Abstract
Musa acuminata commonly known as banana plant is a storehouse of various phytoconstituents and one such constituent is gallic acid. Gallic acid is believed to have antibacterial property and hence can be used as an alternative to antibiotics and in case of antibiotic resistance. This article is an attempt to provide for an alternative method for extraction of gallic acid and evaluating it for desired phytoconstituents. The process of extraction begins with collecting the plant materials for extraction that is in our case peels and stem of banana plant. These plant materials need to be dried before extraction. Various methods can be used for extraction but in this attempt, sonication was used as a method for extracting phytoconstituent. After extraction the extract was standardized using Thin Lyer Chromatography (TLC), UV-Spectroscopy and High-Performance Liquid Chromatography (HPLC). Preliminary photochemical screening was preformed to detect presence of various phytoconstituents. The extract was then evaluated for antibacterial property. This extract can be incorporated into various topical formulations to give antibacterial action to the final product.
Keywords
Gallic acid, Sonication, TLC, HPLC, UV-Spectroscopy, Antibacterial
Introduction
Banana is a herbaceous plant belonging to the Musaceae family and has three genera (Musa, Musella and Ensete). Musa acuminata and M. balbisiana are important banana species. Bananas are classified as diploid, triploid and tetraploid according to their ploidy structure. According to their ploidy, bananas are classified as diploid, triploid, or tetraploid. According to the most recent Food and Agriculture Organization statistic, India is the leading banana producer with an annual production of 30,460,000 tons, followed by China and Indonesia. Globally, banana is the most affordable, widely available crop, and more than 1000 banana varieties, which differ in their colour, taste, and chemical composition, are produced and consumed. Bananas are often referred to as the dessert variety, while plantains are the culinary variety. Banana plants are grown primarily for their fruit and secondarily for wine and natural fibres. They are also used for shading, and the leaf of the tree is suitable for packaging large volumes of food due to its large surface area and waxy nature. Banana tree parts, such as the peels and leaves, have antioxidant activities and biological functions, including anti-diabetic, anti-diarrheal, anti-tumor, anti-mutagenic, and anti-ulcerogenic properties. Bananas have also been shown to be the source of bioactive compounds that inhibit bacterial or fungal growth. This antimicrobial activity is wide-ranging in the diverse nature of this plant. The ongoing evolution of bacterial resistance to currently available antibiotics has necessitated the identification of novel and effective antimicrobial compounds. Furthermore, there is a demand for the efficient and cheap antimicrobial compounds. To our knowledge, most previous reviews discussed the antioxidant capacity of this plant in detail, and few have mentioned its antimicrobial ability and the specific action of different banana extracts against foodborne pathogens. Banana plants contain a wide variety of phenolic compounds with different polarities. Their abundance of hydroxyl groups makes these compounds essential due to their scavenging abilities and potential for multiple biological effects, including antimicrobial activity [1].

Fig. 1 Banana Plant
Sonication is the act of applying sound energy to agitate particles in a sample, for various purposes such as the extraction of multiple compounds from plants, microalgae and seaweeds. Ultrasonic frequencies (> 20 kHz) are usually used, leading to the process also being known as ultrasonication or ultra-sonication. In the laboratory, it is usually applied using an ultrasonic bath or an ultrasonic probe, colloquially known as a sonicator [2]. The equipment used for sonication is known as a sonicator. The following are the three parts of the sonicator.
• A generator
• A transducer
• A probe

Fig. 2 Sonicator
The generator is used for transforming the input electrical power into an electrical signal that drives the transducer. The transducer is used for converting the electrical signal into vibration. This vibration is used in the probe tip by amplifying it into a longitudinal vibration causing a cavity in the sample. The ultrasound energy is the creation of cavitation which causes the disruption of the sample and makes it easy to break down the particles into smaller ones. The sonication process uses ultrasonic sound waves. During the process, there is a production of thousands of microscopic vacuum bubbles in the solution due to applied pressure. The formed bubbles collapse into the solution during the process of cavitation. The collapsing of bubbles takes place in the cavitation field leading to the generation of enormous energy as there is a production of waves. This results in the disruption of the molecular interactions between the molecules of water. As there is a reduction in the molecular interactions, the particles start to separate and allow the mixing process to take place. There is a release of energy from the sound waves that result in friction in the solution. Ice cubes are used during and after the sonication process to prevent the sample from heating up [3]. Using the above-mentioned plant and extraction technique one can probably extract gallic acid and use it for its antibacterial activity.
MATERIAL AND METHOD:
- Plant Profile: Banana plant
- Common Name: Apple of Paradise, Plantain tree, Kele
- Botanical Name: Musa acuminata

