Abstract
Nanotechnology has emerged as a transformative field in medicine, particularly through the development of nanosuspensions. Nano refers to particles sized between 1-100 nm. Nanosuspensions are a key aspect of nanotechnology, addressing the issue of poor aqueous solubility in many drugs. Drug nanosuspensions offer a universal formulation approach to enhance the therapeutic performance of these drugs across various administration routes. A nanosuspension is defined as a finely colloidal, biphasic dispersion of solid drug particles in an aqueous vehicle with particle sizes below 1 µm. These particles are stabilized by surfactants and polymers and are prepared through suitable methods for drug delivery applications. Various methods used in the formulation of nanosuspension can be classified into top-down and bottom-up approaches. Wet milling, high-pressure homogenization, anti-solvent techniques, melt emulsification, supercritical fluid extraction and ultrasonic homogenization are some of the methods for the preparation of nanosuspension. Nanosuspensions can be administered via oral, topical, parenteral, ocular and pulmonary routes. The advantages of nanosuspensions include improved drug dispersibility and solubilization, increased therapeutic efficacy and reduced toxicity. This review emphasizes various advantages and disadvantages of nanosuspension, its methods of preparation, formulation approaches, evaluation parameters, pharmaceutical applications, marketed products and patents of nanosuspension.
Keywords
nanosuspension, bioavailability, solubility, surfactants and polymers
Introduction
In recent years, more than 40% of the new chemical moieties being generated by drug discovery projects are lipophilic in nature or poorly soluble in water. Developing poorly water- soluble drug has always been a challenging issue confronted by the pharmaceutical researchers. to tackle this issue, nano sized formulation of these compounds can be implemented to all drug compounds belonging to biopharmaceutical classification system (BCS) classes II and IV to increase their solubility and attaining higher bioavailability1 There are many conventional methods for increasing the solubility of poorly soluble drugs, which include micronization, solubilization using cosolvents, salt form, surfactant dispersions, precipitation technique and
Oily solution. Other techniques such as liposomes, emulsions, microemulsion, solid dispersion and inclusion complexation using cyclodextrins can enhance the drug solubility markedly. However, these methods have their limitations such as a large amount of additives that may induce stability and toxicity issues. therefore, they are frequently not ideal for clinical treatment. Since nanosuspension drug delivery system was firstly developed in 1994, nanosuspension has attracted more attention as a formation solution for the poorly soluble drugs
A Nanosuspension is a submicron colloidal dispersion of drug particles. A pharmaceutical nanosuspension is defined as very finely colloid, biphasic, dispersed, solid drug particles in an aqueous vehicle, without any matrix material, stabilized by surfactants and polymers, prepared by suitable methods for drug delivery applications, through various routes of administration like oral, topical, parenteral, ocular and pulmonary routes2. Nanosuspensions differ from nanoparticles average particle size is between 200 and 600 nm3. various methods which are generally used to prepare nanosuspension are bottom-up including precipitation and top-down including media milling, emulsion solvent diffusion method, supercritical fluid method, dry co-grinding, high-pressure homogenization, Nano edge. Nanosuspension have two particular advantageous properties, firstly increased saturation solubility and secondly the enlarged surface. Both properties result in an increase in the dissolution rate according to the Noyes-Whitney Law, in general it is advantageous to use nanoparticles that are as small as possible to achieve a maximum improvement in the oral bioavailability or a very rapid dissolution rate4
Need of nanosuspension
In case of drugs that are insoluble in both water and inorganic media instead of using lipidic systems, nanosuspensions are used as a formulation approach. It is most suitable for the compounds with high log P value, high melting point and high dose. Nanosuspensions can be used to enhance the solubility of drugs that are poorly soluble in aqueous as well as lipid media. As a result, the rate of flooding of the active compound increases and the maximum plasma level is reached faster (e.g., oral or intravenous (IV) administration of the nanosuspension). This is one of the unique advantages that it has over other approaches for enhancing solubility. It is useful for molecules with poor solubility, poor permeability or both which poses a significant challenge for the formulators.5
Nanosuspension advantages6
- Reduced tissue irritation in the case of subcutaneous/intramuscular administration
- Enhanced dissolution rate and saturation solubility of the drug.
