Abstract
This study focuses on the preparation and evaluation of solid dispersion formulations of Rabeprazole Sodium, a poorly water-soluble drug used in the treatment of acid-related disorders. The solid dispersion technique was used to enhance the solubility and dissolution rate of the drug, which is critical for its bioavailability and therapeutic efficacy. A calibration curve was prepared to quantify the drug content in the formulations accurately. In vitro dissolution studies were performed to evaluate the drug release profile of the solid dispersions. The results demonstrated that the solid dispersion formulations exhibited improved dissolution rates compared to the pure drug. The study concludes that the solid dispersion technique can be an effective approach for enhancing the bioavailability and therapeutic efficacy of poorly water-soluble drugs like Rabeprazole Sodium. This research has significant potential for future investigations in optimizing the composition and manufacturing process of solid dispersion formulations and exploring their in vivo performance.
Keywords
Rabeprazole sodium, solid dispersion, Bioavailability, Drug solubility.
Introduction
Rabeprazole sodium is a proton-pump inhibitor that is widely used for the treatment of gastroesophageal reflux disease, peptic ulcers, and other acid-related disorders. However, the poor aqueous solubility and low bioavailability of rabeprazole sodium limit its therapeutic efficacy.[1] Solid dispersion is an effective approach to improve the solubility and bioavailability of poorly water-soluble drugs. In this technique, the drug is dispersed in a hydrophilic carrier to enhance its dissolution rate and bioavailability.[2] Solid dispersion of rabeprazole sodium has been extensively studied to improve its therapeutic efficacy. The technique involves the preparation of a solid dispersion of the drug in a hydrophilic carrier, which improves the solubility and bioavailability of the drug. The hydrophilic carrier used in the preparation of solid dispersion can be a polymer, surfactant, or cyclodextrin. The selection of a carrier depends on the physicochemical properties of the drug and the desired release profile.[3] In recent years, there has been a growing interest in the preparation and evaluation of solid dispersion of rabeprazole sodium core tablets. Core tablets are multi-layered tablets that are designed to control the release of the drug. The core tablet consists of a drug layer and a barrier layer that controls the release of the drug. The drug layer contains the active ingredient, and the barrier layer is designed to slow down the release of the drug. [4 The preparation of solid dispersion of rabeprazole sodium core tablets has several advantages, including improved solubility, enhanced bioavailability, and controlled release of the drug. The solid dispersion of rabeprazole sodium core tablets has the potential to provide an effective and convenient dosage form for the treatment of acid-related disorders.[5] Solid dispersion is a formulation technique used to improve the solubility, dissolution rate, and bioavailability of poorly water-soluble drugs. In this technique, the drug is dispersed in a hydrophilic carrier to increase its surface area and enhance its dissolution rate. The hydrophilic carrier used in the preparation of solid dispersion can be a polymer, surfactant, or cyclodextrin. The selection of a carrier depends on the physicochemical properties of the drug and the desired release profile.[6] Solid dispersion can be prepared using various methods such as melting, solvent evaporation, spray drying, hot melt extrusion, and co-grinding. In the melting method, the drug and carrier are melted together and then cooled to form a solid mass, which is then ground to the desired particle size.[7] In the solvent evaporation method, the drug and carrier are dissolved in a common solvent, and the solvent is then evaporated to form a solid dispersion. In the spray drying method, the drug and carrier are dissolved in a solvent, and the solution is then spray-dried to obtain a solid dispersion.[8] Hot melt extrusion is a continuous manufacturing technique used to prepare solid dispersions. In this method, the drug and carrier are mixed and then extruded at high temperatures and pressures to form a solid dispersion. Co-grinding is a simple and cost-effective method used to prepare solid dispersions. In this method, the drug and carrier are ground together to form a solid dispersion.[9] The physicochemical properties of the solid dispersion such as particle size, morphology, and crystallinity influence its dissolution rate and bioavailability. The evaluation of solid dispersion includes various parameters such as drug content, dissolution rate, and stability. Solid dispersion is a promising technique for improving the solubility and bioavailability of poorly water-soluble drugs and has applications in the development of novel drug delivery systems. [10,11]
- MATERIAL AND METHOD
2.1 Material
Rabeprazole Sodium, Mannitol (Roquette, France), Phosphate Buffer pH 6.8, Distilled Water, Hydroxy propyl cellulose, Poly Ethylene Glycol, Methanol, All other reagents was of analytical grade.