Fig. 3 Banana Plant
- Description: The height of cultivated banana plants varies depending on the variety and growing conditions. Most plants are about 5 m (16 ft) tall, ranging from "Dwarf Cavendish" plants at about 3 m (10 ft) to "Grand Michel" plants at 7 m (23 ft) or more. The leaves are arranged in a spiral and can reach 2.7 meters (8.9 ft) long and 60 cm (2.0 ft) wide. They are easily broken down by the wind and form the familiar leaves. When a banana plant grows, the seed stops producing new leaves and begins to form a flower or inflorescence. The stem grows into a pseudostem and bears immature inflorescences until they appear at the top. Each false stem often produces an inflorescence, also known as a "banana heart". After fruiting, the false stem dies, but branches usually form from the base, so all plants are perennials. In the plantation cultivation system, only one branch is allowed to be established in order to maintain distance. The inflorescence consists of numerous bracts (sometimes incorrectly called petals) on rows of flowers. Female flowers (which can grow into fruit) appear on stem rows farther away (closer to the leaves) than the male flower row. The ovary is abnormal, meaning small worms and other blooms appear at the tip of the ovary. The banana fruit develops from the heart of the banana into a large cluster of hanging layers (called "hands"), with up to 20 fruits in each layer. Hanging piles, called piles, have 3-20 layers, or "banana stalks" as they are commercially known, and can weigh up to 30-50 kg (66-110 lb). A banana fruit (often called a banana or “finger”) weighs an average of 125 grams (4 + 1 × 2 ounces), which is about 75% water and 25% dry matter (Nutrition, bottom right).
- Chemical Constituents: Phenolic compounds, carotenoids, Biogenic amines and Phytosterols.
- Uses: Anti-Bacterial, Anti-Oxidant, to treat burns, Hepatoprotective, Sweetener, Flavouring agent, etc [4].
- Chemical Profile: Gallic Acid
- Synonym: 3,4,5- trihydroxy benzoic acid
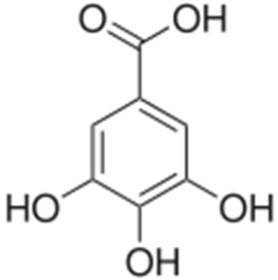
Fig. 4 Structure of Gallic Acid
- Molecular formula: C7H6O5.
- Molecular Weight: 170.12 g/mol
- Description: Gallic acid is an odourless white solid. Sinks in water. A colourless or slightly yellow crystalline compound obtained from nutgalls. It is used in photography, pharmaceuticals, and as an analytical reagent.
- Boiling Point: 260 °C
- Density: 1.694 gm/cm3
- Log p: 0.70
- Solubility in Water: 1.19 gm/ 100ml, 20°C (Anhydrous)
- Solubility: Soluble in alcohol, ether, glycerol, acetone. Negligible in benzene, chloroform, petroleum ether [5].
3) Chemicals:
Table 1 Chemicals used
4) Glassware:

Table 2 Glassware used
5) Equipments:
Table 3 Equipments used
6) Method of Extraction:
- The alcoholic extract of gallic acid was prepared using banana peels and stems that were purchased in form of powders.
- Both the peel powder and stem powder were mixed in 1:1 ratio and were soaked in N-hexane for 24 hours to remove any impurities and for defatation.
- After 24 hours the powder was separated by filtration and kept for air drying to remove N-hexane.
- The dried powder was then mixed with 70% methanol and sonicated for 1 hour.
- The sonicated extract was filtered to get an alcoholic extract of gallic acid [6].