- Enhance the physical and chemical stability of drugs
- Higher drug loading can be achieved.
- Higher bioavailability for ocular administration and drug delivery by inhalation
- Nanosuspensions can be incorporated in tablets, pellets, hydrogel and suppositories are suitable for various routes of administration.
- Maintenance of drug release over a prolonged period of time
Disadvantages of nanosuspension
- Physical stability, sedimentation & compaction cause problems.
- It is bulky sufficient care must be taken during handling & transport.
- Uniform & accurate dose cannot be achieved.
Criteria for selection of drug for nanosuspension: -
- API should be water insoluble but soluble in oil phase
- API is insoluble in both water and oil
- Drug should have reduced affinity of the crystal to dissolve irrespective of solvent
- API with very bulky dose
- Drugs with low bioavailability, either due to solubility or permeability issues,
- Drugs with a short to moderate half-life are often better candidates for nanosuspensions because the formulation can help extend the duration of drug action and improve bioavailability.7
Formulation consideration of nanosuspension
The preparation of nanosuspension involves the following agents:
- Stabilizer
- Organic solvents
- Surfactant
- Co-surfactant
- Other additives
Stabilizer:
The main function of a stabilizer is to wet the drug particles thoroughly and to prevent Ostwald’s ripening and agglomeration of Nanosuspensions in order to yield a physically stable formulation by providing steric or ionic barrier. The type and amount of stabilizer has a pronounced effect on the physical stability and in vivo behavior of nanosuspension. Stabilizers that have been used are poloxomers, polysorbate, cellulosic, povidones and lecithins.8
Organic solvents:
Organic solvents are used in the formulation of Nanosuspension if emulsions or micro emulsions are used as a template. The pharmaceutically acceptable less hazardous water miscible solvent, such as ethanol, chloroform, propanol, carbonate, benzyl alcohol, are preferment in the formulation over the conventional hazardous solvents, such as dichloromethane and methanol.
Surfactants
Surfactants play a crucial role in the formulation of nanosuspensions as they help stabilize the small drug particles, prevent aggregation, and enhance the solubility and bioavailability of poorly soluble drugs. In nanosuspension formulations, surfactants reduce surface tension and provide steric or electrostatic stabilization to the nanoparticles.
Commonly Used Surfactants in Nanosuspensions:10.11
1. Non-ionic Surfactants:
-
-
- Polysorbates (e.g., Tween 20, Tween 80): Commonly used for their biocompatibility and effective steric stabilization.
- Polyvinyl Alcohol (PVA):
Provides good steric stabilization and is widely used in formulations.
-
-
- Pluronic (Poloxamer):
A block copolymer with both hydrophilic and hydrophobic segments, ideal for steric stabilization.
-
-
- Polyethylene Glycol (PEG):
Used to enhance stability and prolong the circulation time of nanosuspensions by creating a hydrophilic barrier around particles.
2. Ionic Surfactants:
-
-
- Sodium Lauryl Sulfate (SLS):
An anionic surfactant that provides electrostatic stabilization, commonly used in nanosuspension formulations.
-
-
- Docusate Sodium:
Another anionic surfactant that helps in reducing surface tension and stabilizing nanoparticles.
-
-
- Benzalkonium Chloride:
A cationic surfactant used for electrostatic stabilization, though less common due to potential toxicity.
3. Amphiphilic Stabilizers:
i. Lecithin (Phospholipids):
Natural surfactants that have both hydrophilic and hydrophobic parts, commonly used in parenteral nanosuspension formulations for biocompatibility and safety.
- Cremophor EL:
A non-ionic surfactant derived from castor oil, often used in formulations to improve the solubility and stability of hydrophobic drugs.
