Abstract
The purpose of this research work is to formulate and evaluate Mucoadhesive film by using Zolmitriptan. It is an triptan group, that relieves the migraine, when pro-inflammatory neuropeptide reease. It is a Biopharmaceutics Classification System(BCS), which is a classification system that categorizes drugs based on their solubility and permeability properties. BCS class II drugs are characterized by high permeability but low solubility. The mucoadhesive film formulation was studied using HPMC K4M polymers, Carbopol 934 and poly ethylene glycol used as plasticizer. There are three levels, i.e. were studied for mucoadhesive strength, swelling index, in-vitro drug release and in-vitro residence time. Preformulation study was carried out includes drug excipients compatibility study, drug estimation method and melting point determination. The drug estimation was found to be linear between 3 to 25 ?g/ml and ?max was found to be 222 nm. The FTIR spectrum of drug-excipient was found to be satisfactory, which indicated that excipients were compatible with the drug. Zolmitriptan mucoadhesive films were prepared by solvent casting method with different concentration of excipients Carbopol 934 (0.5-2%) and HPMC K4M (3-6%).The mucoadhesive strength of the films was found in the range of 9.95-23.095%, In-vitro residence time 80-247 min, swelling index 12.79-30.2%, In-vitro drug release at 7thhr 93.099%. Among the nine formulations F3 was selected as optimized formulation.
Keywords
Zolmitriptan, Carbopol 934, HPMC K4M, Solvent casting method, Design of experiment software.
Introduction
A migraine is a type of headache. It may occur with symptoms such as nausea, vomiting, or sensitivity to light and sound. In most people, a throbbing pain is felt only on one side of the head. It is a one side headache it lasts for one day or two, three days. Causes for migraine headache is caused by abnormal brain activity. This activity can be triggered by many things. But the exact chain of events remains unclear. Most medical experts believe the attack begins in the brain and involves nerve pathways and chemicals. The changes affect blood flow in the brain and surrounding tissues. Migraine headaches tend to first appear between the ages of 10 and 45. Sometimes, they begin earlier or later. Migraines may run in families. Migraines occur more often in women than men. Some women, but not all, have fewer migraines when they are pregnant. Symptoms are There are two main types of migraines Migraine with aura (classic migraine), Migraine without aura (common migraine).An aura is a group of nervous system (neurologic) symptoms. These symptoms are considered a warning sign that a migraine is coming. Most often, the vision is affected and can include any of temporary blind spots or colored spots, blurred vision, eye pain, seeing stars, zigzag lines, or flashing lights, tunnel vision (only able to see objects close to the center of the field of view). Mucoadhesive drug delivery systems prolong the residence time of the dosage form at the site of application or absorption. They facilitate an intimate contact of the dosage form with the underlying absorption surface and thus improve the therapeutic performance of the drug. In recent years, many such mucoadhesive drug delivery systems have been developed for oral, buccal, nasal, rectal and vaginal routes for both systemic and local effects. Dosage forms designed for mucoadhesive drug delivery should be small and flexible enough to be acceptable for patients and should not cause irritation. Other desired characteristics of a mucoadhesive dosage form include high drug loading capacity, controlled drug release (preferably unidirectional release), good mucoadhesive properties, smooth surface, tastelessness, and convenient application. Erodible formulations can be beneficial because they do not require system retrieval at the end of desired dosing interval. Mucoadhesive film is a absorption of drug released from the film occurs either trans-mucosal or in the GI tract, governed by the properties of the film. The mucoadhesive buccal film is classified as a prolonged release formulation, which can be single-layer or multi-layer in action. Mucoadhesive buccal films are multi-layered systems that release drugs into the oral cavity over a prolonged period. The film's properties determine whether the drug is absorbed trans-mucosally or in the gastrointestinal (GI) tract. The films can be single-layer or multi-layer, and multi-layer films are often designed as oral patches. The non-dissolvable layer of a multi-layer film can promote uni-directional drug release, either for transmucosal absorption or for local effect in the oral cavity. Triptans are a class of medications used to treat migraines and cluster headaches. They are also known as 5HT1-receptor agonists because they work by binding to serotonin receptors in the brain. Triptans work by Changing how blood circulates in the brain, changing how the brain processes pain signals, limiting pain signaling, releasing serotonin, which causes blood vessels around the brain to contract. Lasmiditan Triptan drugs are Almotriptan, Eletriptan, Frovatriptan, Naratriptan, Rizatriptan, Sumatriptan, Zolmitriptan and Lasmiditan is a new drug approved in OCT.11, 2019.