Fig. 5 Sonication of Plant extract

Fig. 6 Extract after Sonication
7) Methods of Standardization:
- Thin Layer Chromatography (TLC):
- Prepare a TLC plate by mixing silica gel-G and water to form a runny paste that is spreaded evenly on the glass slide to form the stationary phase. The prepared slides were dried and activated by keep them in hot air oven at temperature of 100°C for 15 minutes. The mobile phase was prepared by mixing a single or multiple solvents depending on the constituent to be extracted. The plates are kept in the mobile phase to allow the solvent to run over the stationary phase. The plates are dried and a suitable reagent is spread for elution of a desired analyte.

Fig. 7 Mobile phase running over Stationary phase
Sample: Alcoholic extract of gallic acid
Solvent system: Toluene: Ethyl acetate: Formic acid: Methanol (4.2:4.2:1.1:0.2) [7].
Reagent: Folin-Ciocalteu / Folin-Denis
Rf value: 0.31
- UV-Spectroscopy:
- Turn on the uv-spectrophotometer and start the uv analysis in computer. Remove the cuvette and give it a good washing with the solvent used. Pour the sample in the cuvette and insert it inside the uv-chamber. Along with the sample put the solvent in another cuvette as reference. Close the uv-chamber and start the analysis on the software.
- The sample was prepared by mixing 1ml of extract with 70 % of methanol and this sample was poured in the cuvette and kept for uv analysis.

Fig. 8 UV-Spectrophotometer along with analysing software
- High-Performance Thin Layer Chromatography:
- A liquid sample is injected into a stream of solvent (mobile phase) flowing through a column packed with a separation medium (stationary phase).
- Sample components separate from one another by a process of differential migration as they flow through the column.
- As bands emerge from the column, flow carries them to one or more detectors which deliver a voltage response as a function of time.
- This is called a chromatogram. For each peak, the time at which it emerges identifies the sample constituent with respect to a standard. The peak’s area represents the quantity.
- The sample was prepared by diluting 1ml of extract with 9ml of 70% methanol and was allowed to run for 10 minutes at a flow rate of 1 and pressure 50 psi at a wavelength of 230-260 nm.

Fig. 9 HPLC Instrument
RESULT AND DISCUSSION:
- Preliminary Phytochemical Screening:
Preliminary phytochemical analysis of banana extract was positive for phenolics and tannins, indicating the presence of phenolics and tannins in the extract. [8].
- Tests for Phenols and Tannins
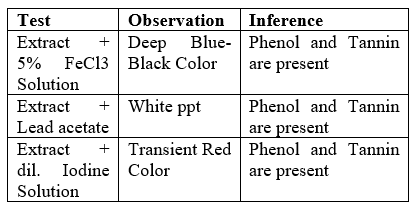
Table 4 Tests for Phenols and Tannin
- Thin Layer Chromatography (TLC):
After performing TLC for Gallic acid, the Rf value was found to 0.31 which was then compared with Rf value of standard gallic acid i.e. 0.35±0.02 given in reference articles and it was concluded that the given extract might contain Gallic acid [9].