Co-surfactants:
The influence of cosolvent should be monitored for selected nanosuspension composition, which depends on various factors like uptake of the internal phase and drug loading. After going through the literature, it describes certain cosurfactants for various stabilizers can be safely used in the formulation of microemulsion, co-surfactant such as salts (dipotassium glycyrrhizinate) can be safely used with stabilizers like transcutol, glycofurol, ethanol, and isopropanol.12
Other additives:
Different type of additives are used in the formulation of nanosuspensions such as, osmogent, cryoprotectant, polyols, buffers and salts which depends upon either the route of administration or properties of the drug moiety.13
Methods of preparation of nanosuspension
Nanosuspensions can be prepared using bottom-up and top-down approaches (Fig.1) These methodologies are widely applied in nanomedicine due to its consistency to produce nanoparticles of controlled aspect ratio, as shown in (Fig.1) The bottom-up methods are called as conventional precipitation methods whereas the top-down methods are referred to as disintegration methods.14,18.
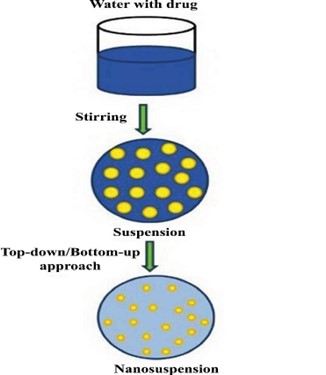
Fig 1. Manufacturing process of nanosuspension
Top-down techniques
The top-down process involves the disintegration from large particles, microparticles to nanosized particles. The top-down techniques further classified are
- Media milling
- High-pressure homogenization Nanoedge
- Nanopure
- Dry-co-grinding
Media milling (Nano-crystals)
Nanosuspensions are formulated by high shear media mills or pearl mills. It consists of milling chamber, recirculation chamber and milling shaft. Milling media consists of balls or pearls which are made up of ceramic sintered aluminium oxide or zirconium oxide. Milling chamber charged with milling media, water, drug and stabilizer. Balls rotated at high shear rate under control temperature the balls have an impact on the sample. Due to the both forces of friction and impact particle size reduction occurs and nanosized particles will obtained.15
High-pressure homogenation(dissocubes)
The high-pressure homogenization method is the most widely used technique for preparing nanosuspensions of many poorly water-soluble drugs. Several methods have been developed based on this principle, including Discubes, Nanopure, Nanoedge, and Nanojet technologies. In high-pressure homogenization, a suspension of a drug and surfactant is forced through a nanosized aperture valve of a high-pressure homogenizer. This method relies on cavitation in the aqueous phase, where the forces generated are strong enough to break down drug microparticles into nanoparticles. However, this method has certain limitations, such as the requirement for small sample particles before loading and the need for multiple homogenization cycles.16
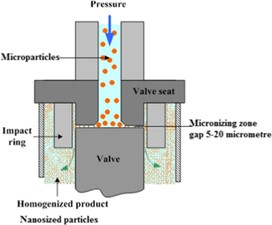
Fig 2. High pressure homogenation process
Nanoedge
The drug is dissolved in an organic solvent and this solution is mixed with a miscible anti-solvent for precipitation. In the water-solvent mixture, the solubility is low and the drug precipitates. Precipitation has also been coupled with high shear processing. The basic principles of Nanoedge are the same as that of precipitation and homogenization. This is accomplished by a combination of rapid precipitation and high-pressure homogenization. The Nanoedge patented technology by Baxter depends on the precipitation of friable materials for fragmentation under conditions of high shear and/or thermal energy. Rapid addition of a drug solution to an anti-solvent leads to sudden super saturation of the mixed solution, and generation of fine crystalline or amorphous solids. Precipitation of an amorphous material may be favored at high super saturation when the solubility of the amorphous state is exceeded. The basic principles of Nanoedge are the same as that of precipitation and homogenization. A combination of these techniques results in smaller particle size and better stability in a shorter time. The major drawback of the precipitation technique, such as crystal growth and long-term stability, can be resolved using the Nanoedge technology
Nanopure:
It involves homogenization in water mixtures or water free media and is prepared for the thermolabile compound. Nano pure is also called as deep freezing because homogenization of drug suspension is carried out in non-aqueous media at 0?.