MATERIALS, EQUIPMENTS AND METHODS
MATERIALS:

EQUIPMENTS:

METHODS:
Preformulation studies
Identification of Drug by FT-IR:Weighed amount of drug was mixed with IR grade of Potassium Bromide (1:10) and compressed under 10-ton pressure in a hydraulic press to form a transparent pellet. The pellets were scanned by IR spectrophotometer over a range 400 cm-1 to 4000 cm-1.
Melting point determination
Melting point of Zolmitriptan was determined by capillary tube method. Fine powder of the drug was filled into a glass capillary tube which was previously sealed at one end. The capillary tube tied to a thermometer was subjected to increasing temperatures and the temperature at which Zolmitriptan melts was recorded. This was repeated for three times and average reading recorded.
Preparation of Calibration curve of Zolmitriptan
Stock-1 solution:
100mg of Zolmitriptan was accurately weighed and dissolved in 30ml of methanol in a 100ml volumetric flask. Then the volume was made up to 100ml using pH 7.4 phosphate buffer and shaken well to get a 1000?g/ml solution (primary stock solution).
Stock-2 solution:
2.5ml was pipetted out from primary stock solution into 25ml volumetric flask and the volume was made up to the mark with phosphate buffer (pH 7.4) to give a 100?g/ml solution (secondary stock solution). Serial dilution: From this solution (100?g/ml) 0.3,0.6,0.9,1.2,1.5,1.8,2.1 and 2.4ml was pipetted out into and diluted to 10ml volumetric flask and using pH 7.4 to get aliquots of 3,6,9,12,15,18,21 and 25?g/ml respectively. The absorbance was measured at 222 nm using UV visible spectrophotometer. The standard graph was repeated three times (n=3). The mean data was plotted by taking the concentration on X-axis and absorbance on Y-axis.
Preparation of drug loaded mucoadhesive films
The drug Zolmitriptan loaded mucoadhesive films were prepared by solvent casting method. HPMC K4M and Carbopol 934 were used as film forming and mucoadhesive polymer respectively. Polyethylene glycol-400 used as plasticizer. Methanol used as solvent (drug solubility). It was designed to prepare a film 1×1 cm2 to contain 2.5 mg of drug. The total area of casting is 49 square cm. According to this, calculated amount of drug equivalent to 0.1225 g of drug Zolmitriptan was weighed and transferred in to 100 ml beaker added with 5 ml methanol to that and mix it well. Required quantity of HPMC K4M and Carbopol 934 were weighed and dissolved in 20 ml of methanol and stirred with magnetic stirrer until it gets fully dissolved. The beaker was sealed using aluminium foil to avoid evaporation. After that the prepared drug solution was slowly added into the polymeric mixture and stirrer for 2 hrs and 0.15 ml of polyethylene glycol-400 (10% of polymer concentration) was added in to the drug polymer solution and mix it well. After completely dissolving, the mixture was poured square shaped plate made up of aluminium foil with side 7cm and the total area would be 49 square cm. This was placed on horizontal surface adjusted using spirit level. The polymer solution containing drug was carefully spread over 49 square cm area without any air bubble entrapment. The formulation kept in room temperature for drying till 16 hr. Each mucoadhesive film are cut by the measurement of 1×1 cm2 which contains dose equivalent to 2.5 mg. Total nine formulation of mucoadhesive films done by different concentration of polymers. The minimum concentration of HPMC K4M 3% and Carbopol 934 0.5% and the maximum concentration of HPMC K4M 6% and Carbopol 934 2%. Formulations were prepared using Face Centered Central Composite Design using Design Expert Version 11.
Table 1: Formulation chart of Zolmitriptan mucoadhesive films (F1-F9)

Evaluation of mucoadhesive films of Zolmitriptan Weight Uniformity
Ten films of each batch of formulation were weighed individually by using a digital weighing balance, and average weight of the films was calculated.
Folding endurance
The folding endurance was measured manually. A small strip of film measuring 1×1cm2 of each formulation was taken and folded at the same place till it broke or folded up to 300 times manually. The number of times a film could be folded at the same place gave the value of folding endurance. Average of three determinations was
Thickness of film
Thickness of the mucoadhesive film was measured by Vernier callipers with the least count of 0.01mm at different spots of the film. The thickness was measured at ten different spots of the plane surface of films and the average film thickness and standard deviation was calculated.
