Abstract
Roxatidine acetate hydrochloride the drug of interest belongs to the category antiulcer agent, suppresses histamine release (thus inhibiting proton secretion) and inhibits the production of VEGF-1, an important marker of inflammation and angiogenesis. Anti-allergic inflammatory effect. It belongs to the class of organic containing a piperidine ring conjugated to a benzyl group through one nitrogen ring atom and is being used in the study extensively in treatment and management of Gastroesophageal reflux disease (GERD) or gastro-oesophageal reflux disease (GORD) works through antagonizing the histamine H2 receptor. It is used to reduce abdominal pain, heartburn, acid indigestion and acid reflux. Therefore, in the present study a multidisciplinary approach has been carried out in formulating the Solid lipid nanoparticles for oral delivery of Roxatidine acetate in the form of capsules to counteract the pre-existing setbacks in oral administration of the drug. The approach is empowered by articulating from conventional to lipid-based nanocarrier formulation and in addition, specific amendment in the functional components. It has been proposed through work that Roxatidine acetate loaded nano particle will work onto overcome the complications in an effective way. Therefore, the presenting work highlights the improved delivery of Roxatidine acetate through oral route providing better oral absorption in same amount of drug. The enhanced solubility and therefore the bioavailability results into dose reduction which also lowers the risk of side effects due to drug toxicity or due to higher doses.
Keywords
Roxatidine acetate, Solid lipid nanoparticles, Gastroesophageal reflux disease, Solubility.
Introduction
The drugs having problems related to their solubility and bioavailability are intended to be delivered through any novel delivery system to overcome the shortcomings and get the desired therapeutic effect on the body. The various techniques involved in enhancing the properties of drug involve precipitation, micronization, nanonization, use of surfactants or drug coating. The lipid matrix made in the SLNs are prepared by the physiologically tolerated fat content which results in the decrease in the potential risk of chronic or acute toxicity which can occur in case of polymeric nanoparticles [1]. Therefore, it can be concluded that solid-lipid nanoparticles have the combined advantages of various delivery systems such as the low toxicity of liposomes and fatty emulsions and are capable of providing sustained release occurred because of the solid matrix just like the polymeric nanoparticles and can exhibit targeted drug delivery if administered parenterally [2]. The SLNs are considered to be the favorable systems of drug delivery in advanced era of submicron-sized emulsions of lipid in which the solid lipid is being used in place of the liquid lipid (oil). These solid-lipid nanoparticles exhibit several properties like nano ranged size, larger surface area, increased drug loading and the interfacial interaction of phases. These systems have the capability to enhance the therapeutic performance of the pharmaceutically active materials [3]. Every oral route solid lipid nanoparticle formulation either involves any aqueous dispersion or they are loaded in any conventional dosage form such as capsules, tablets or pellets. The environment of stomach supports the aggregation of particles because of the high ionic strength and acidity. It is quite possible that the food present in the stomach will have a great effect on the performance of the SLNs, although no such experimental proof or data have been found yet as per our knowledge.
MATERIALS AND METHODS
Physicochemical characterization of the drug
Physical appearance test
The organoleptic properties such as appearance, colour and odour of the drug Roxatidine Acetate was observed and characterized.
Melting point
For the determination of melting point of Roxatidine Acetate, the capillary method was carried out in which the capillary tube was filled with drug up to 4mm at one sealed end. This capillary tube was then placed in the melting point apparatus and turned up the temperature and recorded the temperature at which the drug starts melting and the temperature at which the complete drug gets melted.
Fourier transform infrared spectral analysis
The FTIR analysis of Roxatidine Acetate was done by using the potassium bromide disk. On the KBr-press, the pellets were made while applying 150 Kg/cm2 of hydraulic pressure. These KBr pellets were then scanned under the FTIR, between the range of 4000 to 400 cm-1 wave number and the resulted spectra was compared with the standard FTIR specta of Roxatidine Acetate to ensure the authenticity and purity of the drug.