Fig. 10 TLC plate of Gallic Acid
- UV-Spectroscopy:
When UV Spectroscopy of gallic acid was performed the lambda max was obtained at 246nm which was then compared with range lambda max given in various reference paper for gallic acid i.e. 230-270nm and conclusion was drawn that given extract might contain gallic acid.
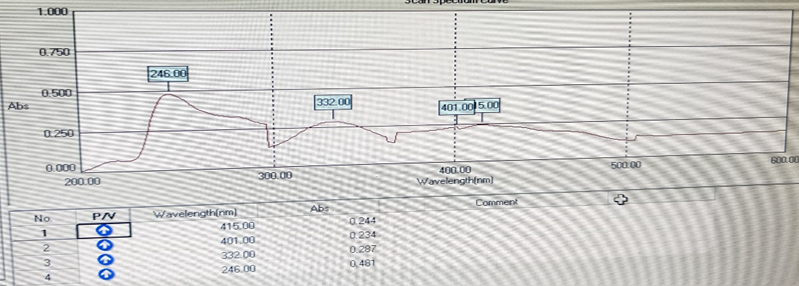
Fig. 11 UV spectrum of Gallic Acid
- High-Performance Liquid Chromatography:
After performing HPLC for Gallic acid the retention time was found to be 3.1min at 265nm which was compared with retention time of standard gallic acid i.e. 5.3min at 270nm and it was concluded that the given extract might contain Gallic Acid [10].
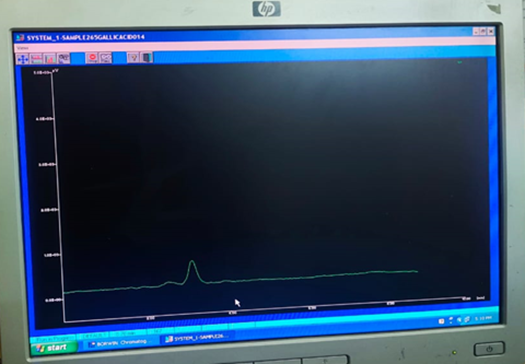
Fig. 12 HPLC peak of Gallic Acid
- Evaluation of Antibacterial Activity:
The antibacterial activity was evaluated using agar well diffusion method and staphylococcus aureus and the zone of inhibition of sample and standard drug were compared Zone of inhibition of the sample- 1.8mm Zone of inhibition of the standard drug- 3.5mm.

Fig. 13 Zone of Inhibition of Sample

Fig. 14 Zone of Inhibition of Standard
By utilizing the above-mentioned data one can conclude that Banana peels and stems do contain gallic acid which can be extracted by sonication.
REFERENCE
- Sayed M. [2021], ‘Banana plant as a source of valuable antimicrobial compounds and its current application in the food sector’, Journal of food sciences, 86(9), pp..3778-3797.
- Google [2024] Sonication, Available at: https://en.wikipedia.org/wiki/Sonication (Accessed: 20 June 2024).
- Google [2024] Sonication: Definition, Working, Principle, Application and Method, Available at: https://byjus.com/physics/sonication/( Accessed: 20 June 2024).
- Google [2023] Banana-Wikipedia. Available at: https://en.m.wikipedia.org/wiki/Banana(Accessed: 20 June 2024).
- Google [2024] Gallic Acid. Available at- https://en.m.wikipedia.org/wiki/Gallic_acid (Accessed: 20 June 2024).
- Rahmatia L [2022], ‘Application of Ultrasound Assisted Extraction of Ripe Banana Peels (Musa balbisiana Colla) and It’s Potential as Anti-oxidant and Anti-gout.’ Research Journal of Pharmacy and Technology, 15(2).
- Sawant L [2010], ‘Determination of Gallic Acid in Phyllanthus Embilica Linn. Dried Fruit Powder By HPTLC’, Journal of Pharmacy and Bioallied Sciences, 2(2), pp..105-108.
- Khandelwal R. [2008], ‘Preliminary Phytochemical Screening’, Practical Pharmacognosy Techniques and Experiments, Pune, Nirali Publication, pp..149.
- Sajeet C.I [2010], ‘Quantitative Estimation of Gallic Acid, Rutin and Quercetin in Certain Herbal Plants by HPTLC method.’ Der Chemica Sinica 1(2) pp... 80-85.
- Singh M [2019], ‘Qualitative analysis of Gallic Acid by HPLC Method in Different Extract of Terminalia Bellerica Roxb. Fruit.’ FABAD Journal of Pharmaceutical Sciences 44(2), pp... 101-106.


 Pratham S. Shinde*
Pratham S. Shinde*
 Jeeshan J. Shaikh
Jeeshan J. Shaikh
 Yash N. Sharma
Yash N. Sharma
 Poonam R. Shewale
Poonam R. Shewale
 Chaitanya S. Shisode
Chaitanya S. Shisode


















 10.5281/zenodo.12699589
10.5281/zenodo.12699589