Mucoadhesive strength
In this study, Porcine buccal mucosa was employed as a biological membrane due to similarities with human buccal tissue. A modified physical balance method was used for determining the ex-vivo mucoadhesive strength. Fresh porcine buccal mucosa was obtained from a local slaughter house and used within 2 h of slaughter. The mucosal membrane was separated by removing the underlying fat and loose tissues using blunt forceps and scissors intermittently dipping the tissue in hot water. The collected tissues were washed with distilled water, placed in normal saline solution at 5 ?C to maintain freshness and immediately used for experiment. Then the tissues were cut by scissors to approximate size of mucoadhesive film.
Modification of physical balance:
left hand side pan was removed and replaced with glass block with flat surface below. Height of glass block was adjusted such that when balanced it would exactly touch the fat surface of the 500 ml beaker kept on inverted position placed exactly below the hanging glass block. Two sides of the balance were made equal before the study by keeping a 6g weight on the right side. Porcine Buccal mucosa was then stuck on to the bottom flat surface of the 500 ml beaker using cyanoacrylate adhesive such that mucosal surface faces upward. The beaker containing mucosal membrane was kept below the left hand set up of the balance. The buccal film was stuck to the lower flat side of hanging glass assembly with cyanoacrylate adhesive glue. The exposed film surface was moistened with distilled water and left for 45 s for initial hydration and swelling. Then the platform was slowly raised until the film surface came in contact with mucosa. A weight of 6 g was removed from the right hand pan, which lowered the pan along with the film over the mucosa. The balance was kept in this position for 2 minutes contact time. Then weights were slowly added to the right-hand pan until the film detached from the mucosal surface. This detachment force gave the mucoadhesive strength of the buccal film in grams. The following parameters were calculated from the mucoadhesive strength.
In-vitro residence time
It was determined using a locally modified USP disintegration test apparatus. A 5cm long segment of porcine buccal mucosa was glued to the surface of a glass slab using acrylic glue, vertically attached to the apparatus. The mucoadhesive film was hydrated from one surface using 16 µl isotonic phosphate buffer and the hydrated surface was brought into contact with the mucosal membrane. The glass slab was vertically fixed to the apparatus and allowed to move up and down in 850 ml isotonic phosphate buffer pH 7.4 maintained at 38°, so that the film was completely immersed in the buffer solution at the lowest point and was out at the highest point. The time taken for complete erosion or detachment of the films from the mucosal surface was recorded (mean of triplicate determinations) was taken.
Swelling index
The films were weighed and placed in a 2.7% agar gel plate. The 2.7% agar gel plate were prepared by hydrating 2.7g of agar powder in 98 ml hot distilled water and 27ml taken from the agar solution and poured to petri-plate which was kept in room temperature till it gets solidified. And after solidification one film from each formulation was kept over the agar gel plate. At regular time intervals, the films were removed from the plates and excess surface water was removed carefully using a filter paper. The swollen films were then reweighed and the degree of swelling was calculated using the following formula:
In-vitro drug release
Modification of the USP Dissolution apparatus type I, was used throughout the study. films (1cm2 diameter) of each formulation was fixed to base of the central shaft of the basket (without using basket) using by a cyanoacrylate adhesive. The dissolution medium consisted of 100 ml of phosphate buffer pH 7.4in a 100ml beaker kept inside the dissolution jar. The release study was performed at 38 ± 0.5°C with a rotation speed of 25 rpm and the release study was carried out for 10 hrs. After every hour, 6 ml sample was withdrawn from each beaker and the same volume was replaced (with the dissolution medium) to the beaker. Each withdrawn sample was filtered, diluted in 25 ml volumetric flask using pH 7.4 phosphate buffer and analysed spectrophotometrically at 222 nm. Each formulation, 2 films were tested for in-vitro release and average release was calculated.
