emulsions is their versatility; they can be formulated to deliver both hydrophilic and lipophilic drugs. Additionally, emulsions can provide controlled release properties, allowing for sustained therapeutic effects. However, the stability of emulsions can be a concern, as they may undergo phase separation or creaming over time. Proper formulation techniques and storage conditions are essential to maintain emulsion stability and ensure consistent drug release.Microemulsions are transparent, thermodynamically stable systems composed of oil, water, and surfactants, with droplet sizes typically in the range of 10 to 100 nm. These systems are often referred to as “self-emulsifying” due to their ability to spontaneously form upon mixing the components. Microemulsions offer several advantages over traditional emulsions, including higher stability, improved bioavailability, and enhanced drug solubilization. The small droplet size in microemulsions increases the surface area available for dissolution, facilitating faster absorption of the encapsulated drug.The stability of microemulsions is attributed to their unique structure, which minimizes the energy required to maintain the emulsion. This stability allows microemulsions to remain homogeneous over extended periods, making them suitable for long-term storage. The ability to solubilize significant amounts of hydrophobic drugs makes microemulsions an attractive option in drug formulation, especially for drugs with poor aqueous solubility. However, the formulation of microemulsions requires careful optimization of surfactant and co-surfactant ratios to achieve the desired stability and performance.Self-emulsifying drug delivery systems (SEDDS) are a subclass of lipid-based formulations designed to enhance the solubility and bioavailability of hydrophobic drugs. SEDDS typically consist of a mixture of oils, surfactants, and co-surfactants that spontaneously form emulsions upon contact with aqueous media, such as gastrointestinal fluids. This spontaneous emulsification is a significant advantage, as it simplifies the formulation process and reduces the need for mechanical energy input during preparation.SEDDS can solubilize substantial quantities of lipophilic drugs, leading to increased effective concentrations in the gastrointestinal tract. Once ingested, the components of SEDDS interact with the aqueous environment, forming fine emulsions that facilitate drug absorption. The surfactants used in SEDDS play a crucial role in promoting drug solubilization and enhancing permeability across biological membranes. Additionally, the ability of SEDDS to improve lymphatic transport allows for bypassing first-pass metabolism, increasing the bioavailability of sensitive compounds. One of the key benefits of SEDDS is their adaptability; they can be formulated as soft or hard capsules, making them convenient for oral administration. Their relatively simple preparation process and effectiveness in enhancing bioavailability make SEDDS a popular choice in pharmaceutical development. Lipid nanoparticles, including solid lipid nanoparticles (SLNs) and nanostructured lipid carriers (NLCs), have emerged as advanced lipid-based formulations for drug delivery. SLNs are composed of solid lipids that provide a stable matrix for drug encapsulation. In contrast, NLCs are formulated with a combination of solid and liquid lipids, allowing for improved drug loading and release characteristics. Both formulations offer controlled drug release, enhanced stability, and improved bioavailability.The use of lipid nanoparticles allows for encapsulation of hydrophobic drugs in a protective lipid matrix, preventing degradation and facilitating transport across biological membranes. The small size of lipid nanoparticles enhances their ability to penetrate tissues and reach targeted sites of action. Additionally, lipid nanoparticles can improve the solubility of poorly soluble drugs and enhance their pharmacokinetic profiles, making them particularly valuable in the delivery of anticancer agents and other therapeutics.The stability of lipid nanoparticles is a significant advantage; they are less prone to aggregation or precipitation compared to traditional emulsions. This stability allows for long-term storage and consistent drug release profiles. However, the formulation of lipid nanoparticles requires careful optimization of lipid ratios and processing techniques to achieve the desired characteristics. Lipid-based injections are another category of lipid-based formulations that have gained traction in the pharmaceutical field. These formulations involve the intravenous or intramuscular administration of lipid emulsions or lipid nanoparticles. Lipid-based injections can provide rapid drug delivery, making them suitable for various therapeutic applications, including parenteral nutrition, pain management, and the delivery of hydrophobic drugs. The ability of lipid-based injections to solubilize poorly soluble drugs allows for higher drug concentrations and improved bioavailability compared to conventional aqueous formulations. Furthermore, lipid injections can enhance the therapeutic efficacy of drugs by enabling sustained release profiles, which can reduce the frequency of administration and improve patient compliance. Lipid-based formulations have found applications in numerous therapeutic areas, including oncology, infectious diseases, and chronic conditions. For instance, lipid formulations have been utilized for the delivery of poorly soluble anticancer drugs, improving their therapeutic effectiveness and reducing side effects. Additionally, lipid-based systems have been explored for the delivery of vaccines, where they enhance immunogenicity and stability. One notable example is the use of lipid nanoparticles in mRNA vaccine development, as seen in the COVID-19 vaccines. These formulations effectively encapsulate and deliver mRNA, facilitating its uptake by cells and promoting an immune response. The success of lipid-based formulations in vaccine delivery underscores their potential in addressing modern pharmaceutical challenges. While lipid-based formulations offer significant advantages in drug delivery, regulatory considerations play a crucial role in their development and commercialization. Regulatory agencies, such as the U.S. Food and Drug Administration (FDA) and the European Medicines Agency (EMA), require thorough characterization of lipid-based products, including their composition, stability, and bioavailability. Preclinical and clinical studies must demonstrate the safety and efficacy of lipid-based formulations, ensuring compliance with Good Manufacturing Practices (GMP). lipid-based formulations represent a diverse and promising approach for enhancing the solubility and bioavailability of poorly water-soluble drugs. Through various types, including lipid solutions, emulsions, microemulsions, SEDDS, lipid nanoparticles, and lipid-based injections, these formulations provide unique advantages that address the challenges of drug delivery. By utilizing lipids to solubilize drugs and improve their pharmacokinetic profiles, lipid-based formulations hold the potential to revolutionize pharmaceutical development and improve patient outcomes. As research and technology continue to advance, the future of lipid-based formulations appears bright, with ongoing innovations poised to enhance the efficacy and safety of therapeutic agents in various clinical applications.
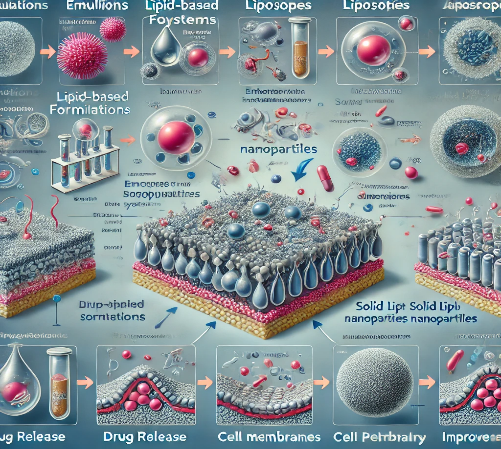
Fig-1 Here is the image representing lipid-based formulations, showing their types (emulsions, liposomes, solid lipid nanoparticles) and the mechanisms by which they enhance solubility and absorption of drugs. The image illustrates how each formulation type works to improve drug delivery.
The mechanisms of action of lipid-based formulations are integral to their effectiveness in enhancing the solubility, bioavailability, and therapeutic efficacy of hydrophobic drugs. These formulations utilize the unique physicochemical properties of lipids to solubilize poorly soluble drugs, facilitate their absorption, and improve their pharmacokinetic profiles. Understanding these mechanisms is essential for optimizing drug delivery systems and addressing the challenges posed by lipophilic compounds in pharmaceutical development.The primary mechanism by which lipid-based formulations enhance drug bioavailability is through solubilization. Hydrophobic drugs typically have low solubility in aqueous environments, leading to limited absorption in the gastrointestinal (GI) tract. Lipid-based formulations, such as lipid solutions, emulsions, microemulsions, and self-emulsifying drug delivery systems (SEDDS), can solubilize these drugs by creating a favorable environment that reduces the energy barrier for dissolution.When a poorly soluble drug is incorporated into a lipid formulation, the lipids serve as a solubilizing agent, increasing the effective concentration of the drug in the GI fluids. In emulsions and microemulsions, for example, the drug can be encapsulated within lipid droplets, allowing it to exist in a solubilized state that can be more readily absorbed by the intestinal epithelium. The droplet size and lipid composition can be optimized to maximize drug solubilization, improving the overall bioavailability of the formulation.
In addition to solubilization, lipid-based formulations significantly enhance the permeability of hydrophobic drugs across biological membranes. The intestinal epithelium consists of tightly packed cells with lipid bilayers that can act as barriers to drug absorption. Lipid formulations can interact with these membranes, fluidizing their structure and enhancing the diffusion of drugs through the lipid bilayer.This interaction is primarily due to the amphiphilic nature of surfactants used in emulsions and microemulsions. Surfactants reduce the surface tension between the lipid and aqueous phases, allowing the lipid-based formulations to interact more effectively with cell membranes. As a result, hydrophobic drugs can penetrate the epithelial cells more readily, facilitating their passage into systemic circulation.Furthermore, lipid-based formulations can also increase the absorption of drugs via transcellular transport mechanisms. When drugs are solubilized in lipids, they can diffuse through the lipid bilayers of the intestinal cells, bypassing the more restrictive paracellular pathways. This enhanced permeability is crucial for improving the bioavailability of hydrophobic compounds that would otherwise be poorly absorbed. Another significant mechanism of action for lipid-based formulations is the promotion of lymphatic transport. Certain lipids, particularly long-chain triglycerides and certain types of emulsions, can facilitate the transport of lipophilic drugs through the lymphatic system. This is particularly beneficial for drugs that undergo extensive first-pass metabolism in the liver, as it allows for a greater proportion of the active drug to reach systemic circulation.When lipid-based formulations are ingested, they are emulsified in the gastrointestinal tract, forming small lipid droplets that can be absorbed by intestinal lymphatics. These lipid droplets enter the lymphatic system instead of the portal circulation, bypassing the liver's metabolic processes. Consequently, drugs delivered via this route can achieve higher plasma concentrations, enhancing their therapeutic effectiveness and reducing the risk of side effects associated with hepatic metabolism.This mechanism is especially advantageous for poorly soluble drugs that require high systemic concentrations for therapeutic efficacy. By leveraging lymphatic transport, lipid-based formulations can improve the pharmacokinetics of these compounds, providing a more effective delivery strategy. Lipid-based formulations also offer the ability to provide sustained or controlled release of hydrophobic drugs. This is particularly important for maintaining therapeutic drug levels over an extended period, improving patient compliance, and reducing the frequency of dosing. Various lipid-based systems, such as solid lipid nanoparticles (SLNs) and nanostructured lipid carriers (NLCs), are designed to release their drug payload in a controlled manner.The mechanisms behind sustained release in lipid nanoparticles involve diffusion and erosion processes. As the lipid matrix slowly dissolves in the biological environment, the drug is released gradually over time. The choice of lipid composition, particle size, and surface properties can be optimized to achieve the desired release profile. For example, using a combination of solid and liquid lipids in NLCs can improve drug loading and provide a more favorable release kinetics compared to SLNs alone.The sustained release properties of lipid-based formulations can significantly enhance the therapeutic efficacy of poorly soluble drugs by maintaining drug concentrations within the therapeutic range over extended periods. This approach can lead to improved patient adherence and reduced side effects associated with peak plasma concentrations. Lipid-based formulations provide enhanced stability for hydrophobic drugs, protecting them from degradation due to environmental factors such as moisture, heat, and light. Many hydrophobic drugs are susceptible to hydrolysis and oxidation, which can compromise their efficacy. By encapsulating these drugs within lipid matrices, formulations can minimize exposure to these degrading factors.For instance, solid lipid nanoparticles can shield the drug from environmental stresses, extending its shelf life and maintaining its stability over time. The lipid matrix can act as a barrier against moisture and oxygen, reducing the likelihood of degradation reactions. This improved stability is particularly important for the successful development of lipid-based drug delivery systems, as it ensures consistent therapeutic effects and safety for patients.
The design of lipid-based formulations also allows for high drug loading capacities, particularly in nanoparticle systems. By employing various lipid compositions and formulations, drug developers can optimize the loading efficiency of hydrophobic drugs. The ability to achieve high drug loading is particularly advantageous in the development of formulations intended for intravenous or subcutaneous delivery, as it allows for smaller injection volumes and reduced dosing frequency.The high drug loading capacity of lipid nanoparticles is attributed to their unique structural characteristics, which provide a large surface area for drug adsorption. This property can be particularly beneficial for formulating combination therapies, where multiple drugs may need to be delivered simultaneously.