Dry co-grinding
Stable nanosuspensions can be prepared using the dry co-grinding technique with various polymers and copolymers such as polyvinyl pyrrolidone, hydroxypropyl methylcellulose (HPMC), polyethylene glycol, sodium dodecyl sulfate, and cyclodextrin derivatives. Unlike wet grinding processes, dry grinding methods are more economical and do not require the addition of toxic solvents. A significant advantage of this method is the enhancement of surface polarity and the conversion of a large portion of the drug's crystalline state to an amorphous form. This controlled stability of the amorphous phase can greatly improve the saturation solubility and dissolution rate of poorly soluble drug nanosuspensions.17

Fig 3. Methods of preparation nanosuspension
Bottom-up technique:
It is the technique in which nano size is obtained by increasing the size of particle from molecular range to nano range. The convectional method of precipitation (Hydrosol) are called as Bottom up techniques. Using a precipitation technique, the drug is dissolved in an organic solvent & this solution is mixed with miscible anti-solvent. In the water solvent mixture, the solubility is low & drug precipitates. Basic challenge is that during the precipitation procedure growing of the crystals need to be controlled by addition of surfactant to avoid formation of microparticles. The use of simple & low cost equipments is the advantage of bottom up technique. But the drug needs to be soluble in at least one solvent & the solvent needs to be miscible with non-solvent. Moreover, it is not applicable to the drugs, which are poorly soluble in both aqueous & non-aqueous media.
Precipitation (solvent-antisolvent method) method:
The precipitation method is commonly used to prepare submicron particles, particularly for poorly soluble drugs. Initially, the drug is dissolved in a solvent and then mixed with a miscible antisolvent in the presence of surfactants. Rapid addition of the drug solution to the antisolvent causes sudden supersaturation, resulting in the formation of ultrafine crystalline or amorphous drug solids. This method involves two phases: nucleation and crystal growth. To achieve a stable suspension with minimal particle size, a high nucleation rate and a low growth rate are required, both of which are temperature-dependent. The drug must be soluble in at least one solvent that is miscible with the nonsolvent.
Advantages:
- Simple process
- Ease of scale-up
- Economical production
- Disadvantages
- Crystal growth must be limited by the addition of surfactants
- The drug must be soluble in at least one solvent
Supercritical fluid process:
This method utilizes solubilization and nanosizing technologies through the supercritical fluid process for particle size reduction. Supercritical fluids (SCF) are non-condensable dense fluids whose temperature and pressure are greater than their critical temperature (Tc) and critical pressure (TP). This process allows the micronization of drug particles to the submicron level. Recent advances in the SCF process are to create a nanoparticulate suspension of particle size of 5 to 200nm in diameter. The low solubility of poorly water-soluble drugs and surfactants in supercritical CO2 and the high pressure required for these processes restrict the utility of this technology in the pharmaceutical industry18
Microemulsion as template/lipid emulsion:
Microemulsions are thermodynamically stable and isotropically clear dispersions of two immiscible liquids, such as oil and water, stabilized by an interfacial film of surfactant and co-surfactant. Their advantages, such as high drug solubilization, long shelf-life and ease of manufacture, make them an ideal drug delivery vehicle. Taking advantage of the microemulsion structure, one can use microemulsions even for the production of nanosuspensions. Oil-in-water microemulsions are preferred for this purpose. The internal phase of these microemulsions could be either a partially miscible liquid or a suitable organic solvent, as described earlier. The drug can be either loaded in the internal phase or pre-formed microemulsions can be saturated with the drug by intimate mixing. The suitable dilution of the microemulsion yields the drug nanosuspension by the mechanism described earlier. The influence of the amount and ratio of surfactant to co-surfactant on the uptake of internal phase and on the globule size of the microemulsion should be investigated and optimized in order to achieve the desired drug loading. The nanosuspension thus formed has to be made free of the internal phase and surfactants by means of diultrafiltration in order to make it suitable for administration19
Emulsion as template:
In addition to their use as drug delivery vehicles, emulsions can also serve as templates to produce nanosuspensions. This approach is suitable for drugs that are soluble in volatile organic solvents or partially water-miscible solvents, which can be used as the dispersed phase of the emulsion. There are two primary methods for creating drug nanosuspensions via emulsification. In the first method, an organic solvent or a mixture of solvents containing the drug is dispersed in an aqueous phase with appropriate surfactants to form an emulsion. The organic phase is then evaporated under reduced pressure, causing the drug particles to precipitate instantaneously, forming a nanosuspension stabilized by surfactants. Since one particle forms in each emulsion droplet, the particle size of the nanosuspension can be controlled by managing the size of the emulsion. Optimizing the surfactant composition enhances the intake of the organic phase and ultimately increases drug loading in the emulsion. While organic solvents like methylene chloride and chloroform were originally used, concerns about environmental hazards and human safety have limited their use in routine manufacturing processes. However, relatively safer solvents such as ethyl acetate and ethyl formate can still be considered for use.2,20.