Numerical Optimization
The goal of optimization is to find a good set of conditions that will meet all the goals. The possible goals are maximize, minimize, target, within range, none (for responsible only) and set to an exact value (factor only). Numerical optimization will search the design space, using the models created during analysis to find factor settings that meet defined goals. The numerical optimization finds a point that maximizes the desirability function. Desirability simply a mathematical method to find the optimum. A desirability of 1.00 means the goals were easy to reach and better results may be available. So, the data obtained were subjected to numerical optimization technique using design expert software version 11. The criteria for factors are set HPMC K4M and Carbopol 934 in the range and in that mucoadhesive strength should be maximum and invitro residence time should be maximum and swelling index in range and In-vitro drug release 7th hr maximum. The software recommended 5% of HPMC K4M 2?rbopol 934 as optimum formulation which coincidence with F3 in design matrix.
Table 2: Constraints for factors and responses of Zolmitriptan mucoadhesive films for Numerical Optimization.

RESULT AND DISCUSSION
Preformulation studies Identification of drug by FT-IR
The IR spectrum of pure drug was found to be similar to the standard spectrum of Zolmitriptan.FT-IR of Zolmitriptan showed three main characteristic absorption bands of at 1735
cm-1 due to C=O stretching vibrations of amide functional group and N-H stretching band of secondary and tertiary amine appears at 3350.46 cm-1 as a single sharp band and C-O stretching of ester group at 1264.60 cm-1 and C-H band at 1463.31 cm-1 as shown in figure 1.

Figure 1: FT-IR spectra of Zolmitriptan
Melting point Determination
Melting point of Zolmitriptan was determined by capillary method . The melting point was found to be 181 ? C which matches with the standard melting point (175-182 c) indicating the purity of the drug sample.
Calibration Curve of Zolmitriptan
The ? max was found to be 222 nm. So, the standard calibration curve of Zolmitriptan was developed at this wave length. The calibration curve was linear between 3-25 µg/ml concentration ranges. The standard calibration curve of Zolmitriptan was determined in pH 7.4 phosphate buffer, by plotting absorbance against concentration at 222 nm. Results were tabulated in (table no 3). The R2 and slope were found to be 0.1001 and 0.0935 respectively.
Table 3: Calibration curve of Zolmitriptan in pH 7.4 phosphate buffer

Evaluation of Zolmitriptan mucoadhesive films Weight uniformity
The weight of the film was found to be in the range of 24.06 to 55.45mg. Formulation F3 showing more weight as both the polymers are at maximum level.
Folding endurance
Film did not show any cracks even after folding for more than 300 times. Hence it was taken as the end point. Folding endurance did not vary when the comparison was made between plane film and drug loaded films. It reveals that all films having satisfactory flexibility.
Thickness of films
The mean thickness of the mucoadhesive film was found to be in the range of 0.130 ±0.013 mm to 0.241 ± 0.061 mm. The formulation F3, F6 and F9 showing more thickness compared to other formulation. Because the Carbopol 943 concentration is maximum on these formulations.
Mucoadhesive strength
Mucoadhesive strength was determined by using modified physical balance method. The procedure was followed by using porcine buccal mucosa. All the films had shown good mucoadhesive strength with the range of 10.0222.11gm. Formulation F3 having maximum concentration of Carbopol and HPMC K4M shows maximum mucoadhesive strength. The ANOVA (Analysis of variance) for the Linear model was used to analyse the mucoadhesive strength of prepared mucoadhesive films. The statistical ANOVA evaluation showed that the model is significant as P-value was found to be 0.0015. The Model F-value of 25.04 implies the model is significant. There is only a 0.15% chance that an Fvalue this large could occur due to noise. The Predicted R2of 0.7110 is in reasonable agreement with the Adjusted R2of 0.9538; i.e. the difference is less than 0.2.
Mucoadhesive strength = +17.09 + 4.97*Carbopol 934 + 0.9300 *HPMC K4M
The coded equation is useful for identifying the relative impact of the factors by comparing the factor coefficients.

Figure 2:3D surface graph of mucoadhesive strength from design expert of factors A&B
It was conformed that increase in degree of hydration increases the adhesion but at the same time over hydration results sudden weakening in mucoadhesionand it could be owing to the disentanglement at the tissue interface. The film made using Carbopol 934 as the mucoadhesive polymer showed the highest mucoadhesive strength, and the reason for this could be optimum hydration and swelling of Carbopol 934. Carbopol 934 is a high molecular weight polymer of crosslinked acrylic acid with polyalkenyl alcohols.Carbopol 934 hydrates and swells in aqueous phase due to hydrogen bonding and electrostatic repulsion after neutralization reaction (conversion of carboxylic acids in polymer chain to carboxylates). Thus, the hydration potential of Carbopol 934 permits the film to rapidly establish contact with the mucus upon application. Hydrogen bonding and interchain penetration between Carbopol 934 and mucin components make the mucoadhesion consolidate.