Lipid-based formulations interact with biological systems in complex ways, influencing pharmacokinetics and pharmacodynamics. The interaction of lipids with biological membranes can alter the permeability of drug molecules, impacting their distribution, metabolism, and elimination. Lipids can also modulate the activity of various transport proteins, enzymes, and receptors, further affecting the pharmacological properties of the drugs delivered via lipid-based systems.For instance, certain lipid formulations can enhance the absorption of co-administered drugs by modulating their transport across cell membranes. Additionally, lipid-based formulations can improve the stability and efficacy of biologics, such as peptides and proteins, by providing a protective environment that minimizes degradation.lipid-based formulations leverage multiple mechanisms of action to enhance the solubility, bioavailability, and therapeutic efficacy of hydrophobic drugs. Through processes such as solubilization, enhanced membrane permeability, lymphatic transport, sustained release, and improved stability, these formulations address the challenges posed by poorly soluble compounds in drug development. As research continues to explore the potential of lipid-based systems, these formulations are expected to play an increasingly vital role in the delivery of innovative therapeutics, improving patient outcomes and expanding the therapeutic landscape for hydrophobic drugs. Understanding these mechanisms is crucial for optimizing lipid-based formulations and ensuring their successful application in clinical settings.
Formulation Approaches: Lipid Emulsions, Solid Lipid Nanoparticles, and Liposomes
Lipid-based formulations have proven to be effective strategies for enhancing the solubility and bioavailability of poorly water-soluble drugs, especially those that are hydrophobic. Among the various lipid-based systems, three primary formulation approaches—lipid emulsions, solid lipid nanoparticles (SLNs), and liposomes—stand out due to their versatility and effectiveness. Each of these formulations has unique characteristics, preparation methods, and mechanisms of drug delivery that make them suitable for addressing specific challenges in pharmaceutical development. A deeper understanding of these systems can offer valuable insights into how they contribute to overcoming bioavailability issues and improving therapeutic outcomes for hydrophobic drugs.
Lipid emulsions are among the most widely used lipid-based formulations in drug delivery. They are colloidal dispersions consisting of two immiscible liquids—usually oil and water—stabilized by surfactants to prevent phase separation. The emulsions can be categorized into oil-in-water (O/W) emulsions, where the oil phase is dispersed as droplets in the continuous water phase, and water-in-oil (W/O) emulsions, where the water phase is dispersed in the oil. Lipid emulsions have significant applications in the pharmaceutical industry, particularly for the delivery of hydrophobic drugs that have poor water solubility.The main advantage of lipid emulsions lies in their ability to improve the solubility of hydrophobic drugs by incorporating them into the oil phase. Once administered, these emulsified droplets enhance the drug's dissolution rate in the gastrointestinal (GI) tract, leading to improved absorption and bioavailability. Additionally, lipid emulsions protect the encapsulated drug from chemical and enzymatic degradation, which is particularly important for drugs that are sensitive to hydrolysis or oxidation. This makes them ideal for the oral and parenteral delivery of lipophilic drugs.Lipid emulsions are also used in parenteral nutrition and drug delivery systems, where they provide a biocompatible and metabolizable vehicle for drug administration. These emulsions are often formulated using natural lipids such as soybean oil, medium-chain triglycerides, or omega-3 fatty acids, which offer additional health benefits beyond drug delivery. Parenteral lipid emulsions are commonly used for the intravenous delivery of poorly soluble drugs like anesthetics (e.g., propofol) and chemotherapeutic agents.In addition to their ability to enhance solubility, lipid emulsions offer versatility in terms of droplet size. Traditional emulsions have droplet sizes ranging from 100 nm to several micrometers. However, nanoemulsions, which have droplet sizes in the range of 20-200 nm, are gaining popularity due to their superior stability, enhanced drug absorption, and improved biodistribution. Nanoemulsions are thermodynamically stable, meaning they require less energy for production and have a longer shelf life compared to conventional emulsions. The smaller droplet size also provides a larger surface area for drug release, making nanoemulsions highly efficient delivery systems for hydrophobic drugs.
Solid lipid nanoparticles (SLNs) are another emerging formulation approach for improving the delivery of poorly water-soluble drugs. SLNs are submicron-sized particles made from solid lipids, typically with diameters ranging from 50 to 1000 nm. The solid core of these nanoparticles is composed of lipids that remain solid at room and body temperature, providing a matrix for drug encapsulation. The outer layer is stabilized by surfactants or emulsifiers, which prevent particle aggregation and improve the physical stability of the formulation.The solid lipid matrix in SLNs offers several advantages over other lipid-based systems, particularly in terms of drug protection and controlled release. Because the lipids used in SLNs are in a solid state, they can effectively encapsulate hydrophobic drugs and protect them from degradation, making SLNs ideal for delivering sensitive or unstable drugs. Additionally, the solid lipid core allows for sustained drug release, as the drug must diffuse through the lipid matrix over time, leading to prolonged therapeutic effects.One of the most significant benefits of SLNs is their ability to improve drug bioavailability. By reducing the particle size to the nanometer range, SLNs increase the surface area available for drug absorption, leading to enhanced dissolution rates and improved bioavailability. The small size of SLNs also facilitates their uptake by cells and tissues, making them suitable for a wide range of applications, including oral, topical, and intravenous drug delivery.SLNs are particularly useful in the delivery of hydrophobic anticancer drugs, which often suffer from poor solubility and low bioavailability. For example, SLNs have been used to encapsulate paclitaxel, a potent chemotherapeutic agent with low water solubility. By incorporating paclitaxel into SLNs, researchers have been able to enhance its solubility, improve its bioavailability, and reduce its toxicity, making SLNs a promising delivery system for cancer therapy.Despite their numerous advantages, SLNs do have some limitations. One of the main challenges is the potential for drug expulsion during storage, as the solid lipid matrix may undergo polymorphic transitions over time, leading to a loss of drug from the nanoparticle. However, advancements in formulation techniques, such as the use of lipid blends or the incorporation of polymers, have helped to mitigate this issue and improve the stability of SLNs.
Liposomes are one of the most extensively studied lipid-based drug delivery systems, and they have gained widespread use due to their ability to encapsulate both hydrophilic and hydrophobic drugs. Liposomes are spherical vesicles composed of one or more phospholipid bilayers surrounding an aqueous core. Hydrophilic drugs can be encapsulated in the aqueous core, while hydrophobic drugs can be incorporated into the lipid bilayers, making liposomes versatile carriers for a wide range of therapeutic agents.The unique structure of liposomes allows them to improve the solubility, stability, and bioavailability of drugs. For hydrophobic drugs, liposomes offer a lipid bilayer environment that mimics biological membranes, facilitating drug incorporation and transport across cellular barriers. Liposomes can also protect the encapsulated drug from degradation, thereby improving its stability in biological fluids and enhancing its therapeutic efficacy.One of the key advantages of liposomes is their ability to provide targeted drug delivery. Liposomes can be modified with ligands, such as antibodies, peptides, or small molecules, to selectively target specific cells or tissues. This targeting capability is particularly beneficial in cancer therapy, where liposomes can deliver chemotherapeutic agents directly to tumor cells while minimizing off-target effects. For example, liposomal formulations of doxorubicin, such as Doxil, have been developed to improve the selective delivery of the drug to tumors, reducing its cardiotoxicity and improving patient outcomes.Liposomes also offer controlled drug release, as the drug can be released slowly from the liposome over time. This controlled release is achieved through the use of lipids with varying degrees of fluidity, which can influence the rate of drug release. For instance, liposomes made from saturated lipids with high transition temperatures tend to release drugs more slowly, while those made from unsaturated lipids with lower transition temperatures release drugs more rapidly. This flexibility allows for the design of liposomal formulations with tailored release profiles, depending on the therapeutic needs of the patient.In addition to their use in cancer therapy, liposomes have been employed in a variety of other therapeutic areas, including antifungal treatment, vaccine delivery, and gene therapy. Liposomal formulations of antifungal drugs, such as amphotericin B, have been shown to reduce the toxicity of the drug while maintaining its antifungal activity, making them safer for patients. Liposomes are also being explored as carriers for mRNA vaccines, where they can protect the fragile mRNA molecules and enhance their delivery to target cells.Despite their many advantages, liposomes do face challenges in terms of stability and cost. Liposomes can be prone to leakage of the encapsulated drug, particularly during storage, and they can be expensive to manufacture on a large scale. However, ongoing research is focused on improving the stability and scalability of liposomal formulations, making them more accessible for clinical use. Lipid emulsions, solid lipid nanoparticles (SLNs), and liposomes represent three key formulation approaches for enhancing the solubility and bioavailability of hydrophobic drugs. Each of these lipid-based systems offers unique advantages in terms of drug encapsulation, protection, and release, making them versatile tools for drug delivery. Lipid emulsions provide a simple and effective way to solubilize hydrophobic drugs, while SLNs offer enhanced stability and controlled release. Liposomes, with their ability to encapsulate a wide range of drugs and provide targeted delivery, are particularly valuable in precision medicine. Together, these lipid-based formulations are driving advances in drug delivery, enabling more effective treatment of diseases that were previously difficult to manage due to solubility and bioavailability issues.
Table-1 This table summarizes the key characteristics and uses of each type of lipid-based formulation approach.