Evaluation parameter
Mean Particle Size and Particle Size Distribution:
The mean particle size and the span of the particle size distribution (polydispersity index, PI) are two important characteristic parameters because they affect the saturation solubility, dissolution rate, physical stability, even in-vivo behaviour of nanosuspensions. The particle size distribution can be determined by photon correlation spectroscopy (PCS), laser diffraction (LD), and coulter counter multisizer. PCS can even be used for determining the width of the particle size distribution (polydispersity index, PI). The PI is an important parameter that governs the physical stability of Nano suspensions and should be as low as possible for the long-term stability of Nano suspensions. a PI value of 0.1– 0.25 indicates a fairly narrow size distribution whereas a PI value greater than 0.5 indicates a very broad distribution.21
Particle Charge (Zeta Potential):
Zeta potential gives certain information about the surface charge properties and furthers the long-term physical stability of the nanosuspension. Particle charge determines the stability of Nano suspension. For electrostatically stabilized Nano suspension a minimum zeta potential of ±30mV and for combined steric and electrostatic stabilization it should be a minimum of ± 20Mv.
Crystal Morphology:
To characterize the polymorphic changes due to the impact of high- pressure homogenization in the crystalline structure of the drug, techniques like X-ray diffraction analysis in combination with differential scanning calorimetric or differential thermal analysis can be utilized. The change in the solid-state of the drug particles as well as the extent of the amorphous fraction can be determined by X-ray diffraction analysis and supplemented by differential scanning calorimetry. To get an actual idea of particle morphology, scanning electron microscopy is preferred.22
Dissolution Velocity and Saturation Solubility:
Nanosuspensions have a critical gain over other strategies, that it could increase the dissolution pace in addition to the saturation solubility. These parameters have to be determined in diverse physiological answers. The assessment of saturation solubility and dissolution velocity facilitates in figuring out the In-vitro behavior of the system. Böhm et al. suggested an increase within the dissolution pressure as well as dissolution pace with a discount in the particle length to the nanometer variety. size reduction results in an increase in the dissolution stress.23
In vitro drug release:
Studies of the release of drugs In vitro were formed in a dissolution apparatus using paddle method at 50 rpm rotational speed. The dissolution medium volume and temperature were 900 ml, and 37.0±0.2 ° C, respectively. Samples were collected at fixed times and were filtered using filter of 0.45µm. The amount of drug dissolved can be determined by measuring absorbance using UV spectroscopy or HPLC. Establishment of an IVIVC, described as a correlation between In vitro release and In vivo behavior, enhances the utility of an In vitro study. Water, aqueous surfactant solutions, buffer solutions (PH 6.8&7.4) or other simulated biological fluids can be chosen as dissolution media24
Drug content:
Drug content of nanosuspension formulation can be carried out by extracting the nanosuspension in suitable solvent mixture, like Methanol: THF (1:1) mixture, shaken well and then centrifuged. The supernatants can be separated and diluted with same solvent mixture and the absorbance can be measured at suitable ? max. The drug content then can be calculated using the calibration curve.25
Osmolarity:
The osmolarity of nanosuspension can be measured by using Osmometer. Intravenous dosage form should be iso-osmolar with the blood so the nanosuspension formulation checked for osmolarity. Practically osmolarity was measured using an osmometer.