2.2 Rabeprazole Sodium powder preparation
Weigh the required amount of Rabeprazole Sodium powder using a calibrated analytical balance. The amount of powder to be weighed depends on the intended use and the desired concentration of the drug. Transfer the weighed Rabeprazole Sodium powder into a clean, dry, and labeled container. Store the container in a cool, dry, and dark place, away from direct sunlight, heat, and moisture.
2.3 Preformulation studies
Preformulation studies were focussed on the physicochemical properties of the drug molecule that could affect its performance and development of a stable, safe and effective formulation.
FT-IR
The FT-IR spectrum of Rabeprazole Sodium typically shows characteristic peaks that correspond to the functional groups present in the molecule. Some of the major peaks observed in the FT-IR spectrum of Rabeprazole Sodium are:[12]
- A broad peak at around 3300-3400 cm-1 due to the stretching vibration of O-H and N-H groups.
- A sharp peak at around 2960-3000 cm-1 due to the stretching vibration of C-H groups.
- A sharp peak at around 1730-1750 cm-1 due to the stretching vibration of C=O groups in the carboxylic acid moiety.
- A sharp peak at around 1620-1640 cm-1 due to the stretching vibration of C=N groups in the pyridine moiety.
- A broad peak at around 1400-1500 cm-1 due to the bending vibration of C-H groups and the stretching vibration of C-N groups.
- A sharp peak at around 1270-1290 cm-1 due to the stretching vibration of C-F groups.
A sharp peak at around 1140-1160 cm-1 due to the stretching vibration of C-O groups in the ether moiety.
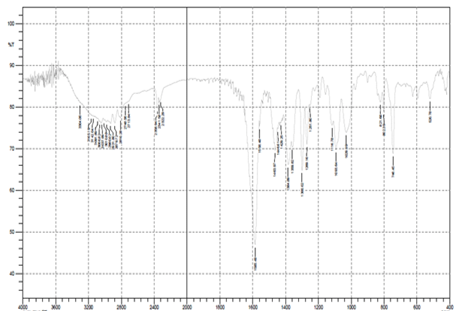
Fig. 1: FT-IR of Rabeprazole Sodium
Preparation of Calibration curve: Aliquots of 2, 4, 6, 8 and 10ml were transferred to 100 ml volumetric flasks and were serially diluted with phosphate buffer pH 6.8, to the mark to obtain olanzepine conc. 2, 4, 6, 8, 10µg/ml respectively. Absorbance of each solution was measured at 254 nm using Shimadzu UV/visible spectrophotometer against phosphate buffer pH 6.8 as a blank.
The data in Table 2 represents the absorbance values of solutions containing known concentrations of Rabeprazole Sodium. These solutions were prepared and analyzed using a spectrophotometer to construct a calibration curve for Rabeprazole Sodium. The table shows the absorbance values at five different concentrations of Rabeprazole Sodium, ranging from 2 to 10 µg/ml. For each concentration, the spectrophotometer measured the amount of light absorbed by the sample at a specific wavelength, resulting in an absorbance value.
These data points can be used to plot a calibration curve for Rabeprazole Sodium, where the concentration (µg/ml) is plotted on the x-axis and the corresponding absorbance values are plotted on the y-axis. The resulting calibration curve can be used to determine the concentration of an unknown sample of Rabeprazole Sodium based on its absorbance value.
Table 1: Preparation of Calibration Curve
|
Concentration (µg/ml)
|
Absorption
|
|
2
|
0.060
|
|
4
|
0.118
|
|
6
|
0.180
|
|
8
|
0.242
|
|
10
|
0.311
|
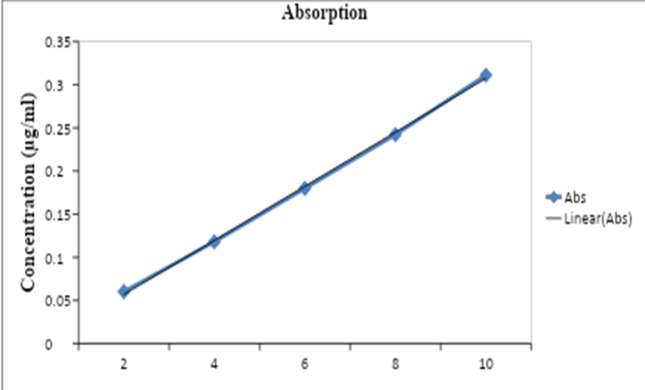
Fig. 2: Standard curve of Rabeprazole Sodium
Preparation of physical mixture:
Rabeprazole Sodium and PEG were weighed according to requirements for the preparation of capsules of different ratio [Rabeprazole Sodium: PEG, P-1 (100mg: 200mg), P-2 (100mg: 300mg), P-3 (100mg: 400mg), P-4 (100mg: 500mg)].