|
Feature
|
Lipid Emulsions
|
Solid Lipid Nanoparticles (SLNs)
|
Liposomes
|
Particle Size
|
Composition
|
Stability
|
Applications
|
|
Definition
|
Colloidal dispersions of oil and water stabilized by surfactants
|
Nanoparticles made from solid lipids
|
Spherical vesicles with one or more lipid bilayers
|
100 nm - 10 µm
|
Oils, water, surfactants
|
Moderate to high
|
Drug delivery, parenteral nutrition
|
|
Drug Solubilization
|
Solubilizes hydrophobic drugs in the oil phase
|
Encapsulates drugs within solid lipid matrix
|
Encapsulates drugs in aqueous core or lipid bilayer
|
50 nm - 1 µm
|
Solid lipids, surfactants
|
High
|
Cancer therapy, vaccine delivery
|
|
Drug Release Profile
|
Quick release due to diffusion from oil phase
|
Sustained release due to solid lipid matrix
|
Sustained release based on bilayer composition
|
50 nm - 200 nm
|
Phospholipids, cholesterol
|
Moderate to high
|
Gene delivery, antimicrobial delivery
|
|
Preparation Methods
|
High-pressure homogenization, ultrasonication
|
High-pressure homogenization, microemulsion technique
|
Thin-film hydration, reverse phase evaporation
|
100 nm - 200 nm
|
Oils, lipids, surfactants
|
High
|
Targeted drug delivery
|
|
Encapsulation Efficiency
|
Moderate (depends on drug solubility)
|
High (especially for lipophilic drugs)
|
High for both hydrophilic and lipophilic drugs
|
100 nm - 1 µm
|
Phospholipids, cholesterol
|
Moderate
|
Vaccine carriers, anti-cancer therapy
|
|
Toxicity
|
Generally low, dependent on emulsifier used
|
Generally low, depends on lipid toxicity
|
Low, biocompatible lipids
|
10 nm - 300 nm
|
Natural/ synthetic lipids, surfactants
|
High
|
Gene therapy, transdermal drug delivery
|
|
Challenges
|
Physical instability (creaming, coalescence)
|
Drug expulsion during storage, crystallization
|
Stability issues, oxidation of lipids
|
50 nm - 1 µm
|
Phospholipids, stabilizers
|
Moderate to high
|
Gene therapy, hydrophobic drug delivery
|
Mechanisms of Solubilization: How Lipids Enhance Drug Absorption
The solubilization and absorption of poorly water-soluble drugs present one of the most significant challenges in pharmaceutical formulation and drug development. Hydrophobic drugs, which are insoluble in water, often exhibit poor bioavailability because they cannot be easily dissolved and absorbed in the aqueous environment of the gastrointestinal (GI) tract. Lipid-based formulations have emerged as a promising solution to these issues by improving the solubility, dissolution, and absorption of these drugs. Lipids enhance drug absorption through several interconnected mechanisms, including solubilization in micelles, promotion of drug dissolution, modification of gastrointestinal fluid properties, facilitation of lymphatic absorption, and inhibition of drug efflux. By leveraging these mechanisms, lipid-based drug delivery systems (like lipid emulsions, micelles, solid lipid nanoparticles, and liposomes) significantly improve the pharmacokinetic profiles of hydrophobic drugs, ultimately enhancing their therapeutic efficacy. Solubilization in Micelles and Mixed Micelles One of the most well-recognized mechanisms by which lipids enhance the solubilization of poorly water-soluble drugs is through the formation of micelles and mixed micelles. When lipids, such as triglycerides, fatty acids, or phospholipids, are introduced into the GI tract, they are broken down by bile salts and pancreatic enzymes. This digestion process leads to the formation of monoglycerides, free fatty acids, and bile salt-lipid complexes, which self-assemble into micelles.Micelles are colloidal aggregates composed of amphiphilic molecules, which have both hydrophilic (water-loving) and hydrophobic (water-repelling) regions. In an aqueous environment like the GI tract, the hydrophobic tails of these molecules cluster together to form the core of the micelle, while the hydrophilic heads face outward, interacting with water. The hydrophobic core of the micelle can solubilize poorly water-soluble drugs, essentially trapping the drug molecules inside and allowing them to exist in a water-based solution. Mixed micelles are formed when bile salts combine with the digested lipid products. These mixed micelles have an even greater capacity to solubilize hydrophobic drugs because they provide a stable environment in which the drug can dissolve. As the mixed micelles travel through the intestinal lumen, they transport the solubilized drug to the intestinal epithelial cells, where the drug can be absorbed into the bloodstream. By increasing the concentration of dissolved drug in the GI fluids, micelles and mixed micelles greatly enhance the bioavailability of hydrophobic drugs. Improved Drug Dissolution rates is the Another mechanism by which lipids enhance drug absorption is by improving the dissolution rate of poorly water-soluble drugs. In the case of hydrophobic drugs, dissolution is often the rate-limiting step in drug absorption because these drugs tend to form crystalline solids that do not readily dissolve in aqueous environments. Lipid-based formulations can improve the dissolution rate of such drugs in several ways.First, when drugs are incorporated into lipid-based formulations such as emulsions or solid lipid nanoparticles (SLNs), they are often present in a more amorphous or non-crystalline form. In contrast to crystalline drugs, which require energy to break the lattice structure, amorphous drugs dissolve more readily because they lack the ordered, rigid structure of crystals. This reduction in crystallinity decreases the energy barrier for dissolution, resulting in a faster dissolution rate and, consequently, improved absorption.Second, lipid-based formulations provide a large surface area for drug release. In emulsions and nanoparticles, the drug is dispersed as small droplets or particles, significantly increasing the surface area in contact with the surrounding aqueous environment. The increased surface area enhances the rate of drug release and dissolution, enabling the drug to be more readily absorbed across the intestinal epithelium. Modulation of Gastrointestinal Fluid Properties is the Lipid-based formulations can also modify the properties of gastrointestinal fluids in ways that favor enhanced drug solubilization and absorption. When lipids are ingested, they stimulate the secretion of bile salts and pancreatic lipase, which are critical for the digestion and absorption of fats. Bile salts act as natural surfactants, reducing the surface tension between hydrophobic drug particles and the aqueous environment, thereby enhancing the dissolution of the drug.Moreover, the presence of lipids in the GI tract slows gastric emptying and prolongs the residence time of the drug in the small intestine. This extended residence time increases the duration during which the drug is exposed to the solubilizing effects of bile salts and mixed micelles, providing more time for drug dissolution and absorption. Additionally, lipid-rich formulations can increase the permeability of the intestinal epithelium, facilitating the transport of drug molecules across the epithelial barrier into the bloodstream. Lymphatic Absorption Pathway is the one of the most significant advantages of lipid-based formulations is their ability to promote lymphatic absorption of hydrophobic drugs. Typically, most drugs are absorbed through the hepatic portal vein and undergo first-pass metabolism in the liver, which can significantly reduce the bioavailability of orally administered drugs. However, when drugs are incorporated into lipid-based systems, they can bypass the liver by entering the lymphatic system, thereby avoiding first-pass metabolism and enhancing systemic drug exposure.The lymphatic system, which is responsible for the transport of dietary lipids, plays a crucial role in the absorption of long-chain fatty acids and lipophilic compounds. After lipids are digested and absorbed in the small intestine, they are re-esterified into triglycerides within enterocytes and incorporated into chylomicrons—lipoprotein particles that transport lipids through the lymphatic system. Hydrophobic drugs that are formulated with lipids can be incorporated into these chylomicrons and absorbed into the lymphatic system rather than the portal circulation. This lymphatic transport pathway significantly enhances drug bioavailability by reducing the extent of drug metabolism and increasing the drug’s access to the systemic circulation.Lipid-based formulations, particularly those containing long-chain triglycerides, are well-suited for promoting lymphatic transport of hydrophobic drugs. The absorption of these drugs into the lymphatic system results in prolonged drug circulation, increased drug levels in target tissues, and reduced liver exposure, making this mechanism particularly beneficial for drugs that are extensively metabolized in the liver. Inhibition of Drug Efflux and Metabolism is the Lipid-based formulations can also enhance drug absorption by inhibiting drug efflux and metabolism in the intestine. The intestinal epithelium contains various efflux transporters, such as P-glycoprotein (P-gp), which pump drugs out of the cells and back into the intestinal lumen, reducing the overall absorption of the drug. In addition, enzymes such as cytochrome P450 (CYP) isoforms are present in the intestinal cells and can metabolize drugs before they are absorbed into the bloodstream, further limiting bioavailability.Certain lipid excipients, such as medium-chain triglycerides and some surfactants, have been shown to inhibit the activity of efflux transporters and metabolic enzymes. By inhibiting P-gp and CYP enzymes, these lipid-based formulations can increase the intracellular concentration of the drug, enhancing its absorption across the intestinal epithelium. This inhibitory effect is particularly beneficial for drugs that are substrates for efflux transporters or metabolizing enzymes, as it allows for greater systemic exposure and therapeutic efficacy. Formation of Drug-Lipid Complexes is the another important mechanism by which lipids enhance drug absorption is through the formation of drug-lipid complexes. Some hydrophobic drugs can interact with lipid molecules to form stable complexes that are more soluble and bioavailable than the free drug. These complexes can be formed between the drug and lipid components such as fatty acids, phospholipids, or cholesterol. For example, phospholipids can form complexes with hydrophobic drugs through hydrophobic interactions and hydrogen bonding. The resulting drug-phospholipid complex can enhance the solubility and permeability of the drug, improving its absorption. Similarly, fatty acids can form micelles or vesicles with hydrophobic drugs, enhancing drug solubilization and transport across the intestinal epithelium.These drug-lipid complexes not only enhance solubility but also protect the drug from degradation in the GI tract. By shielding the drug from hydrolysis or enzymatic breakdown, these complexes improve the stability and bioavailability of the drug, making lipid-based formulations an effective strategy for delivering labile or poorly soluble drugs. Lipid-based formulations provide a multifaceted approach to improving the solubility, dissolution, and absorption of poorly water-soluble drugs. By leveraging mechanisms such as micelle formation, enhanced drug dissolution, modulation of gastrointestinal fluid properties, lymphatic absorption, inhibition of drug efflux, and the formation of drug-lipid complexes, lipid formulations significantly improve the pharmacokinetic profiles of hydrophobic drugs. These mechanisms not only increase the bioavailability of drugs but also extend their therapeutic efficacy, making lipid-based drug delivery systems an essential tool in modern pharmaceutical development. As research in this area continues, lipid-based formulations are expected to play an even greater role in overcoming the challenges associated with poorly soluble drugs.
Table-2 This table breaks down various lipid mechanisms that enhance drug solubilization and absorption, focusing on different systems, benefits, and limitations.
|
Mechanism
|
Lipid-Based System
|
How It Enhances Absorption
|
Drug Type Targeted
|
Absorption Site
|
Bioavailability Impact
|
Challenges/Limitations
|
|
Micelle Formation
|
Lipid Emulsions, Mixed Micelles
|
Lipids form micelles with bile salts, improving solubility of hydrophobic drugs
|
Lipophilic (poorly water-soluble) drugs
|
Small intestine (via bile interaction)
|
Significantly increases solubility
|
Requires bile secretion, stability issues
|
|
Lymphatic Transport
|
Triglycerides, Long-Chain Fatty Acids
|
Lipids bypass liver metabolism by directing drugs into lymphatic system
|
Highly lipophilic drugs
|
Small intestine (lymphatic system)
|
Enhances bioavailability by avoiding first-pass metabolism
|
Limited to highly lipophilic drugs
|
|
Improved Membrane Permeability
|
Phospholipids, Liposomes
|
Lipid systems interact with cell membranes, increasing permeability
|
Both hydrophobic and amphiphilic drugs
|
Gastrointestinal tract, cell membranes
|
Enhances membrane fluidity, promoting drug uptake
|
Potential for destabilization of membranes
|
|
Self-Emulsification
|
Self-Emulsifying Drug Delivery Systems (SEDDS)
|
Lipids self-emulsify in aqueous environments, forming fine droplets
|
Lipophilic, poorly soluble drugs
|
Gastrointestinal tract
|
Rapid drug solubilization and absorption
|
Requires high lipid content, complex formulation
|
|
Protection from Degradation
|
Solid Lipid Nanoparticles (SLNs)
|
Lipids provide a protective matrix around drugs, preventing degradation
|
Labile or unstable drugs
|
Gastrointestinal tract, bloodstream
|
Protects from enzymatic and acidic degradation
|
Potential for drug expulsion from the lipid matrix
|
|
Increased Dissolution Rate
|
Lipid-Based Nanoparticles
|
Lipid nanoparticles increase surface area, enhancing dissolution of drugs
|
Poorly water-soluble drugs
|
Gastrointestinal tract
|
Improves solubility and dissolution rate
|
Agglomeration, stability challenges
|
|
Prolonged Release
|
Lipid Micelles, Nanoparticles, Liposomes
|
Lipids provide sustained or controlled release of drugs
|
Both hydrophilic and hydrophobic drugs
|
Throughout the GI tract or bloodstream
|
Extends drug presence in the systemic circulation
|
Potential for burst release or inconsistent dosing
|
Characterization Techniques for Lipid-Based Drug Delivery Systems
Lipid-based drug delivery systems (LBDDS) have gained widespread attention due to their ability to improve the solubility, stability, and bioavailability of poorly water-soluble drugs. LBDDS include a range of formulations such as lipid emulsions, liposomes, solid lipid nanoparticles (SLNs), nanostructured lipid carriers (NLCs), and self-emulsifying drug delivery systems (SEDDS). These systems leverage the amphiphilic nature of lipids to encapsulate hydrophobic drugs, promoting enhanced drug dissolution and absorption, particularly for oral administration. However, the complexity of these formulations necessitates thorough characterization to ensure their quality, efficacy, and safety.The characterization of LBDDS involves a series of techniques aimed at analyzing the physicochemical properties, structural attributes, stability, and performance of these systems. These techniques include particle size analysis, surface charge measurement, encapsulation efficiency, drug release studies, and thermal, morphological, and stability assessments. The following detailed explanation outlines the key characterization techniques used for lipid-based drug delivery systems and their importance in formulation development.
Particle Size Analysis: The size of the particles or droplets in lipid-based drug delivery systems significantly impacts the formulation’s performance, particularly its bioavailability, stability, and drug release profile. Smaller particles have a larger surface area relative to their volume, which enhances the dissolution and absorption of hydrophobic drugs in the gastrointestinal (GI) tract. Therefore, particle size analysis is one of the most crucial characterization techniques for LBDDS.Dynamic light scattering (DLS), also known as photon correlation spectroscopy (PCS), is the most commonly used method for determining the particle size and size distribution of LBDDS. In this technique, particles are suspended in a liquid medium and illuminated by a laser. The scattered light is measured over time, and the fluctuations in the scattered light intensity are used to calculate the particle size distribution. DLS is suitable for measuring particles in the nanometer to micrometer range, making it ideal for characterizing lipid emulsions, SLNs, NLCs, and liposomes.Other methods for particle size analysis include laser diffraction and electron microscopy. Laser diffraction is particularly useful for measuring larger particles (e.g., in emulsions), while electron microscopy (e.g., scanning electron microscopy (SEM) and transmission electron microscopy (TEM)) provides detailed images of the particle morphology and structure.