Surface Hydrophilicity:
For intravenously injected nanosuspensions, additional parameters need to be determined which affect the In vivo fate of the drug nanoparticles. Surface hydrophilicity / hydrophobicity is considered as one of the important parameters affecting the In vivo organ distribution after i.v. injection. The surface hydrophobicity determines the interaction with cells prior to phagocytosis and in addition, it is a relevant parameter for the adsorption of plasma proteins the key factor for organ distribution. To avoid artifacts, the surface hydrophobicity needs to be determined in the original environment of the drug nanoparticles, which means in aqueous dispersion medium.
Stability studies:
Because of the reduction in size, the surface area of the suspended particle becomes very large. An increase in surface area causes an increase in surface free energy. This enhanced surface free energy makes nanosuspension's stability issue worse. Nanosuspension particles try to reduce the surface free energy, in order to do so particles form agglomeration and crystal growth starts. Hence to reduce agglomeration and increase stability different stabilizers are used. The accelerated stability testing recommendations of ICH can be used to study the stability of nanosuspension. Thermal cycling may be used to store nanosuspensions under various stress conditions at temperatures such as 15, 25, 35, and 45 °C. The effect of thermal cycling on mean particle size can also be periodically investigated.26
Applications of nanosuspension

Fig 4. Applications of Nanosuspensions
Oral drug delivery
In the conventional dosage form (i.e oral drug administration) there are number of problems such as solubility is poor, inadequate dissolution and does not have sufficient efficacy. To overcome these problem oral nanosuspension is used, particle size should be very small and large surface area as compare to conventional which help to increase the bioavailability and solubility of poorly soluble drugs (BCS class-II). advantages of Nanosuspension like high drug loading, enhanced solubility, enhanced stability (physical and chemical) and enhanced bioavailability. with the help of simple manufacturing techniques Nanosuspension can be easily incorporated in to numerous dosage forms such as tablets, capsules. Recently Jeong et al developed a new celecoxib (CXB)- loaded nanosuspensions, various nanosuspensions were set up with different polymers and surfactants utilizing a wet media milling methods, therefore determined the particle size. the pharmacokinetic results of prepared formulation are compared with powder form of same drug. the nanosuspension under the appropriate conditions showed the particle size 190 nm, which is increase physical stability (8 week). this Nanosuspension showed higher plasma concentration and AUC rate which is compared with powder form of conventional product. therefore, nanosuspension form of CXB- loaded is increase stability and oral bioavailability27
Parenteral Drug Delivery
One of the important applications of nanosuspension technology is the formulation of intravenously administered products. IV administration results in several advantages, such as administration of poorly soluble drugs without using a higher concentration of toxic co-solvent, improving the therapeutic effect of the drug available as convectional oral formulations & targeting the drug to macrophages & the pathogenic micro-organism residing in the microphages. Injectable nanosuspensions of poorly soluble drug tarazepide have been prepared to overcome the limited success achieved using convectional solubilizing techniques, such as use of surfactants, cyclodextrins etc. To improve bioavailability11
Pulmonary Drug Delivery