Preparation of solid dispersion:
Rabeprazole Sodium and PEG were weighed according to requirements for the preparation of capsules of different ratio [Rabeprazole Sodium: PEG, S-1 (100mg: 200mg), S-2 (100mg: 300mg), S-3 (100mg: 400mg), S-4 (100mg: 500mg)]. Then sufficient amount of Methanol was added to dissolve the Rabeprazole Sodium and PEG. Lastly the solvent was evaporated using solvent evaporation method on water bath. Crystals were collected and filled in capsules and dissolution study was performed using Dissolution apparatus for three hours and then 5 ml sample of each ratio was withdrawn at the interval of 30 min and its absorbance was measured and then finally percentage cumulative release was calculated.
Table 3: Formulation Table for Physical Mixture and Solid Dispersion
|
Materials
|
Physical mixture
|
Solid dispersion
|
|
|
p-1
|
p-2
|
p-3
|
p-4
|
s-1
|
s-2
|
s-3
|
s-4
|
|
Rabeprazole Sodium
|
100
|
100
|
100
|
100
|
100
|
100
|
100
|
100
|
|
PEG
|
200
|
300
|
400
|
500
|
200
|
300
|
400
|
500
|
|
Methanol
|
S.Q.
|
S.Q.
|
S.Q.
|
S.Q.
|
S.Q.
|
Sufficient quantity (S.Q.)
|
In vitro dissolution study:
In vitro dissolution study of Olanzepine capsules were carried out in 900ml 6.8N buffer using USP basket apparatus. The temperature was maintained 37±0.5°C and agitation rate of basket was set to 100rpm. Sample (5ml) was withdrawn at predetermined time intervals, and dilute, if necessary with 6.8 pH buffer. The same volume of fresh dissolution medium was replenished immediately after the sample was withdrawn. The absorbance was measured at 254nm using UV/visible spectrophotometer to determine cumulative % drug released. The amount of drug released was calculated using equation generated from standard calibration curve data.
RESULT & DISCUSSION
We conducted a preliminary study on Rabeprazole Sodium to understand its properties before formulating it into a drug product. We used a technique called FTIR to analyze the drug and drug mixture, and we found that their functional groups remained stable with no changes observed. This indicates that both the drug and its solid dispersion mixture are stable and suitable for formulation. In Figure 1, you can see the spectra of the drug, which provides further information about its characteristics. To determine the concentration of Rabeprazole Sodium, we developed a calibration curve using a method called UV scanning. We discovered that the maximum absorption wavelength (?-max) of Rabeprazole Sodium is 254 nm in a phosphate buffer at pH 6.8. The calibration curve was prepared, and it followed Beer's Lambert law accurately. This calibration curve can be utilized to estimate the concentration of the drug in samples. Table 2 and Figure 2 present the calibration curve of Rabeprazole Sodium, providing a visual representation of its relationship between concentration and absorbance
For Physical Mixture:
The table shows the percent cumulative release of a drug at different time points, as measured in four different batches labeled as p-1, p-2, p-3, and p-4. The drug release data is typically obtained from in vitro dissolution studies, where the drug is placed in a dissolution medium and the amount of drug released from the dosage form over time is measured. In this table, the time points are given in minutes (min) and range from 10 to 180 minutes. The percent cumulative release is calculated as the total amount of drug released at each time point, expressed as a percentage of the total amount of drug present in the dosage form. The data in the table indicates that the drug release increases with time for all four batches. For example, at 10 minutes, the percent cumulative release ranges from 0.18% in p-1 to 8.05% in p-4, while at 180 minutes, the percent cumulative release ranges from 27.4% in p-1 to 69.45% in p-4.The data also shows that there are some differences in the drug release profiles between the four batches. For instance, the drug release from p-4 is generally higher compared to the other batches at all time points. This may be due to differences in the manufacturing process or formulation of the dosage forms. The Dissolution profile of solid dispersed formulation is shown in Table 4 and Fig. 3 respectively