Table-3 This table outlines the primary methods of particle size analysis in lipid-based drug delivery systems, illustrating the principle, applications, and limitations of each technique.
|
Method
|
Principle
|
Typical Size Range Measured
|
Accuracy/Resolution
|
Application in Lipid-Based Systems
|
Impact on Drug Delivery
|
Limitations/Challenges
|
|
Dynamic Light Scattering (DLS)
|
Measures particle size based on light scattering from Brownian motion
|
1 nm – 10 µm
|
High accuracy for nanosized particles
|
Commonly used for nanoparticles, liposomes, emulsions
|
Determines stability, uniformity, and bioavailability
|
Sensitive to aggregation and polydispersity
|
|
Laser Diffraction (LD)
|
Measures particle size by analyzing light diffraction patterns
|
0.1 µm – 3 mm
|
High accuracy across a wide range
|
Used for larger emulsions, suspensions, solid lipid nanoparticles
|
Assesses size distribution and drug loading potential
|
Less sensitive for small nanoparticles (below 100 nm)
|
|
Electron Microscopy (SEM/TEM)
|
Visualizes particles using electron beams for high-resolution imaging
|
1 nm – 100 µm
|
Extremely high resolution (down to 1 nm)
|
Provides direct visualization of liposomes, nanoparticles
|
Confirms morphology, structure, and surface properties
|
Expensive, time-consuming, requires sample preparation
|
|
Atomic Force Microscopy (AFM)
|
Uses a mechanical probe to scan the surface of particles
|
1 nm – 1 µm
|
Very high resolution
|
Characterizes surface topology and particle size of lipid-based systems
|
Evaluates surface properties affecting drug interaction
|
Limited to surface analysis, small sample size
|
|
Coulter Counter
|
Measures particle size based on electrical resistance as particles pass through an aperture
|
0.1 µm – 1 mm
|
Moderate resolution for a broad size range
|
Used for solid lipid nanoparticles, larger liposomes
|
Measures size distribution, concentration
|
Limited sensitivity to small particles (<0>
|
|
Nanoparticle Tracking Analysis (NTA)
|
Tracks the movement of nanoparticles to calculate size distribution
|
10 nm – 2 µm
|
High accuracy for small particles
|
Widely used for liposomes, emulsions, solid lipid nanoparticles
|
Provides size distribution and concentration
|
Sensitive to particle aggregation and refractive index variations
|
|
Field Flow Fractionation (FFF)
|
Separates particles based on size and hydrodynamic properties in a flow field
|
1 nm – 100 µm
|
High resolution for small to large particles
|
Suitable for complex lipid-based systems like liposomes and emulsions
|
Separates particles by size for analysis of polydispersity
|
Time-consuming and requires specialized equipment
|
Zeta Potential (Surface Charge) Measurement:Zeta potential is a measure of the surface charge of particles in a colloidal system and provides insight into the stability of LBDDS. A higher absolute zeta potential (positive or negative) indicates greater electrostatic repulsion between particles, which helps prevent aggregation and ensures the stability of the formulation over time. On the other hand, systems with low zeta potential are more prone to particle aggregation, which can lead to phase separation, reduced bioavailability, and formulation instability.Zeta potential is typically measured using electrophoretic light scattering (ELS), which determines the velocity of particles moving under an applied electric field. The electrophoretic mobility of the particles is then used to calculate the zeta potential. This measurement is particularly important for lipid emulsions, liposomes, and SLNs, as it helps predict their colloidal stability and shelf-life. Encapsulation Efficiency and Drug Loading:Encapsulation efficiency (EE) refers to the percentage of the total drug incorporated into the lipid-based system, while drug loading (DL) is the amount of drug relative to the total weight of the formulation. Both parameters are essential for determining the effectiveness of the LBDDS in delivering the intended dose of the drug.To measure encapsulation efficiency, the lipid-based formulation is typically separated into its free drug and encapsulated drug fractions. This can be done using techniques like ultracentrifugation, filtration, or dialysis. The amount of free drug in the supernatant is quantified using analytical methods such as high-performance liquid chromatography (HPLC) or ultraviolet-visible (UV-Vis) spectroscopy. The encapsulation efficiency is then calculated as the ratio of encapsulated drug to the total drug added to the formulation.High EE is desirable for LBDDS, as it indicates efficient drug incorporation into the lipid matrix. Drug loading, on the other hand, helps optimize the formulation's design by balancing the drug-to-lipid ratio, which can affect solubility, release rate, and stability. In Vitro Drug Release Studies: In vitro drug release studies are critical for predicting the in vivo behavior of lipid-based drug delivery systems. These studies help determine how the drug is released from the lipid matrix, which influences the pharmacokinetics, bioavailability, and therapeutic efficacy of the formulation.The most common method for evaluating drug release from LBDDS is the dialysis technique, where the formulation is placed in a dialysis membrane and immersed in a release medium (e.g., simulated gastrointestinal fluids). The drug that diffuses through the membrane is collected at various time intervals, and the drug concentration is measured using HPLC or UV-Vis spectroscopy. This technique is particularly useful for assessing the sustained or controlled release properties of formulations such as liposomes, SLNs, and NLCs.Other methods for studying drug release include diffusion cells (e.g., Franz diffusion cells) and dissolution testing apparatuses (e.g., USP dissolution apparatus). These methods are particularly relevant for LBDDS intended for oral or transdermal delivery, as they simulate the conditions of the gastrointestinal tract or skin.
Thermal Analysis:Thermal analysis techniques, such as differential scanning calorimetry (DSC) and thermogravimetric analysis (TGA), are used to evaluate the thermal behavior of lipid-based drug delivery systems. These techniques provide information about the melting point, crystallinity, and thermal stability of both the drug and the lipid excipients.DSC measures the heat flow associated with phase transitions in the formulation as a function of temperature. This is important for determining the melting point of the lipids and the crystallization behavior of solid lipid nanoparticles and nanostructured lipid carriers. Changes in the melting point can indicate interactions between the drug and lipid matrix, which can affect drug release and stability.TGA, on the other hand, measures the weight loss of the formulation as it is heated, providing information about the thermal stability and decomposition of the formulation components. TGA is particularly useful for assessing the stability of lipid-based formulations under various storage conditions. Morphological Characterization: The morphology of lipid-based drug delivery systems, particularly those with nanostructured components, plays a significant role in their stability and performance. Morphological characterization techniques provide insights into the shape, size, and structure of the particles or vesicles in the formulation. Transmission electron microscopy (TEM) and scanning electron microscopy (SEM) are commonly used to obtain high-resolution images of lipid-based systems. TEM provides detailed images of the internal structure of nanoparticles, liposomes, and emulsions, while SEM gives information about the surface morphology of these particles. Atomic force microscopy (AFM) is another technique that provides topographical information about the surface structure of lipid-based formulations at the nanoscale.Cryo-electron microscopy (cryo-EM) is particularly useful for visualizing liposomes and other lipid vesicles in their hydrated state, preserving their native structure. This technique allows for the direct observation of the lipid bilayer and drug encapsulation, providing valuable insights into the formulation’s architecture.
Stability Studies:Stability is a critical consideration in the development of lipid-based drug delivery systems, as these formulations are prone to degradation due to factors such as oxidation, hydrolysis, and particle aggregation. Stability studies assess the physical, chemical, and microbiological stability of the LBDDS over time, under different storage conditions (e.g., temperature, humidity, and light exposure).Accelerated stability studies are commonly conducted by storing the formulation under stress conditions (e.g., elevated temperature and humidity) and monitoring changes in key parameters such as particle size, zeta potential, encapsulation efficiency, and drug release. These studies help predict the shelf-life of the formulation and identify potential degradation pathways.In addition, chemical stability studies involve monitoring the degradation of the drug and lipid excipients over time using techniques such as HPLC, mass spectrometry, and nuclear magnetic resonance (NMR) spectroscopy. Oxidation of lipids is a particular concern, and antioxidants may be added to the formulation to improve stability.
Permeability and Absorption Studies:In vitro permeability studies are conducted to evaluate the ability of the drug to permeate biological membranes, which is critical for determining the bioavailability of the LBDDS. The Caco-2 cell model, which uses human intestinal epithelial cells, is commonly employed to assess drug permeability across the intestinal barrier. This model mimics the absorption process in the human gut, providing insights into the formulation’s potential for oral delivery.In addition, liposomal formulations and other vesicle-based systems are often tested using membrane models such as the parallel artificial membrane permeability assay (PAMPA), which evaluates passive diffusion across lipid membranes. The successful development of lipid-based drug delivery systems requires comprehensive characterization to ensure the formulation’s efficacy, stability, and safety. Techniques such as particle size analysis, zeta potential measurement, encapsulation efficiency, drug release studies, thermal analysis, and morphological characterization provide critical insights into the performance of LBDDS. These characterization techniques, combined with stability and permeability assessments, help optimize the formulation and ensure consistent drug delivery, ultimately improving the therapeutic outcomes for hydrophobic drugs.
Enhanced Bioavailability: Clinical Implications of Lipid Formulations
Enhanced bioavailability through lipid-based formulations is a crucial strategy in overcoming the challenges of poorly water-soluble drugs, which make up nearly 40% of the drugs on the market and an even higher percentage of drugs in development pipelines. Lipid formulations improve the solubility, absorption, and overall bioavailability of these drugs, making them an essential tool in modern drug development, especially for oral administration. This detailed explanation explores the mechanisms by which lipid formulations enhance bioavailability, their clinical implications, and their impact on therapeutic efficacy. Challenges of Poor Bioavailability: Poor bioavailability is one of the main limitations of many orally administered drugs. It occurs when a drug does not dissolve adequately in the gastrointestinal (GI) tract, preventing it from being absorbed into the bloodstream in sufficient quantities to exert its therapeutic effect. Many drugs, particularly those classified as Biopharmaceutics Classification System (BCS) Class II (poor solubility, high permeability) and Class IV (poor solubility, poor permeability) compounds, face significant bioavailability challenges due to their low solubility in water.The gastrointestinal tract presents additional barriers, such as the first-pass metabolism in the liver, pH variation along the digestive tract, and efflux transporters like P-glycoprotein (P-gp), which can pump drugs back into the intestinal lumen, further reducing their absorption. These obstacles necessitate innovative drug delivery systems that can bypass these barriers and improve the bioavailability of poorly soluble drugs. Mechanisms of Bioavailability Enhancement by Lipid-Based Formulations: Lipid-based formulations enhance the bioavailability of drugs through several mechanisms. These includeIncreased Solubilization in the GI Tract Lipid formulations, such as emulsions, liposomes, solid lipid nanoparticles (SLNs), and self-emulsifying drug delivery systems (SEDDS), enhance the solubility of hydrophobic drugs by dissolving them in lipid matrices. In the gastrointestinal environment, the lipid components interact with bile salts, phospholipids, and digestive enzymes, forming mixed micelles that facilitate the solubilization and subsequent absorption of the drug. Facilitation of Lymphatic Transport Lipid formulations can promote the uptake of drugs into the lymphatic system, bypassing the liver and avoiding first-pass metabolism. The lymphatic system offers a direct route for drug absorption, particularly for highly lipophilic drugs. This results in improved systemic bioavailability, as the drug avoids hepatic degradation. Triglycerides, long-chain fatty acids, and certain surfactants in lipid formulations play a role in triggering lymphatic transport. Prolonged Drug Residence Time in the GI Tract Lipid-based formulations often have the ability to prolong the residence time of drugs in the stomach or small intestine, which can lead to increased drug absorption. This is particularly true for formulations that form a gel-like matrix or remain in the GI lumen for extended periods, allowing more time for the drug to dissolve and be absorbed.