Nanosuspensions may prove to be an ideal approach for delivering drugs that exhibit poor solubility in pulmonary secretions. Aqueous nanosuspensions can be nebulized using mechanical or ultrasonic nebulizers for lung delivery. Because of their small size, it is likely that in each aerosol droplet at least one drug particle is contained, leading to a more uniform distribution of the drug in lungs. The nanoparticulate nature of the drug allows the rapid diffusion and dissolution of the drug at the site of action. At the same time, the increased adhesiveness of the drug to mucosal surfaces offers a prolonged residence time for the drug at the absorption site. This ability of nanosuspensions to offer quick onset of action initially and then controlled release of the active moiety is highly beneficial and is required by most pulmonary diseases. Budesonide drug nanoparticles were successfully delivered using an ultrasonic nebulizer28
Targeted Drug Delivery:
Targeted drug delivery can be used for the anti?mycobacterial, fungal, or leishmanial drugs to macrophages if the infectious pathogen is persisting intracellular. The further plan of action for targeted drug delivery system is by using various surface coatings for active or passive targeting., atovaquone nanosuspension concentration in brain, lungs, sera, liver is high and has improved therapeutic efficacy against toxoplasma encephalitis in murine Mold infected with toxoplasma gonidia.29
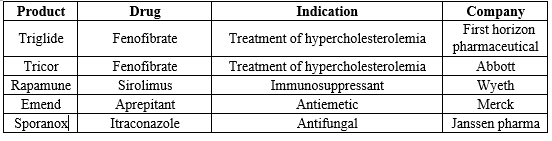
Tab No 1: Current Marketed Pharmaceutical Products Based on Nanosuspensions30
CONCLUSION
In conclusion, the utilization of nanosuspension technology provides a viable strategy for developing formulations of poorly soluble drugs, ultimately leading to enhanced bioavailability. Large-scale production techniques, including media milling and high-pressure homogenization, have proven successful in the manufacturing of nanosuspensions. Moreover, nanosuspensions can be tailored for diverse routes of administration, encompassing oral, parenteral, ocular, topical, and pulmonary routes. Given the escalating number of molecules facing solubility and bioavailability challenges, nanotechnology emerges as a pivotal tool in drug discovery initiatives, facilitating the improvement of aqueous solubility and bioavailability for poorly soluble drugs.
ACKNOWLEDGMENT:
We, the authors are very much thankful to SJM College Of Pharmacy, chitradurga. karnataka), for their support to make this article with full of information in every aspect.
REFERENCES:
- Azimullah S, Sudhakar CK, Kumar P, Patil A, Usman MR, Usman MZ, Jain BV. Nanosuspensions as a promising approach to enhance bioavailability of poorly soluble drugs: An update. Jour of Drug Deliv and Therapeutics. 2019; 9(2):574-82
- Quadri N, Abdullah MM. Review on polyherbal nanosuspension and approaches to enhance solubility of drugs. W Jour of Pharma Res. 2023;12(10) 313-35.
- Nayak B, Mohanty B, Roy H, Patnaik A. Nanosuspension: bioavailability enhancing novel approach. Inte Jour of Pharm and Bio Sciences. 2018; 1(3);8-16.
- Pawar SS, Dahifale BR, Nagargoje SP, Shendge RS. Nanosuspension technologies for delivery of drugs. Nano sci Nano tech Res. 2017;4(2):59-66.
- Rahman A, Hussain A, Iqbal Z, Kumar Harwansh R, Ratnakar Singh L, Ahmad S. Nanosuspension: a potential nanoformulation for improved delivery of poorly bioavailable drug. Micro and Nanosystems. 2013; 5(4):273-87.
- Haranath C, Ahad HA, Kalpana K. Nanosuspension as promising and potential drug delivery: a review. Int J Pharma Bio Sci. 2021;11(1):59-66.
- Prasad RD, Charmode N, Shrivastav OP, Prasad SR, Moghe A, Sarvalkar PD, Prasad NR. A review on concept of nanotechnology in veterinary medicine. ES Food & Agroforestry. 2021:11(4):28-60.
- Srinivasakumari C, Sireesha P, Nafees SS, Jagadeesh P, Dasthagiri S, Basha KS. Nanosuspension–an effective approach for solubility enhancement. World Jour of Pharma Res. 2016; 5(9):1518-44.
- Jayaprakash R, Krishnakumar K, Dinesh Kumar B, Jose R, Nair SK. Nanosuspension in drug delivery-A review. Sch. Acad. J. Pharm. 2016; 5:138-41.