Determination of absorbance maxima (?max)
Accurately weighed 100 mg of Roxatidine Acetate using digital weighing balance and then dissolved in small amount of methanol. This solution was poured into the 100 ml volumetric flask. The volume was made up to 100 ml with methanol to give stock solution of 1mg/ml or 100 ?g/ml. One ml of stock solution (1000 ?g/ml) was transferred to 10 ml volumetric flask and the volume was made up to the mark by adding methanol. Thus, the dilution with concentration 100 ?g/ml was prepared. 0.5 ml of stock solution (1000 ?g/ml) was transferred to 10 ml volumetric flask and the volume was made up by adding methanol. The resulting dilution was 50 ?g/ml. [4]
Method validation for Roxatidine Acetate in 0.5% Iodine solution in DCM
Calibration plot for Roxatidine Acetate in 0.5% Iodine solution in DCM
100 mg of Roxatidine Acetate was accurately weighed on calibrated digital weighing balance and was dissolved in small quantity of methanol. The solution was then transferred to 100 ml of volumetric flask. The volume was made up to 100 ml to give stock solution of 1mg/ml or 1000 ?g/ml. Now from the stock solution, 0.04, 0.08, 0.12, 0.16 and 0.2 ml of solution was transferred into 10 ml volumetric flasks and added 1.2 ml 0.5% w/v iodine solution (prepared in DCM) to each volumetric flask.
Linearity and Range
Linearity is the ability of the method to elicit the results of test samples that are directly proportional to analyte concentration within a given range [ICH, Q2 (R1) guidelines, 2005].
Accuracy
It represents the closeness of agreement between the value which is accepted either as a conventional true value or an accepted reference value and the value found [ICH, Q2 (R1) guidelines, 2005]. Accuracy was determined by performing recovery studies. It was performed by preparing different concentration levels (0.4, 1.2 and 2.0) ?g/ml.
Precision
The precision of an analytical procedure expresses the closeness of agreement (degree of scatter) between a series of measurement obtained from multiple sampling of the same homogeneous sample under the prescribed conditions [ICH, Q2 (R1) guidelines, 2005]. [5]
Robustness
It is the measure of capacity to remain unaffected by small, but deliberate variations in the method parameters and provides an indication of its reliability during normal usage [ICH, Q2 (R1) guidelines, 2005]. The robustness of proposed method was estimated by evaluating the influence on solvent if handled by two different analysts. The % R.S.D was determined for solution of 0.4. 1.2 and 2.0 ?g/ml by two different analysts.
Screening studies
Screening of the method for preparation of SLNs
METHOD 1 – Accurately weighed amount of GMS (Glyceryl mono stearate) was melted on water bath with soy lecithin by keeping at 60?C [6] and then mixed into chloroform. Solution of excess amount of distilled water with PVA and tween 80, preheated at same temperature was added to molten GMS. The mixture was homogenized at 1200 rpm for 20 mins and then sonicated on bath sonicator for 40 minutes. The solvent was evaporated by spray drying to obtain the solid lipid nanoparticles.
METHOD 2 – Accurately weighed amount of GMS and soy lecithin were melted together on the water bath by keeping at chloroform and then mixed mixture was added to the water, tween 80 and PVA solution (preheated at 70?C) using a syringe with needle no. 21 and stirring at 1800 rev/min for 30 mins. The solution was then sonicated for one hour to obtain a clear solution. The solvent was evaporated by spray drying to obtain the solid lipid nanoparticles.
METHOD 3 – Accurately weighed amount of GMS was melted with soy lecithin on water bath by keeping at 60?C and then mixed into chloroform. Preheated solution of water, tween 80 and PVA at same temperature was added to lipid phase using syringe and needle. The solution was homogenized at 1800 rpm for one hour and then sonicated on a bath sonicator for two hours to get the clear solution. The solvent was evaporated by spray drying to obtain the solid lipid nanoparticles.
Effect of preparation technique on SLN formulation
The composition of SLN formulations prepared by using solvent evaporation technique. GMS and soy lecithin were weighed accurately and weighed amount of Roxatidine Acetate was mixed with them. The ratios of the components were varied. The solid mixtures were molten in water bath by keeping the temperature at 60°C and then mixed in to the organic solvent chloroform. Excess amount of distilled water (25 ml) with polymer PVA and surfactant Tween 80, preheated at the same temperature was added into the lipid phase using syringe and needle while stirring. The mixture was then homogenized at 1800 rpm for one hour. The obtained dispersion was then sonicated on a bath sonicator for two hours to reduce the size of the particles and get a clear solution. The solvent was evaporated by spray drying technique and obtained solid lipid nanoparticles in powdered form.