In-vitro Residence time
In-vitro residence time test was carried out using modified USP disintegration test apparatus. The ANOVA (Analysis of variance) for the 2 FI model was used to analyse the in-vitro residence time of mucoadhesive films. It shown within the range of 80 to 247 minute. The P-value was found to be <0>
In-vitro residence time = +150.19+ 58.93 *Carbopol 934 +15.17 *HPMC K4M + 22.65 *Carbopol 934 &HPMC K4M
The coded equation is useful for identifying the relative impact of the factors by comparing the factor coefficients. It was observed In-vitro residence time increased by increased concentration of Carbopol 934 and HPMC K4M.
The least in-vitro residence time were found to be with formulation F1, and the reason might be over hydration of HPMC K4M containing carboxylic groups, which form hydrogen bonds with the tissue.

Figure 3: 3D surface graph of In-vitro residence time from design expert of factor A&B
Swelling index was carried out by 2.5% agar gel plate method. The ANOVA (Analysis of variance) for the linear model was used to analyse the swelling index of the prepared mucoadhesive films. Swelling index of mucoadhesive films shown with in the range of 13.09% to 30.30%. The P-value and R2 value was found to be 0.0018 and 0.8945 respectively. Adequate precision measures the signal to noise ratio. The ration greater than 4 is desirable.The ratio of 11.994 indicates an adequate signal.This model can be used to navigate the design space. The model F-value of 25.10 implies the model significant. There is only a 0.13% chance that an F-value this large could occur due to noise. P-values less than 0.0555 indicate model terms are significant in this case B is significant model term. Values greater than 0.01000 indicates the model terms (not counting those required to support hierarchy), model reduction may improve your model. The predicted R2of 0.8520 is in reasonable agreement with the Adjusted R2 of 0.9420; i.e. the difference is less than 0.2. Swelling index = +21.42 + 7.12 *Carbopol 934 +2.07 *HPMC K4M The equation in term of coded factors can be used to make prediction about the response for given levels of each factor.The coded equation is useful for identifying the relative impact of the factors by comparing the factor coefficients. It was assumed that the high hydrophilicity nature of polymer in F9 resulted in extreme swelling as compared to other formulations. There exists a direct relationship between the swelling index and presence of polymer Carbopol. It is formed by ‘polyacrylic acid chains’ cross linking. It is well known statement that swelling reduces generally due to cross-linking of polymeric chains. But the presence of carboxylic acid (COOH) group in the polymeric chains of Carbopol gets converted to carboxylate groups (COO-) after neutralization that leads to selfrepulsion of polymeric chains themselves and forms hydrogen bonds with water. This particular characteristics of Carbopol marks this polymer as one of the swellable polymers despite being crosslinked. The quicker the polymer swells, the quicker the bonds between mucus and the polymer form, hence the adhesion to mucus occurs quicker. dissolution media is placed in the dissolution basket.