- Banavath H, Sivarama RK, Ansari T, Ali S, Pattnaik G. Nanosuspension: an attempt to enhance bioavailability of poorly soluble drugs. Inter jour of pharm scie and research. 2010;1(9):119-26
- Mane AN, Gilda SS, Ghadge AA, Bhosekar NR, Bhosale RR. Nanosuspension A Novel Carrier For Lipidic Drug Transfer. Scholars Aca Jour of Phar. (SAJP) ISSN. 2014;3(1):2320-4206
- Tejas P, Prasad N, Shivraj J, Dhananjay P. Review on nanosuspension in drug delivery. Inter Jour of Rec Advas in Multidisc Rese. 2022;9(1) 7442-7450
- Wanole OS. Review on nanosuspension. World Journal of Pharmaceutical Research. 2023; 12(3): 282-307
- Patil AS, Hegde R, Gadad AP, Dandagi PM, Masareddy R, Bolmal U. Exploring the solvent- anti-solvent method of nanosuspension for enhanced oral bioavailability of lovastatin. Turkish Journal of Pharmaceutical Sciences. 2021;18(5):541
- Shah CN. Nanosuspension: a novel approach to enhance solubility of poorly water soluble drugs. Pharma science monitor. 2016;7(3): 321-334
- Jacob S, Nair A B and Shah J. Emerging role of nanosuspensions in drug delivery systems. Biomaterials Research 2020; 24:3
- V. B. Patravale, Abhijit A. Date and R. M. Kulkarni. Nanosuspensions: a promising drug delivery strategy. Journal of pharmacy and pharmacology. 2004; 56: 827–840
- Suri GS, Kaur A, Sen T. A recent trend of drug-nanoparticles in suspension for the application in drug delivery. Nanomedicine. 2016;11(21):2861-76.
- Chin WW, Parmentier J, Widzinski M, Tan EH, Gokhale R. A brief literature and patent review of nanosuspensions to a final drug product. Journal of Pharmaceutical Sciences. 2014:1;103(10):2980-99.
- Kalepu S, Nekkanti V. Improved delivery of poorly soluble compounds using nanoparticle technology: a review. Drug delivery and translational research. 2016; 6:319-32.
- P?nar SG, Canp?nar H, Tan Ç, Çelebi N. A new nanosuspension prepared with wet milling method for oral delivery of highly variable drug Cyclosporine A: development, optimization and in vivo evaluation. European Journal of Pharmaceutical Sciences. 2022;171: 0928-0987
- Daebis N, El-Massik M, Abdelkader H. Formulation and characterization of itraconazole oral nanosuspension: methyl cellulose as promising stabilizer. Ely J pharm res. 2015;1(1):102-110
- Paarakh MP, Jose PA, Setty CM, Christoper GP. Release kinetics–concepts and applications. International Journal of Pharmacy Research and Technology. 2018;8(1):12-20
- Ajay JY, kumar Gajula P. Sustained release nanosuspension of acetaminophen-formulation and in-Vitro evaluation. Int J Res Pharm Sci. 2012;3(1):67-71
- Gouda R, Baishya H, Qing Z. Application of mathematical models in drug release kinetics of carbidopa and levodopa ER tablets. Journal of Developing Drugs. 2017;6(2):1-8.
- Oktay AN, Karakucuk A, Ilbasmis-Tamer S, Celebi N. Dermal flurbiprofen nanosuspensions: Optimization with design of experiment approach and in vitro evaluation. European journal of pharmaceutical sciences. 2018; 122 :254-63.
- Gurpreet S, Amritvir K, Tapas S, A recent trend of drug-nanoparticles in suspension for the application in drug delivery. Nano-biomaterials?Research?Group, Nanomedicine (Lond.) (2016) 11(21), 2861–2876
- Shery J, Anroop B. N, Jigar S. Emerging role of nanosuspensions in drug delivery systems. Biomaterials Research (2020) 24:3: 1-16
- Chin et al. A Brief Literature and Patent Review of Nanosuspensions to a Final Drug Product. Jour of Phar Sciences. 2014: 1-20
- Jadhav SP, Singh SK, Chawra HS. Review on Nanosuspension as a Novel Method for Solubility and Bioavailability Enhancement of Poorly Soluble Drugs. Advances in Pharmacology and Pharmacy. 2023;11(2):117-30.


 Vittal C.*
Vittal C.*




 10.5281/zenodo.13936529
10.5281/zenodo.13936529