Reduction of P-gp Efflux Many lipid-based formulations have the potential to inhibit the action of P-glycoprotein (P-gp), a major efflux transporter in the intestinal lining. P-gp actively pumps drugs back into the intestinal lumen, limiting their absorption. Certain lipids, surfactants, and excipients used in lipid-based systems can inhibit P-gp, allowing more of the drug to be absorbed into the bloodstream. Improved Permeability Across Biological Membranes Lipid formulations can enhance the permeability of drugs across biological membranes by interacting with and disrupting the lipid bilayers of cells, making it easier for drugs to cross cellular barriers such as the intestinal epithelium. This is particularly beneficial for drugs with poor permeability, as it facilitates their entry into systemic circulation. Lipid-based drug delivery systems can be categorized into various types, each with specific mechanisms of action that contribute to improved bioavailability: Lipid emulsions are mixtures of oil and water stabilized by surfactants. They provide a medium for the solubilization of hydrophobic drugs and enable the formation of fine droplets that increase the drug’s surface area, facilitating faster dissolution and absorption. Emulsions are widely used in parenteral formulations but can also be applied for oral delivery. Self-Emulsifying Drug Delivery Systems (SEDDS) are mixtures of oils, surfactants, and co-solvents that spontaneously form fine oil-in-water emulsions upon contact with gastrointestinal fluids. These systems are particularly effective for enhancing the bioavailability of poorly soluble drugs by promoting rapid drug dissolution and improving solubilization in the GI tract. SEDDS formulations also enhance lymphatic transport and reduce drug degradation during transit through the digestive system. Solid Lipid Nanoparticles (SLNs) and Nanostructured Lipid Carriers (NLCs) are solid particles composed of lipids, often stabilized by surfactants. They serve as carriers for poorly soluble drugs, protecting the drug from degradation and enhancing absorption. These nanocarriers also offer sustained release properties, providing a controlled release of the drug over time, which can lead to prolonged therapeutic effects. Liposomes are vesicular structures composed of one or more lipid bilayers surrounding an aqueous core. Hydrophobic drugs can be incorporated into the lipid bilayers, while hydrophilic drugs are encapsulated in the aqueous core. Liposomes improve bioavailability by enhancing drug solubility, protecting the drug from enzymatic degradation, and facilitating targeted drug delivery, particularly for cancer therapies. Lipid micelles are spherical structures formed by the self-assembly of amphiphilic molecules, such as surfactants, in aqueous environments. They are used to solubilize hydrophobic drugs and enhance their absorption by forming micellar solutions in the GI tract, thus improving the drug’s bioavailability. The clinical implications of lipid-based formulations are significant, as they directly impact the therapeutic efficacy and dosing regimens of many drugs. Some key clinical benefits include: Improved Therapeutic Efficacy by increasing the bioavailability of poorly soluble drugs, lipid formulations enhance the drug’s therapeutic efficacy at lower doses. This is particularly important for drugs with narrow therapeutic windows, where maintaining a precise drug concentration in the bloodstream is essential for efficacy and safety. Lipid-based formulations enable the drug to reach the target concentration more efficiently, leading to improved clinical outcomes. Reduction in Dosing Frequency Lipid-based formulations, particularly those with sustained or controlled release properties, allow for extended drug release over time. This reduces the need for frequent dosing, improving patient compliance and convenience. For chronic conditions requiring long-term drug therapy, reduced dosing frequency can enhance patient adherence to treatment regimens. Lower Risk of Dose-Related Toxicity Poor bioavailability often necessitates high doses of a drug to achieve therapeutic plasma concentrations. However, this increases the risk of dose-related side effects and toxicity. Lipid formulations enable better absorption at lower doses, reducing the likelihood of adverse effects and improving the drug’s safety profile. Targeted Drug Delivery Certain lipid-based systems, such as liposomes and SLNs, can be engineered to target specific tissues or cells, such as cancerous tissues or inflamed regions. This targeted delivery enhances the drug’s efficacy at the site of action while minimizing systemic side effects. For example, liposomal formulations of anticancer drugs like doxorubicin have been shown to improve tumor targeting, reducing cardiotoxicity compared to conventional formulations. Overcoming Food Effects many lipid-based formulations can mitigate the impact of food on drug absorption. Some poorly soluble drugs exhibit significantly different absorption profiles when taken with or without food. Lipid formulations help to minimize these variations by enhancing solubilization and absorption, leading to more predictable pharmacokinetics, regardless of food intake.
Several drugs on the market use lipid-based formulations to improve their bioavailability:
Cyclosporine (Neoral®): Cyclosporine, an immunosuppressant used in organ transplantation, has poor water solubility and bioavailability. The lipid-based formulation Neoral® (a microemulsion) improves the drug’s absorption, leading to more consistent and predictable plasma concentrations. Amphotericin B (AmBisome®): AmBisome® is a liposomal formulation of the antifungal drug amphotericin B. The liposomal formulation enhances the drug’s solubility, reduces nephrotoxicity, and allows for targeted delivery to fungal-infected tissues.
Paclitaxel (Abraxane®): Abraxane® is a nanoparticle albumin-bound formulation of paclitaxel, a chemotherapeutic agent. This formulation improves the solubility of paclitaxel, allowing for its administration without toxic solvents and enhancing its therapeutic efficacy in cancer treatment. Lipid-based formulations offer a powerful solution to the challenge of poor bioavailability for many hydrophobic drugs. By enhancing solubility, promoting lymphatic transport, reducing efflux, and improving permeability, these formulations enable more efficient drug absorption and therapeutic action. The clinical implications are profound, with benefits including improved therapeutic efficacy, reduced dosing frequency, lower risk of toxicity, and targeted drug delivery. As lipid-based formulations continue to evolve, they will play an increasingly important role in overcoming the bioavailability challenges of new and existing drugs, leading to better patient outcomes across a wide range of therapeutic areas.

Fig-2 Here is the image depicting the clinical implications of lipid-based formulations in enhancing bioavailability. It visualizes how lipid-based carriers like liposomes and solid lipid nanoparticles improve solubility, absorption, and drug delivery, bypassing first-pass metabolism and enhancing bioavailability.
Case Studies: Successful Applications of Lipid-Based Formulations in Pharmacotherapy
Lipid-based formulations (LBFs) have become a crucial aspect of drug development, particularly for poorly water-soluble drugs, due to their ability to enhance bioavailability, solubility, and overall therapeutic effectiveness. Several drugs on the market leverage these formulations to overcome pharmacokinetic challenges and provide improved clinical outcomes. This section delves into case studies of successful applications of lipid-based formulations in pharmacotherapy, exploring how they have been utilized to address issues related to solubility, absorption, and therapeutic efficacy.
Cyclosporine (Neoral®): Enhancing Consistency and Bioavailability
Cyclosporine, an immunosuppressant used to prevent organ transplant rejection, is notoriously challenging due to its low solubility and erratic bioavailability. Initially, cyclosporine was formulated as an oil-based preparation (Sandimmune®), but it had significant inter-patient variability in absorption, leading to inconsistent therapeutic outcomes.To address this, Novartis developed Neoral®, a microemulsion pre-concentrate that enhances the solubility and bioavailability of cyclosporine. Neoral® spontaneously forms a microemulsion in the gastrointestinal (GI) tract, allowing cyclosporine to be absorbed more efficiently. This lipid-based formulation significantly reduced the variability in cyclosporine absorption, providing more predictable pharmacokinetics and improving therapeutic efficacy. As a result, Neoral® became the standard treatment for transplant patients, allowing for better control over immunosuppressive therapy.
Amphotericin B (Am Bisome®): Reducing Toxicity with Liposomal Formulation
Amphotericin B is an effective antifungal agent used to treat severe fungal infections, but its clinical use is limited by its significant nephrotoxicity. The traditional formulation of amphotericin B, administered in a deoxycholate suspension, often leads to kidney damage due to its accumulation in renal tissues.To mitigate this, liposomal formulations of amphotericin B were developed, with AmBisome® being the most notable. Liposomes are lipid bilayer vesicles that encapsulate the drug, protecting it from rapid degradation and reducing its interaction with non-target tissues. In the case of AmBisome®, the liposomes allow for the targeted delivery of amphotericin B to fungal cells, while minimizing its exposure to the kidneys and other organs.Clinical studies have demonstrated that Am Bisome® significantly reduces the incidence of nephrotoxicity compared to conventional formulations of amphotericin B. This liposomal approach has allowed patients to receive effective antifungal treatment with fewer side effects, making it a valuable option for those with life-threatening infections, such as cryptococcal meningitis and invasive aspergillosis.
Paclitaxel (Abraxane®): Overcoming Solvent-Related Toxicity
Paclitaxel is a chemotherapeutic agent used to treat various cancers, including breast, lung, and ovarian cancers. However, its poor solubility required the use of toxic solvents, such as Cremophor EL, in the original formulation (Taxol®). Cremophor EL is associated with severe hypersensitivity reactions, necessitating pre-medication with steroids and antihistamines, which can be harmful for patients undergoing long-term cancer treatment. To eliminate the need for toxic solvents, the drug Abraxane® was developed. This formulation uses albumin-bound nanoparticles to deliver paclitaxel in a solvent-free form. Albumin, a naturally occurring protein in the body, facilitates the transport of paclitaxel through the bloodstream and enhances its solubility and bioavailability. Additionally, Abraxane® allows for higher doses of paclitaxel to be administered safely, without the need for pre-medication. Abraxane® has demonstrated superior therapeutic efficacy compared to Taxol® in several cancers, including metastatic breast cancer and non-small cell lung cancer (NSCLC). Moreover, the absence of solvents reduces the risk of hypersensitivity reactions and other adverse effects, improving the overall tolerability of paclitaxel therapy.
Fenofibrate (Tricor®): Improving Solubility and Absorption with Microemulsions
Fenofibrate, a lipid-lowering agent used to treat hyperlipidemia and reduce cardiovascular risk, is poorly soluble in water and has variable absorption depending on food intake. The original formulation of fenofibrate required patients to take the drug with meals to enhance absorption, which posed challenges for patient compliance and therapeutic consistency. The development of lipid-based formulations of fenofibrate, such as Tricor®, addressed these issues. Tricor® uses a microemulsion system that enhances the solubility of fenofibrate, allowing for improved absorption regardless of food intake. This new formulation provided more consistent pharmacokinetics, enabling patients to take fenofibrate with or without food, thereby improving compliance. Clinical trials have shown that Tricor® effectively reduces triglyceride levels and improves high-density lipoprotein (HDL) cholesterol levels, with fewer side effects and more predictable results than the original formulation. The lipid-based microemulsion approach has thus contributed to better management of dyslipidemia and cardiovascular risk in patients.
Ritonavir (Norvir®): Addressing Drug Solubility and Stability Ritonavir is an antiretroviral protease inhibitor used in the treatment of HIV. However, its poor water solubility and susceptibility to degradation in the GI tract posed significant challenges for achieving therapeutic plasma concentrations. The original formulation of ritonavir required refrigeration and had limited stability, making it inconvenient for patients.To overcome these challenges, Abbott Laboratories developed a soft gelatin capsule formulation of ritonavir, using a lipid-based vehicle to enhance the drug’s solubility and stability. This formulation allows ritonavir to remain stable at room temperature and improves its absorption in the GI tract. The lipid-based system not only enhances bioavailability but also simplifies storage and administration, improving the convenience and adherence to HIV therapy.Ritonavir’s role as a pharmacokinetic enhancer in combination with other antiretroviral drugs has been transformative in HIV treatment. By boosting the plasma levels of co-administered protease inhibitors, ritonavir has enabled lower dosing regimens and improved the overall efficacy of HIV therapy.
Isotretinoin (Accutane®): Enhancing Absorption for Acne Treatment Isotretinoin is a potent retinoid used in the treatment of severe acne. However, its bioavailability is highly variable and dependent on food intake due to its poor water solubility. The original formulation of isotretinoin required patients to take the drug with a high-fat meal to ensure adequate absorption, which was inconvenient and often led to suboptimal therapeutic outcomes.The development of lipid-based formulations, such as Absorica®, addressed these bioavailability challenges. Absorica® utilizes a lipidic excipient system that enhances the solubility and absorption of isotretinoin, allowing for consistent drug delivery regardless of food intake. This improved formulation has made isotretinoin therapy more effective and easier for patients to adhere to, while reducing the risk of under-dosing and acne recurrence.
Saquinavir (Invirase®): Lipid-Based Enhancements in Antiviral Therapy Saquinavir, an early protease inhibitor used in HIV therapy, had poor oral bioavailability due to its low solubility and extensive first-pass metabolism. The original formulation of saquinavir required high doses to achieve therapeutic plasma levels, leading to increased toxicity and side effects.A lipid-based soft gelatin capsule formulation of saquinavir was developed to enhance its solubility and improve its pharmacokinetics. This formulation allowed for more efficient absorption and reduced first-pass metabolism, leading to higher plasma concentrations at lower doses. The lipid-based approach also facilitated co-administration with other antiretrovirals, improving the overall effectiveness of combination HIV therapy.