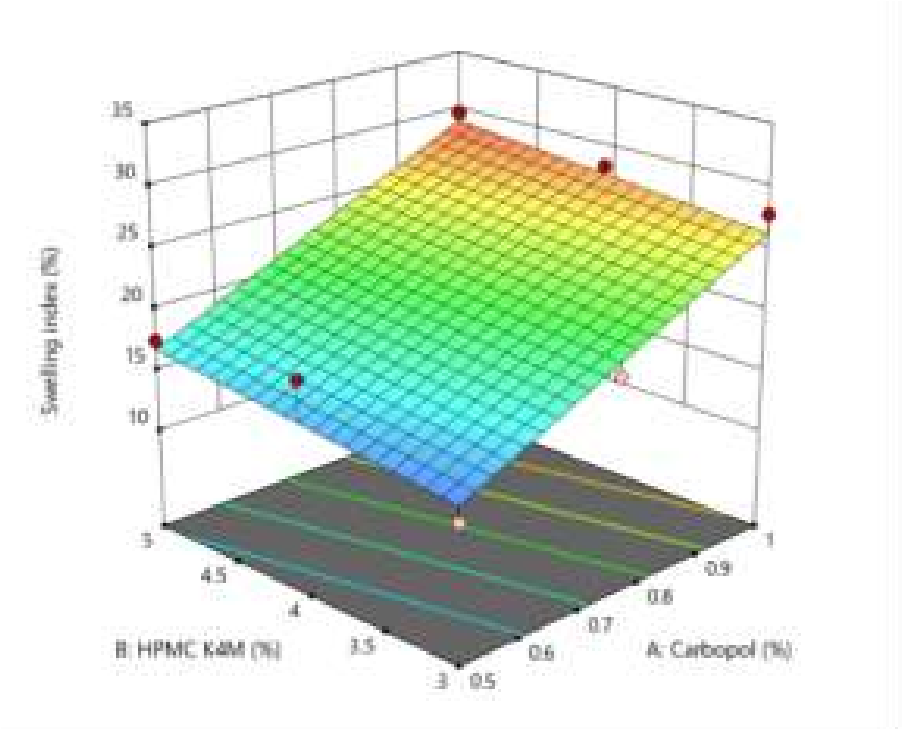
Figure 4: 3D surface graph of swelling index from design expert of factor A&B
In-vitro drug release at 7th hr
In-vitro drug release of mucoadhesive film was performed in phosphate buffer (pH 7.4) using Modification USP dissolution apparatus type-I. Since the dose of the drug in each film 2.5 mg. Volume of the dissolution is reduced to 100 ml and to facilitate the complete immersion of the film in the dissolution media. Separate beaker with the samples were withdrawn at different time intervals up to 10 hrs and was analysed using UV-spectrophotometer at 222nm. ANOVA (Analysis of variance) for the linear model was used to analyse the % in-vitro drug release at 7th hr of the prepared mucoadhesive films. The statistical ANOVA evaluation showed that the model is significant and the P-value of the model was found to be 0.0199. The Model F- value of 9.01 implies the model is significant. There is only a 1.70% chance that an Fvalue this large could occur dur to noise.

Figure 5: 3D plot of In-vitro drug release at 7th hr from design expert of factors A&B
Optimized formulation of mucoadhesive film from design expert

Table 10: Optimized formulation from design expert
Invitro drug release at 7th hr = +70.10 +17.01 *A +5.98 *B
The equation in terms of coded factors can be used to make prediction about the response for given levels of each factor. The coded equation is useful for identifying the relative impact of the factors by comparing the factor coefficients. According to the actual factors equation, concentration of both Carbopol 934 and HPMC K4M are having positive effect on % drug release. Formulation F3 showed maximum drug release in 7th hr (i.e.93.099%). Because the concentration of polymers was maximum in formulation F3. Polymer concentration is a major factor effecting the drug release of mucoadhesive films.(10) Increases the concentration of Carbopol 934 and HPMC K4M showed extended drug release. Formulation F3 found to be as optimized formulation. The optimized formulation suggested the composition as follows: Carbopol (A)= 2%, HPMC K4M (B)= 6%, Mucoadhesive strength= 23.095gm, In-vitro residence time= 246.941min, Swelling index= 30.009%, In-vitro drug release 7th hr= 93.099% with a desirability 0.920.
CONCLUSION
To formulate and evaluate the mucoadhesive film of an migraine drug Zolmitriptan for mucoadhesive drug delivery.
- Pre-formulation studies were carried out for the purpose of identifying the drug by FT-IR, calibration curve was constructed to estimate the drug using UV- visible spectroscopy, melting point determination by using capillary method. FT-IR spectra of drug with other excipients indicated that there is no interaction between the drug and the excipients.
- The mucoadhesive films were formulated using Carbopol 934 and HPMC K4M by solvent casting method. The face centered composite design has planned to evaluate the influence of excipients on various parameters.
- The formulated mucoadhesive films were evaluated for different characterization tests such as swelling index, in-vitro drug release, mucoadhesive strength, in-vitro residence time and all the formulations showcased satisfactory results.
- The excipients concentrations exhibited great effect on the evaluation parameters Mucoadhesive strength, In-vitro residence time, in-vitro drug release and swelling index.
- The results obtained from design expert version 11 in the form of 3D plots indicated that an increase in the concentration of Carbopol 934 increased mucoadhesive strength, in-vitro residence time, in-vitro drug release and swelling index. Comparatively Carbopol 934 has shown greater influence than HPMC K4M as shown by their coefficient of the factors.