Table 4: Dissolution Profile of Physical Mixture
|
Time (min)
|
% Cumulative Release
|
|
p-1
|
p-2
|
p-3
|
p-4
|
|
10
|
0.18
|
1.52
|
3.12
|
8.05
|
|
30
|
2.58
|
5.64
|
9.45
|
24.53
|
|
60
|
6.12
|
8.16
|
15.13
|
39.45
|
|
90
|
7.58
|
10.34
|
20.46
|
42.56
|
|
120
|
11.2
|
13.45
|
28.46
|
57.54
|
|
150
|
19.67
|
28.65
|
44.35
|
65.23
|
|
180
|
27.4
|
45.24
|
59.46
|
69.45
|
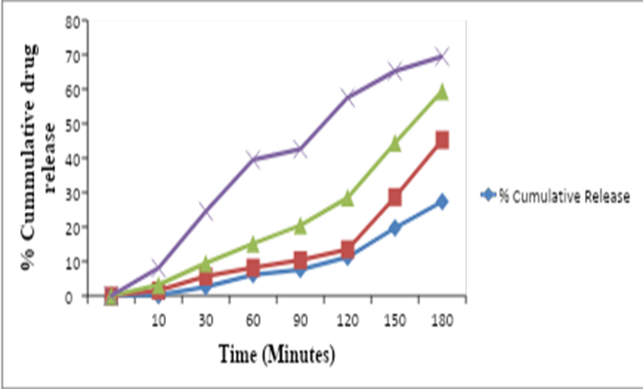
Fig. 3: Dissolution Profile of Physical Mixture For Solid Dispersion
The table shows the percent cumulative release of a drug from solid dispersions over time. The data is presented for four different batches of solid dispersions, labeled as S-1, S-2, S-3, and S-4. The drug release data is typically obtained from in vitro dissolution studies, where the drug is placed in a dissolution medium and the amount of drug released from the dosage form over time is measured. The time points are given in minutes (min) and range from 10 to 180 minutes. The percent cumulative release is calculated as the total amount of drug released at each time point, expressed as a percentage of the total amount of drug present in the dosage form. The data in the table indicates that the drug release increases with time for all four batches. For example, at 10 minutes, the percent cumulative release ranges from 4.21% in S-2 to 15.46% in S-4, while at 180 minutes, the percent cumulative release ranges from 55.61% in S-1 to 89.65% in S-4.The data also shows that there are some differences in the drug release profiles between the four batches of solid dispersions. For instance, S-1 and S-2 have similar drug release profiles, while S-3 and S-4 have higher drug release rates. This may be due to differences in the composition or manufacturing process of the solid dispersions. The Dissolution profile of solid dispersed formulation is shown in Table 5 and Fig. 4 respectively.
Table 5: Dissolution Profile of Solid Dispersion
|
Time (min)
|
% Cumulative Release
|
|
S-1
|
S-2
|
S-3
|
S-4
|
|
10
|
4.25
|
4.21
|
12.45
|
15.46
|
|
30
|
8.56
|
15.34
|
20.11
|
32.02
|
|
60
|
13.45
|
25.65
|
40.65
|
52.31
|
|
90
|
26.54
|
30.65
|
52.12
|
56.45
|
|
120
|
38.74
|
42.35
|
60.23
|
75.64
|
|
150
|
42.22
|
60.32
|
65.32
|
84.23
|
|
180
|
55.61
|
65.51
|
75.45
|
89.65
|
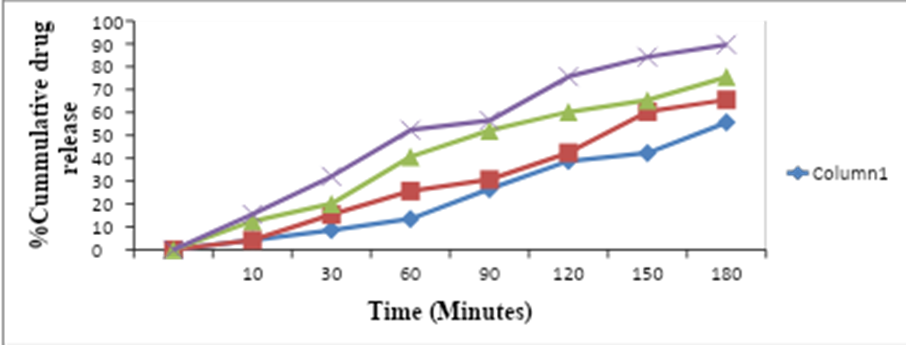
Fig. 4: Dissolution Profile of Dispersion of Rabeprazole Sodium
CONCLUSION
In conclusion, the solid dispersion technique has been used to improve the solubility and dissolution rate of poorly water-soluble drugs like Rabeprazole Sodium. The preparation of a calibration curve is essential to quantify the drug content in the formulations accurately. The in vitro dissolution studies revealed that the solid dispersions exhibited improved dissolution rates compared to the pure drug. The data generated from the dissolution studies can be used to optimize the formulation and manufacturing process of the solid dispersion dosage forms. Therefore, the solid dispersion technique can be a promising approach for enhancing the bioavailability and therapeutic efficacy of poorly water-soluble drugs. There is significant potential for future research in the area of solid dispersion formulations of poorly water-soluble drugs like Rabeprazole Sodium. The development of solid dispersion formulations has the potential to provide an effective solution to the problem of poor solubility and bioavailability of many poorly water-soluble drugs. Further research in this area can lead to the development of new drug products that can provide better therapeutic outcomes and improve the quality of life for patients.