Probucol (Lorelco®): Addressing Poor Absorption in Cholesterol Management Probucol, a lipid-lowering drug used to reduce cholesterol levels, had poor oral absorption and low bioavailability due to its hydrophobic nature. Conventional formulations of probucol required high doses to achieve therapeutic effects, which increased the risk of side effects such as QT interval prolongation.Lipid-based formulations of probucol were developed to enhance its absorption and bioavailability. By incorporating probucol into lipidic carriers, such as emulsions or liposomes, the drug’s solubility in the GI tract was improved, leading to more consistent plasma concentrations and reduced side effects. This lipid-based approach has allowed for safer and more effective cholesterol management in patients.
Clofazimine (Lamprene®): Improving Delivery for Leprosy Treatment Clofazimine is an antimycobacterial agent used in the treatment of leprosy. It has poor water solubility and a propensity to accumulate in tissues, leading to variable pharmacokinetics and adverse effects such as skin discoloration.A lipid-based formulation of clofazimine was developed to enhance its solubility and absorption, providing more consistent plasma levels and reducing tissue accumulation. This formulation improved the drug’s efficacy in treating leprosy while minimizing side effects, making clofazimine therapy more tolerable for patients. The successful applications of lipid-based formulations in pharmacotherapy highlight their transformative impact on drug delivery, especially for poorly water-soluble drugs. These case studies demonstrate how lipid formulations can enhance bioavailability, reduce toxicity, improve therapeutic efficacy, and provide more predictable pharmacokinetics. As drug development continues to evolve, lipid-based delivery systems will remain a critical tool for overcoming the solubility and absorption challenges associated with modern pharmaceuticals. The versatility of lipid formulations in various therapeutic areas, from immunosuppressants to anticancer drugs, underscores their importance in improving patient outcomes and advancing medical treatments.

Fig-3 Here is the graph-based image illustrating successful case studies of lipid-based formulations in pharmacotherapy, comparing various lipid systems like liposomes, solid lipid nanoparticles, and emulsions across therapeutic areas like cancer therapy, vaccine delivery, and antiviral treatments. Key clinical outcomes such as improved bioavailability and patient efficacy are highlighted.
Regulatory Perspectives: Evaluating the Safety and Efficacy of Lipid Formulations
Lipid-based formulations (LBFs) have gained significant attention in the pharmaceutical industry due to their ability to improve the solubility, bioavailability, and therapeutic outcomes of poorly water-soluble drugs. However, the regulatory landscape governing the approval of these formulations requires thorough evaluation to ensure their safety and efficacy. Regulatory agencies, such as the U.S. Food and Drug Administration (FDA), the European Medicines Agency (EMA), and other global counterparts, establish specific guidelines and standards for the development, testing, and approval of LBFs. These regulations are crucial in ensuring that lipid formulations meet stringent safety and efficacy criteria before they are introduced into the market.
Regulatory Framework for Lipid Formulations
LBFs are subject to the same rigorous regulatory scrutiny as conventional drug formulations. Regulatory agencies require comprehensive data on the formulation's safety, efficacy, pharmacokinetics, and quality. In the case of LBFs, there are additional considerations due to the presence of lipid excipients, which can affect the drug's solubility, absorption, and distribution in the body.The regulatory framework for LBFs typically involves several key stages
Preclinical Evaluation: Before a lipid formulation can enter clinical trials, it must undergo extensive preclinical testing. This includes in vitro studies to assess the drug's solubility and stability in lipid excipients, as well as in vivo animal studies to evaluate safety and pharmacokinetics. Toxicology studies are also conducted to identify any potential adverse effects associated with the lipid excipients or the drug-lipid interactions.
Clinical Trials: LBFs must undergo the same phased clinical trial process as other pharmaceuticals, beginning with Phase I trials to assess safety and tolerability in healthy volunteers. Phase II and III trials focus on evaluating the efficacy and safety of the formulation in the target patient population. Regulatory agencies closely scrutinize the results of these trials to ensure that the lipid formulation provides a therapeutic benefit without introducing unacceptable risks.
Regulatory Submission: After successful completion of clinical trials, the pharmaceutical company submits a New Drug Application (NDA) or Marketing Authorization Application (MAA) to regulatory agencies. This submission includes all preclinical and clinical data, along with detailed information on the formulation's manufacturing process, quality control, and stability. The safety of lipid excipients is a critical concern for regulatory agencies when evaluating LBFs. Lipid excipients, such as triglycerides, phospholipids, and surfactants, play a pivotal role in enhancing drug solubility and absorption. However, their potential interactions with the drug, the body's metabolic systems, and immune responses must be carefully assessed.
Lipid Excipient Toxicity: Although many lipid excipients used in LBFs are Generally Recognized as Safe (GRAS) by regulatory authorities, their use in high concentrations or in combination with specific drugs may pose safety risks. For example, certain surfactants used in lipid emulsions can cause gastrointestinal irritation, while high levels of triglycerides may lead to lipid accumulation in tissues, causing inflammation or liver toxicity. Regulatory agencies require toxicology studies to evaluate the potential risks associated with lipid excipients.
Immunogenicity: Lipid-based delivery systems, especially liposomes and solid lipid nanoparticles, can trigger immune responses. Regulatory agencies require immunogenicity studies to assess whether the lipid excipients or the lipid-drug complex can activate the immune system, leading to allergic reactions, anaphylaxis, or other adverse immune responses. For example, liposomal formulations of anticancer drugs, such as Doxil® (liposomal doxorubicin), have been associated with infusion-related reactions due to the immune system's recognition of liposomes as foreign particles.
Stability and Degradation: LBFs must demonstrate stability throughout their shelf life, as lipid excipients can be prone to oxidation and degradation. Regulatory agencies require stability studies to assess how the formulation behaves under various storage conditions, including temperature and humidity. The degradation of lipid excipients can produce toxic by-products, which must be identified and evaluated for their safety. The efficacy of LBFs is primarily evaluated based on their ability to enhance the solubility and bioavailability of poorly water-soluble drugs. Regulatory agencies focus on several key aspects of efficacy during the approval process:
Bioavailability Enhancement: One of the primary goals of LBFs is to improve the bioavailability of hydrophobic drugs by facilitating their solubilization in the gastrointestinal (GI) tract and enhancing their absorption. Regulatory agencies require pharmacokinetic studies to demonstrate that the lipid formulation achieves higher drug concentrations in the bloodstream compared to conventional formulations. These studies typically involve measuring the drug's maximum plasma concentration (Cmax), time to reach maximum concentration (Tmax), and overall exposure (area under the curve, or AUC).
Therapeutic Equivalence: For LBFs that are reformulations of existing drugs, regulatory agencies require data demonstrating therapeutic equivalence to the original product. This means that the lipid formulation must deliver the same clinical efficacy and safety profile as the conventional formulation. For example, the lipid-based formulation of cyclosporine (Neoral®) was required to demonstrate therapeutic equivalence to the original oil-based formulation (Sandimmune®) in terms of preventing organ rejection in transplant patients.
Dose Optimization: LBFs can allow for dose reduction while maintaining therapeutic efficacy, as lipid excipients enhance drug solubility and absorption. Regulatory agencies require clinical data to support any proposed changes in dosing regimens. For example, Abraxane® (albumin-bound paclitaxel) allows for higher doses of paclitaxel to be administered without the need for toxic solvents, but dose optimization studies were required to ensure that the new formulation provided the same or better therapeutic outcomes compared to traditional paclitaxel formulations. The manufacturing process for LBFs is more complex than for conventional formulations, due to the need to carefully control the size, structure, and stability of lipid particles. Regulatory agencies require detailed information on the manufacturing process, including the choice of lipid excipients, methods for producing the lipid-drug complex, and quality control measures.
Particle Size and Distribution: The size of lipid particles, such as liposomes or solid lipid nanoparticles, can significantly impact the drug's bioavailability and distribution in the body. Regulatory agencies require particle size analysis as part of the quality control process to ensure consistency between batches. For example, liposomal formulations must demonstrate a narrow particle size distribution to avoid aggregation and ensure uniform drug release.
Sterility and Contamination: LBFs, particularly those administered via injection, must meet stringent sterility requirements. Regulatory agencies require data on the sterility testing of the formulation, as well as measures to prevent microbial contamination during manufacturing. Lipid-based nanoparticles, such as those used in vaccines or gene therapies, are particularly susceptible to contamination, necessitating rigorous quality control protocols.
Stability and Shelf Life: LBFs must demonstrate long-term stability to ensure that the formulation remains safe and effective throughout its shelf life. Regulatory agencies require stability studies under various environmental conditions to evaluate the formulation's degradation profile, including the potential for lipid oxidation or hydrolysis. The presence of antioxidants or other stabilizing agents in the formulation must be justified with supporting data. While LBFs offer significant advantages in drug delivery, they also present unique challenges for regulatory agencies. The complexity of these formulations requires a multidisciplinary approach to evaluating their safety, efficacy, and quality. Regulatory agencies are continually updating their guidelines to keep pace with advances in lipid-based drug delivery technologies, such as the development of lipid nanoparticles for mRNA vaccines. One of the key challenges is the lack of standardized guidelines for evaluating the safety of lipid excipients, particularly in novel formulations that use new types of lipids or surfactants. Regulatory agencies may require additional preclinical and clinical data for LBFs that use unapproved lipid excipients, which can prolong the approval process. Looking forward, regulatory agencies are expected to focus on the development of harmonized guidelines for LBFs, particularly in the areas of excipient safety, immunogenicity, and particle size control. Advances in lipid-based drug delivery technologies, such as targeted liposomes and lipid nanoparticles for gene therapy, will require updated regulatory frameworks to ensure the safe and effective use of these innovative formulations. The evaluation of the safety and efficacy of lipid-based formulations is a complex process that involves a thorough understanding of both the drug and the lipid excipients used in the formulation. Regulatory agencies play a critical role in ensuring that these formulations meet the necessary safety, efficacy, and quality standards before they are approved for clinical use. By addressing the unique challenges posed by LBFs, regulatory agencies help ensure that these innovative drug delivery systems can provide enhanced therapeutic outcomes for patients while minimizing potential risks.
Emerging Technologies: Novel Approaches in Lipid-Based Drug Delivery
Lipid-based drug delivery systems (LBDDS) have significantly evolved in recent decades, offering promising solutions for enhancing the bioavailability and therapeutic efficacy of poorly water-soluble drugs. With the rise of more complex drug molecules, such as biologics, peptides, and nucleic acids, the demand for innovative delivery systems has increased. Emerging technologies in lipid-based formulations are at the forefront of addressing these challenges, with novel approaches leveraging nanotechnology, targeted delivery, and multifunctional systems that not only improve solubility but also ensure controlled release, enhanced stability, and targeted action. These advancements in LBDDS hold great potential for revolutionizing drug delivery, especially for conditions that require precision treatment.
Nanotechnology in Lipid-Based Drug Delivery
One of the most transformative advancements in LBDDS is the integration of nanotechnology. Nanostructured lipid carriers (NLCs) and solid lipid nanoparticles (SLNs) are key developments that offer improved drug stability, controlled release profiles, and enhanced drug loading capacity. These nanocarriers provide a platform for encapsulating hydrophobic drugs within a lipid matrix, ensuring their solubilization in biological fluids and improving their absorption across biological membranes.
Solid Lipid Nanoparticles (SLNs): SLNs consist of solid lipids that remain solid at both room and body temperature, providing a stable environment for drug molecules. These particles, ranging from 50-1000 nm, protect the drug from degradation, ensuring prolonged circulation in the bloodstream and improved bioavailability. SLNs are especially useful for delivering drugs with poor oral bioavailability or those requiring long-term release, such as anticancer or anti-inflammatory agents.
Nanostructured Lipid Carriers (NLCs): NLCs are a second-generation lipid nanoparticle system designed to overcome some limitations of SLNs, such as limited drug loading and potential drug expulsion during storage. By incorporating a mixture of solid and liquid lipids, NLCs create a less-ordered lipid matrix that can accommodate a higher amount of drug. This results in better drug encapsulation efficiency and stability, making NLCs a promising carrier for hydrophobic drugs, including anticancer, antiviral, and antifungal agents.