- The optimized formulation suggested by numerical optimization method as follows
Carbopol (A)= 2%, HPMC K4M (B)= 6%, Mucoadhesive strength= 23.095gm, In-vitro residence time= 246.941min, Swelling index= 30.009%, In-vitro drug release 7th hr= 93.099% with a desirability 0.920.
With these concentrations, the suggested responses were 23.10 gm for mucoadhesive strength, 246 min for in-vitro residence time, 30.009% for swelling index, 93.099% for in-vitro drug release at 7th hr. The practical results of the optimized formulations were closely related to the predicted results.
- The mechanism of drug release of the optimized formulation F3 was found to be nonfickian transport with Zero order release.
- Future plan of action
- Optimized formulation can be further evaluated for in-vivo studies.
ACKNOWLEDGEMENT:
The authors would like to acknowledge the contribution of the technical staff attached with this study. Sri Vijay Vidyalaya College of Pharmacy, Dharmapuri, Affiliated to The Tamil Nadu Dr. M.G.R. Medical University Chennai -600 032, Tamilnadu.
REFERENCE
- Messoud A. Migraine. The New England Journal of Medicine. 2020;383: 18661876.
- Rahamatullah Shaikh, Thakur Raghu Raj Singh, Martin James Garland, A David Woolfson, and Ryan F. Donnelly. Mucoadhesive drug delivery systems, 2011 Jan-Mar; 3(1): 89–100.
- Poluri Koteswari, G. Puja S ravanthi, M. Mounika, S. K. Mohammed Rafi, and K. Nirosha. Formulation development and evaluation of zolmitriptan oral soluble films using 22 factorial designs, 2016 Oct-Dec; 6(4): 201–206.
- Ansari M, Sadarani B, Majumdar A. Optimization and evaluation of mucoadhesive buccal films loaded with resveratrol. J Drug Deliv Sci Technol. 2018; 44:278–88.
- Dhoot N, Vidyasagar G. Formulation and Optimization of Mucoadhesive Films of Some Anti-fungal Drugs by PVA and Gelatin Based Technique. 2015;(April):184–94.
- S. Kishore Kumar, M.V. Nagabhushanam, K.R.S. Sambasiva Rao, D.V.R.N. Bhikshapathi. Formulation development and in vivo evaluation of zolmitriptan oral dissolving films, January 2013, 4(3):P638-P646.
- Ramji Kurmi, 2Dr. Kuldeep Ganju, 3Devendra S. Lodhi and 4Khushi Chouksey. Formulation, Development And Evaluation Of Fast Dissolving Oral Film Of Anti-Migraine Drug, OCT.2021.
- Kraisit P, Limmatvapirat S, Luangtana-Anan M, Sriamornsak P. Buccal administration of mucoadhesive blend films saturated with propranolol loaded nanoparticles. Asian J Pharma.Sci.2018;13(1):34–43.
- Nair AB, Shah J, Jacob S, Al-Dhubiab BE, Patel V, Sreeharsha N, et al. Development of mucoadhesive buccal film for rizatriptan: In vitro and in vivo evaluation. Pharmaceutics. 2021;13(5).
- Herbal buccal films with in vitro antibacterial and anti-inflammatory effects, Cristina M. Pérez Zamora, Ariel G. Michaluk, Diego A. Chiappetta, María B. Nuñez, DEC.2021.
- Shinde AJ, Waghmare DS, Dalvi RS, More HN. Formulation, Design and Characterization of Mucoadhesive Buccal Film of Nebivolol Using Factorial Design. Int J Pharm Sci Res. 2018;9(5):1797–805.
- Nafee NA, Ismail FA, Boraie NA, Mortada LM. Mucoadhesive buccal patches of miconazole nitrate: In vitro/in vivo performance and effect of ageing. Int J Pharm. 2003;264(1–2):1–14.
- Dowson AJ, Charlesworth B. Review of zolmitriptan and its clinical applications in migraine. Expert Opin Pharmacother. 2002;3(7):993–1005.


 Muthamil C * 1
Muthamil C * 1
 Sreedharan.N.K.K 2
Sreedharan.N.K.K 2
 Dr. Senthilkumar.K.L 3
Dr. Senthilkumar.K.L 3











 10.5281/zenodo.11191903
10.5281/zenodo.11191903