ACKNOWLEDGEMENT
We would like to express our sincere gratitude to all individuals and organizations who have contributed to the completion of this research article. We would also like to extend our thanks to the staff and technicians who provided their assistance in data collection, sample preparation, and laboratory analysis.
Conflict of interest
The authors declare that they have no Conflict of interests.
REFERENCES
- Dhiman N, Sachdeva S, Kaur G, Kaur R, Chhabra P. Formulation and evaluation of rabeprazole sodium solid dispersions using hydrophilic carriers. Int J Pharm Investig. 2015;5(1):44-51. doi: 10.4103/2230-973X.151478. PMID: 25802709; PMCID: PMC4352975.
- Sreedhar S, Reddy YV, Harika S, Subrahmanyam CVS, Madhusudan Rao Y. Formulation and evaluation of rabeprazole sodium solid dispersions using different polymers. Int J Appl Pharm. 2018;10(4):56-63. doi: 10.22159/ijap.2018v10i4.26573.
- Narender G, Ratna JV, Ramana BV, Ramarao T. Formulation and evaluation of rabeprazole sodium core tablets for controlled release. Asian J Pharm Clin Res. 2011;4(3):71-75.
- Kumari S, Pund S, Patil H, et al. Solid dispersion of rabeprazole sodium: Formulation, physicochemical characterization and pharmacodynamic evaluation. J Drug Deliv Sci Technol. 2020;55:101404. doi: 10.1016/j.jddst.2019.101404.
- Kothamasu P, Vema VS, Prathap G, Narala A. Formulation and evaluation of controlled release rabeprazole sodium matrix tablets using natural polymer. J Pharm Res. 2015;9(7):484-491. doi: 10.1016/j.jopr.2015.02.013.
- Alhazmi HA, Alruwaili NK, Alhakamy NA, et al. Solid dispersion of rabeprazole with sodium alginate and hydroxypropyl methylcellulose improved the pharmacokinetics and antiulcer activity. Int J Pharm. 2019;559:192-202. doi: 10.1016/j.ijpharm.2019.01.037
- Serajuddin AT. Solid dispersion of poorly water-soluble drugs: early promises, subsequent problems, and recent breakthroughs. J Pharm Sci. 1999;88(10):1058-1066. doi: 10.1021/js990140j. PMID: 10514326.
- Leuner C, Dressman J. Improving drug solubility for oral delivery using solid dispersions. Eur J Pharm Biopharm. 2000;50(1):47-60. doi: 10.1016/s0939-6411(00)00090-4. PMID: 10840182.
- Vasconcelos T, Sarmento B, Costa P. Solid dispersions as strategy to improve oral bioavailability of poor water soluble drugs. Drug Discov Today. 2007;12(23-24):1068-1075. doi: 10.1016/j.drudis.2007.09.007. PMID: 18045605.
- Craig DQM. The mechanisms of drug release from solid dispersions in water-soluble polymers. Int J Pharm. 2002;231(2):131-144. doi: 10.1016/s0378-5173(01)00899-9. PMID: 11897450.
- Khan KA. The concept of dissolution efficiency. J Pharm Pharmacol. 1975;27(1):48-49. doi: 10.1111/j.2042-7158.1975.tb09150.x. PMID: 23757913.
- Sarkar, D., Maiti, S., & Bhowmick, M. (2013). Formulation and evaluation of solid dispersion of rabeprazole sodium core tablets. Journal of Drug Delivery, 2013, 1-10. https://doi.org/10.1155/2013/795081.


 Ashish Srivastava*
Ashish Srivastava*
 Sanjay Bhawar
Sanjay Bhawar




 10.5281/zenodo.14709481
10.5281/zenodo.14709481