Lipid Nanoparticles for Gene Delivery and mRNA Vaccines
The COVID-19 pandemic highlighted the importance of lipid nanoparticles (LNPs) in gene therapy and vaccine delivery, particularly for mRNA-based vaccines like Pfizer-BioNTech and Moderna’s COVID-19 vaccines. LNPs play a crucial role in protecting fragile mRNA molecules, facilitating their entry into cells, and ensuring efficient release in the cytoplasm where the mRNA is translated into proteins. The success of these vaccines has spurred further research into using LNPs for delivering other types of genetic material, such as DNA and siRNA, for the treatment of various diseases, including cancer, genetic disorders, and infectious diseases.LNPs are composed of various lipid types, including phospholipids, cholesterol, and ionizable lipids, which are designed to interact with the negatively charged RNA or DNA molecules and form stable complexes. The ionizable lipids are particularly important as they remain neutral at physiological pH but become positively charged in acidic environments, such as within the endosomes, enabling the release of the genetic payload into the cell's cytoplasm.
LNPs for Cancer Gene Therapy: Researchers are investigating the use of LNPs for delivering small interfering RNA (siRNA) and microRNA (miRNA) to silence genes involved in cancer progression. This approach can target specific oncogenes, reducing tumor growth and metastasis. LNP-based delivery of gene-editing technologies, such as CRISPR/Cas9, is also being explored as a potential treatment for genetic disorders and cancers.
Targeted Lipid-Based Drug Delivery
One of the key challenges in drug delivery is ensuring that drugs reach the target site while minimizing off-target effects and reducing toxicity. Recent advancements in lipid-based delivery systems focus on targeted drug delivery, where lipids are engineered to deliver drugs specifically to diseased tissues or cells, such as cancer cells or inflamed tissues, while sparing healthy tissues.
Ligand-Conjugated Lipid Nanoparticles: Targeted LBDDS can be achieved by conjugating ligands, such as antibodies, peptides, or small molecules, onto the surface of lipid nanoparticles. These ligands recognize and bind to specific receptors that are overexpressed on the surface of target cells, such as cancer cells. This approach ensures that the drug is preferentially delivered to the diseased tissue, enhancing therapeutic efficacy while minimizing side effects. For example, liposomes conjugated with antibodies that target the HER2 receptor are used in the treatment of HER2-positive breast cancer. pH-Sensitive and Stimuli-Responsive Lipid Systems: Another emerging strategy involves the use of lipid-based carriers that respond to specific stimuli in the body, such as changes in pH, temperature, or the presence of specific enzymes. Tumor tissues often exhibit an acidic microenvironment, and pH-sensitive lipid formulations can release their drug cargo specifically in these acidic conditions. This targeted release minimizes drug exposure to healthy tissues and enhances the therapeutic index of anticancer drugs. Combination Therapy Using Lipid-Based Formulations Combination therapy, where two or more drugs are delivered together to produce synergistic effects, is gaining traction in the treatment of complex diseases like cancer, HIV, and tuberculosis. Lipid-based formulations offer an effective platform for delivering multiple drugs simultaneously, improving therapeutic outcomes and reducing the development of drug resistance. Co-Encapsulation of Drugs: Lipid-based carriers can be engineered to encapsulate both hydrophobic and hydrophilic drugs in a single delivery system. For instance, liposomes or solid lipid nanoparticles can carry a chemotherapeutic agent and a drug that enhances the permeability of the tumor vasculature, thereby improving the accumulation of the therapeutic agent at the tumor site. This approach is being explored to overcome multidrug resistance in cancer therapy by delivering drugs that inhibit resistance mechanisms alongside conventional anticancer agents.
Multifunctional Liposomes: Multifunctional liposomes can be designed to deliver drugs with different mechanisms of action, enhancing the overall therapeutic effect. For example, a liposomal formulation might include a drug that kills cancer cells and an anti-inflammatory agent that reduces the side effects of chemotherapy. These combination strategies not only improve patient outcomes but also simplify treatment regimens.
Advanced Lipid-Based Carriers for Oral Delivery
Oral drug delivery remains the most preferred route for drug administration due to its convenience and patient compliance. However, delivering poorly water-soluble drugs orally poses significant challenges. Emerging technologies in LBDDS focus on developing lipid-based carriers that can enhance the oral bioavailability of such drugs by improving solubility and absorption.
Self-Emulsifying Drug Delivery Systems (SEDDS): SEDDS are isotropic mixtures of oils, surfactants, and solvents that spontaneously form fine oil-in-water emulsions when exposed to the aqueous environment of the gastrointestinal tract. These systems enhance the solubility and absorption of poorly water-soluble drugs by forming a stable emulsion that facilitates drug dissolution and transport across the intestinal epithelium. SEDDS have been successfully used for the oral delivery of lipophilic drugs, such as cyclosporine and ritonavir, improving their bioavailability significantly.
Lipid Micelles for Oral Peptide Delivery: Oral delivery of peptide drugs, such as insulin, has long been a challenge due to the poor stability of peptides in the acidic environment of the stomach and their degradation by digestive enzymes. Lipid micelles, particularly those formed using bile salts, offer a promising solution for encapsulating and protecting peptide drugs, allowing them to pass through the stomach and be absorbed in the intestine.
Lipid-Based Carriers for Central Nervous System (CNS) Drug Delivery
Delivering drugs to the CNS is particularly challenging due to the presence of the blood-brain barrier (BBB), which limits the entry of most drugs into the brain. Emerging lipid-based technologies are being developed to overcome this barrier and deliver drugs effectively to treat neurological conditions such as Alzheimer's disease, Parkinson's disease, and brain cancers.
Lipid Nanoparticles for BBB Penetration: Lipid nanoparticles are being engineered to cross the BBB by modifying their surface with specific targeting ligands or by using transport mechanisms such as receptor-mediated transcytosis. For example, lipid nanoparticles conjugated with transferrin or lactoferrin can bind to receptors on the surface of endothelial cells in the BBB, allowing them to be transported into the brain. These systems are being explored for the delivery of neuroprotective agents, anticancer drugs, and gene therapies targeting brain diseases. Liposomal Delivery of CNS Drugs: Liposomes can be designed to encapsulate CNS-active drugs, protecting them from degradation in the bloodstream and facilitating their delivery to the brain. Liposomal formulations of antiepileptic drugs and neuroprotective agents are being studied for their ability to improve drug delivery to the brain and reduce systemic side effects. The field of lipid-based drug delivery is rapidly evolving, with novel technologies offering exciting possibilities for improving the solubility, bioavailability, and therapeutic efficacy of a wide range of drugs. From nanotechnology and targeted delivery systems to combination therapies and advanced oral formulations, these innovations hold great promise for addressing some of the most pressing challenges in modern pharmacotherapy. As these technologies continue to develop, they have the potential to revolutionize the treatment of various diseases, providing more effective, targeted, and patient-friendly drug delivery solutions.
Future Directions: Research Trends in Lipid-Based Formulations
Lipid-based formulations (LBFs) have emerged as a powerful tool in pharmaceutical sciences, offering promising solutions to many drug delivery challenges, particularly for poorly water-soluble and bioavailable drugs. The continued evolution of LBFs is driven by innovations in nanotechnology, biomaterials, and pharmaceutical sciences, leading to novel strategies for improving drug solubility, stability, bioavailability, and targeted delivery. As drug development moves towards more complex molecules like biologics, peptides, and nucleic acids, the demand for sophisticated and versatile lipid-based delivery systems continues to grow. In this context, several future directions and research trends are emerging in lipid-based formulations, focusing on enhanced therapeutic efficacy, reduced toxicity, and more personalized approaches to drug delivery.
Nanostructured Lipid Carriers (NLCs) and Solid Lipid Nanoparticles (SLNs)
Solid lipid nanoparticles (SLNs) and nanostructured lipid carriers (NLCs) are two lipid-based systems that have been widely studied for their ability to improve the delivery of poorly water-soluble drugs. These carriers offer advantages such as controlled release, improved drug stability, and the capacity to encapsulate both lipophilic and hydrophilic drugs.SLNs are composed of solid lipids that remain in the solid state at both room and body temperatures, providing a stable matrix for drug encapsulation. However, they sometimes suffer from limited drug loading and potential drug expulsion during storage. On the other hand, NLCs, a second-generation lipid carrier, are designed with a mixture of solid and liquid lipids. This less-ordered structure allows for higher drug loading, improved stability, and reduced drug leakage during storage.Future research in this area is likely to focus on optimizing the lipid composition, particle size, and surface modifications of NLCs and SLNs to improve their pharmacokinetic properties, targeting capabilities, and safety profiles. The development of multifunctional lipid nanoparticles that can deliver drugs, genes, and imaging agents simultaneously is another exciting avenue for future research.
Lipid-Based Nanoparticles for Gene Therapy and RNA Delivery
The success of lipid nanoparticles (LNPs) in delivering mRNA vaccines, such as those developed for COVID-19, has spurred renewed interest in lipid-based carriers for gene therapy. LNPs provide a platform for delivering nucleic acids such as mRNA, siRNA, and CRISPR components, offering a non-viral alternative for gene therapy applications.Future research is expected to focus on improving the efficiency of LNPs in delivering genetic material to specific tissues and cells. This includes designing LNPs with better targeting capabilities, reduced toxicity, and enhanced stability. For example, the development of ionizable lipids that change charge in response to the pH environment of target cells is an area of active research. These lipids enable the safe delivery of nucleic acids by protecting them during circulation and releasing them once inside the target cell.Gene editing technologies like CRISPR/Cas9 also benefit from LNPs, which can encapsulate the editing machinery and deliver it to specific tissues. Research will continue to refine these LNP-based delivery systems, potentially enabling new treatments for genetic diseases, cancer, and infectious diseases.
Targeted Lipid-Based Drug Delivery
A significant area of growth in LBFs is the development of targeted delivery systems. Targeted delivery enhances drug accumulation at the desired site of action, thereby reducing off-target effects and minimizing systemic toxicity. This is particularly important in cancer therapy, where traditional chemotherapy can damage healthy tissues as well as cancerous ones.One of the primary strategies for targeted delivery involves modifying the surface of lipid nanoparticles with ligands such as antibodies, peptides, or small molecules that recognize specific receptors on the target cells. For instance, lipid nanoparticles can be conjugated with ligands that target cancer cell markers, such as HER2 or folate receptors, enabling the selective delivery of chemotherapeutic agents to tumor cells.Future research in this area will likely focus on identifying new molecular targets and designing smart lipid carriers that can recognize and bind to these targets with high specificity. Moreover, the development of stimuli-responsive lipid-based formulations that release their payload in response to specific physiological triggers, such as pH changes, temperature, or enzymatic activity, is a promising direction for enhancing the precision of drug delivery.
Personalized Medicine and Lipid-Based Formulations
Personalized medicine, which tailors treatments to individual patient characteristics, is an emerging trend in drug development. Lipid-based formulations offer significant potential in this area due to their versatility in encapsulating a wide range of therapeutic agents and their ability to be modified for specific patient needs.Advances in pharmacogenomics and biomarker discovery are enabling the development of lipid-based formulations that can be customized for patients based on their genetic profile, disease state, and other individual factors. This approach can optimize drug efficacy while minimizing adverse effects. For instance, personalized lipid-based carriers could be designed to improve drug delivery to patients with specific mutations in cancer or to adjust the pharmacokinetics of a drug based on an individual's metabolism.Another promising area of research is the use of lipid-based formulations in developing precision vaccines. The ability to deliver nucleic acids, proteins, or adjuvants in a personalized manner could revolutionize vaccine development, enabling the rapid production of vaccines tailored to emerging pathogens or individual immune responses.
Oral Lipid-Based Formulations for Biologics
Oral drug delivery is the most convenient and preferred route of administration, but it remains a challenge for biologics such as peptides, proteins, and nucleic acids due to their poor stability and absorption in the gastrointestinal tract. Lipid-based formulations, particularly self-emulsifying drug delivery systems (SEDDS) and lipid micelles, offer a potential solution by improving the solubility and stability of biologics in the harsh gastrointestinal environment.Current research is focused on developing advanced lipid-based carriers that can protect biologics from enzymatic degradation and promote their absorption across the intestinal epithelium. This includes designing carriers that can bypass first-pass metabolism, enhance lymphatic uptake, and facilitate the transport of biologics across the intestinal barrier.Moreover, the integration of nanotechnology into oral lipid-based formulations, such as the use of nanoliposomes or nanoemulsions, is expected to improve the bioavailability and therapeutic efficacy of biologics delivered via the oral route. These advancements could open up new possibilities for the oral delivery of vaccines, monoclonal antibodies, and gene therapies.
Sustainable and Biodegradable Lipid-Based Formulations
With growing concerns over the environmental impact of pharmaceutical products, there is increasing interest in developing sustainable and biodegradable lipid-based formulations. Traditional lipid-based carriers, such as those based on synthetic or non-biodegradable materials, can pose environmental risks due to their persistence in the ecosystem.Future research is likely to focus on designing biodegradable lipid carriers that can degrade into non-toxic byproducts after fulfilling their drug delivery function. Natural lipids such as those derived from plant oils, fatty acids, and phospholipids are being explored as sustainable alternatives to synthetic materials. These natural lipids offer the added benefit of being biocompatible and less likely to induce immune responses.In addition to improving the sustainability of lipid-based formulations, researchers are also investigating the use of green chemistry principles in the production and processing of these carriers. This includes reducing the use of harmful solvents, minimizing energy consumption, and developing eco-friendly manufacturing processes.
Lipid-Based Formulations for Central Nervous System (CNS) Drug Delivery
The central nervous system (CNS) poses significant challenges for drug delivery due to the blood-brain barrier (BBB), which restricts the entry of most therapeutic agents into the brain. Lipid-based formulations are being developed to improve the delivery of CNS drugs by enhancing their ability to cross the BBB and reach the brain.Current research focuses on designing lipid nanoparticles that can exploit natural transport mechanisms, such as receptor-mediated transcytosis, to facilitate drug delivery across the BBB. Additionally, the use of surface modifications, such as attaching ligands that target BBB receptors, is being explored to enhance the specificity and efficiency of CNS drug delivery.In the future, lipid-based formulations could play a crucial role in the treatment of neurological disorders, including Alzheimer's disease, Parkinson's disease, and brain cancers. By improving the targeting and delivery of drugs to the CNS, these formulations hold the potential to improve therapeutic outcomes for patients with these challenging conditions.
Hybrid Lipid-Polymer Systems
Another emerging trend in lipid-based drug delivery is the development of hybrid systems that combine the advantages of both lipids and polymers. These systems can be tailored to provide specific drug release profiles, improve drug stability, and enhance the targeting capabilities of the carrier.For example, lipid-polymer hybrid nanoparticles (LPHNPs) combine the biocompatibility and drug solubilization capabilities of lipids with the mechanical strength and controlled release properties of polymers. These hybrid systems can be engineered to deliver a wide range of therapeutic agents, from small molecules to biologics, and offer potential applications in cancer therapy, gene delivery, and vaccine development.
The future of lipid-based formulations is marked by exciting developments that promise to address many of the current limitations in drug delivery. From advanced nanocarriers and targeted delivery systems to sustainable and personalized approaches, lipid-based formulations are poised to play a critical role in shaping the next generation of pharmaceutical therapies. As research continues to evolve, these technologies will likely lead to more effective, safer, and patient-friendly treatment options for a wide range of diseases.
Conclusion
Lipid-based formulations (LBFs) have established themselves as a pivotal component in modern pharmaceutical science, particularly for overcoming the challenges associated with poorly water-soluble drugs. Their ability to enhance solubility, stability, and bioavailability makes them indispensable in the formulation of a wide range of therapeutic agents, from small-molecule drugs to biologics. With the rise of nanotechnology and advanced drug delivery systems, LBFs have evolved beyond simple carriers into sophisticated platforms that offer controlled release, targeted delivery, and protection of sensitive molecules.In recent years, LBFs have shown great promise in addressing critical challenges such as drug permeability, first-pass metabolism, and delivery across biological barriers like the blood-brain barrier. Their application extends into various fields, including cancer therapy, gene therapy, and vaccine development, demonstrating their versatility and potential for innovative treatments.Future research is set to refine LBFs further, with a focus on personalized medicine, sustainable materials, and hybrid systems that combine the benefits of lipids and polymers. Advances in this area will not only improve drug efficacy but also minimize side effects and reduce the environmental impact of pharmaceutical products.lipid-based formulations are at the forefront of modern drug delivery technologies. Their continued development will play a crucial role in advancing therapeutic strategies and expanding the possibilities for more effective, patient-friendly treatments. As the pharmaceutical landscape evolves, LBFs will remain a key driver of innovation and improved patient outcomes.
REFERENCES
- Gaudana R, Ananthula HK, Parenky A, Mitra AK. Ocular drug delivery. AAPS J. 2010;12(3):348-360. doi:10.1208/s12248-010-9183-3.
- Rathore KS, Nema RK. Review on ocular inserts. Int J PharmTech Res. 2009;1(2):164-169.
- Sultana Y, Jain R, Aqil M, Ali A. Review of ocular inserts: Achievements and limitations. Drug Discov Today. 2006;11(5-6):207-17. doi:10.1016/j.drudis.2006.01.039.
- Mittal S, Mishra B. Formulation and in vitro evaluation of gum-based ocular drug delivery systems for controlled release of ciprofloxacin hydrochloride. AAPS PharmSciTech. 2014;15(2):410-9. doi:10.1208/s12249-013-0064-6.
- Rathore MS, Majumdar DK. Design and evaluation of xanthan gum-based ocular delivery systems. Int J Pharm. 2006;336(1):14-8. doi:10.1016/j.ijpharm.2006.01.014.
- Patel A, Patel NM. Preparation and characterization of sulfoxy amine-modified xanthan gum as a potential excipient for drug delivery. Int J Biol Macromol. 2020;144:828-36. doi:10.1016/j.ijbiomac.2019.12.139.
- Vashisth P, Sharma V, Gupta A, Kumar S, Kumar M, Sinha VR. Synthesis and evaluation of modified xanthan gum for ophthalmic applications. Carbohydr Polym. 2012;87(2):1707-1713. doi:10.1016/j.carbpol.2011.09.063.
- Gandhi P, Rathod H, Patel S. A review on: Ocular inserts, a novel drug delivery system. Asian J Pharm Res Dev. 2013;1(1):40-48. Available from: https://ajprd.com/index.php/journal/article/view/6?:contentReference[oaicite:0]{index=0}?:contentReference[oaicite:1]{index=1}.
- Tian B, Bilsbury E, Doherty S, Teebagy S, Wood E, Su W, Gao G, Lin H. Ocular drug delivery: advancements and innovations. Pharmaceutics. 2022;14:1931. doi:10.3390/pharmaceutics14091931.
- Jadhav RL, Patil MV, Shaikh SN. Synthesis, characterization and in vivo evaluation of poly sulfoxy amine grafted xanthan gum: pharmaceutical science-chemistry for new drug discovery. Int J Life Sci Pharma Res. 2022;10(3):20-28. doi:10.22376/ijpbs/lpr.2020.10.3.P20-28.
- Patel J, Maji B, Narayana Moorthy NSH, Maiti S. Xanthan gum derivatives: review of synthesis, properties and diverse applications. RSC Adv. 2020;10:27103-27136. doi:10.1039/D0RA04366D
- Makhmal Zadeh SM, Esfahani G, Salimi A. Permeability of ciprofloxacin-loaded polymeric micelles including ginsenoside as P-glycoprotein inhibitor through a Caco-2 cells monolayer as an intestinal absorption model. Molecules. 2018;23(8):1904. doi:10.3390/molecules23081904.
- Khan GJ, Khan RA, Majeed I, Siddiqui FA, Khan S. Ciprofloxacin: the frequent use in poultry and its consequences on human health. Prof Med J. 2015;22(1):1-5. doi:10.29309/TPMJ/2015.22.01.1403
- Bhadru B, Rao TR, Jadav B. UV spectrophotometric method development and validation for metformin hydrochloride in bulk and its tablet formulation. Chem Res J. 2023;8(6):10-15.
- Uhljar LE, Kan SY, Radacsi N, Koutsos V, Szabó-Révész P, Ambrus R. In vitro drug release, permeability, and structural test of ciprofloxacin-loaded nanofibers. Pharmaceutics. 2021;13(4):556. doi:10.3390/pharmaceutics13040556.
- Gupta P, Rani S, Singh D, et al. Formulation and characterization of ocular inserts containing gatifloxacin. Int J Pharm Sci Res. 2014;5(4):1180-1188.
- Rao P, Srinivasan K. Development and characterization of mucoadhesive ocular inserts of diclofenac sodium. J Drug Deliv Sci Technol. 2017;40:60-68. https://doi.org/10.1016/j.jddst.2017.04.022.
- Das S, Saha A, Saha S, et al. Design and evaluation of ocular inserts for the controlled release of moxifloxacin. Asian J Pharm Clin Res. 2015;8(3):109-115.
- Yeoh JQ, Perveen N, Khan NH. Comparative purity study of UV spectrophotometric and Fourier-transform infrared spectroscopic (FTIR) techniques for the determination of ciprofloxacin hydrochloride tablets. Biomed J Sci Tech Res. 2020;32(3): ISSN: 2574-1241. doi:10.26717/BJSTR.2020.32.005246.
- Jadhav RL, Patil MV, Shaikh SN. Synthesis, characterization, and in vivo evaluation of poly sulfoxy amine grafted xanthan gum: pharmaceutical science-chemistry for new drug discovery. Int J Life Sci Pharma Res. 2022;10(3):20-28. doi:10.22376/ijpbs/lpr.2020.10.3.P20-28.
- Jadhav R. Synthesis, Characterization and Invivo Evaluation of Poly Sulfoxy Amine Grafted Xanthan Gum. Int J Life Sci Pharma Res. 2020;10(3):20-28. doi:10.22376/ijpbs/lpr.2020.10.3.P20-28.
- Ara T, Sharma S, Bhat A. Preparation and evaluation of novel ocular inserts of Diclofenac sodium amino acids conjugate for controlled drug delivery. Int J Sci Res Publ. 2015;5(6).
- Mirzaeei S, Taghe S, Alany RG, Nokhodchi A. Eudragit® L100/Polyvinyl Alcohol nanoparticles impregnated mucoadhesive films as ocular inserts for controlled delivery of erythromycin: Development, characterization and in vivo evaluation. Biomedicines. 2022;10(8):1917. doi:10.3390/biomedicines10081917.
- Jadhav RL, Sonwalkar SG. Formulation optimization and evaluation of ocular inserts prepared with sulfoxyamine modified chitosan. Asian J Pharm Sci. 2020;14(2):195-205.
- Shweta K, Shubham S, Satish S. Formulation and Evaluation of Ocular Inserts of Ciprofloxacin as Drug Delivery System. Int J Res Analytical Rev. 2020;7(2). E-ISSN 2348-1269, P-ISSN 2349-5138.
- Mundada AS, Shrikhande BK. Design and evaluation of soluble ocular drug inserts for controlled release of ciprofloxacin hydrochloride. Indian Drugs. 2006;32(4):443-8.
- Shrirao PD, Rathod NS, Kale AS, Aware NP. Ciprofloxacin ocuserts: design, formulation and evaluation. IJNRD. 2024;9(3):1-5.
- Pillai P, et al. Formulation and evaluation of ocular inserts of ciprofloxacin using natural and synthetic polymers. J Pharm Sci Technol. 2016;8(5):1075-82.
- Bhanja S, Das S, Bhunia M, et al. Formulation and Evaluation of Ocular Inserts of Timolol Maleate using Various Polymers. Int J Drug Dev Res. 2018;10(1):1-9. doi:10.21776/ub.ijddr.2018.10.1.1000.
- Patil A, Dhaneshwar S. Development and Evaluation of Ocular Inserts for Controlled Drug Delivery. Asian J Pharm Clin Res. 2021;14(3):138-143. doi:10.22159/ajpcr.2021.v14i3.42688.


 Dr. K. Rajaganapathy*
Dr. K. Rajaganapathy*
 Vidhyalakshmi R.
Vidhyalakshmi R.



 10.5281/zenodo.14628636
10.5281/zenodo.14628636