Formulation Development trials
Preparation of optimized formulation by DoE technique
A Central composite design (CCD) was selected for four factors at three levels (X1, X2, X3 and X4) to optimize the response variables Y1 and Y2 respectively i.e. entrapment efficiency, in-vitro drug release at second hour. Design expert software was used for employing this design. Summarizes an account of seventeen experimental runs studied. Formulation at central point (0, 0) was studied in quintuplicate. Three levels -1, 0 and +1 were decided. On the basis of the preformulation studies, formulations were designed. CCD for four factors at three levels, each was selected to optimize the varied response. Design expert software was used for employing this design. The translation of coded factors levels and value of variables is listed in table 1.
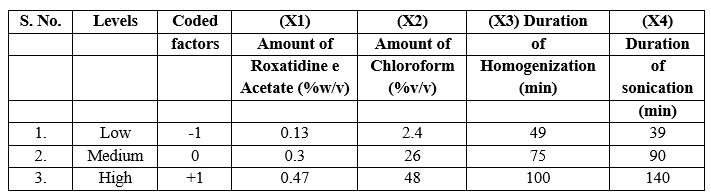
Table No.1 Translation of experimental conditions into physical units
Characterization and evaluation of SLNs
Optical microscopy
Optical microscopy was done by optical Microscope at 100 X using Oil immersion lens for viewing the abundance of solid lipid nanoparticle system and their physical appearance.
Transmission Electron Microscopy (Tem)
A drop of a sample was placed onto a carbon-coated grid and allowed to dry. The grid containing the sample was observed under the transmission electron microscope with an accelerating voltage of 120 kV. The nanoparticles were observed by focusing the lens.
Particle size and size distribution analysis
Particle size was observed by Photon Correlation Spectroscopy (PCS) using Zeta sizer for the optimized SLN formulation, which was prepared by single emulsification solvent evaporation technique. The particle size and the size distribution were observed and were reported for the optimized formulation.
Scanning Electron Microscopy
The physical appearance of the powdered solid lipid nanoparticle product of the optimized formulation was observed under the scanning electron microscope. In vitro drug release of the suggested formulations was determined through diffusion membrane (mol. Size 12000- 14000 Da). 2 ml SLN dispersion was filled in the membrane and the membrane was placed in the dissolution media of 150 ml 0.1 N HCl, 0.02% w/v tween 80 and 5% v/v methanol. The temperature was maintained at 32 °C ± 2 °C and stirring was set up to 100 rpm. 5 ml solution was extracted at out at the time interval of 5, 10, 15, 20, 25, 30, 45, 60, 75, 90, 105 and 120 min and was replaced every time with fresh media to maintain sink condition.[7]
Filling of prepared SLNs in capsules
The solid lipid nanoparticles dried by spray drying were filled in hard gelatin capsule shells along with the excipients. Lactose monohydrate was used as a diluent and 2% talc was used as a glidant. We obtained 5.8 g of SLN product from spray drying in which 1.2 g of drug was present. Roxatidine Acetate has the doses of 120 mg and 60 mg in capsules. To prepare a 60 mg capsule we took 290 mg of SLN product and mixed it with excipients to fill the capsule. Table 2 shows the composition of the Roxatidine Acetate SLN capsule. The capsules were filled manually using capsule filling machine.

Table No. 2 Composition of Roxatidine Acetate SLN capsules
Evaluation of Roxatidine Acetate SLN capsules
Weight variation
20 filled capsules were taken. Weighed one of the capsule on the digital balance and noted it reading. Then emptied the capsule completely and weighed the empty shell. By subtracting the filled capsule weight with empty capsule weight, the net weight of the content was determined. The process was repeated with all other capsules.
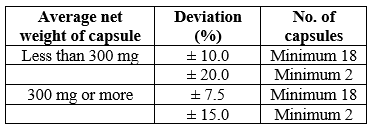
Table No. 3 Limits of weight variation of capsules
Stability study of SLN capsule
The stability study of SLNs were carried at different temperatures i.e. 25 ± 2°C (Refrigerator; RF) and under stress conditions 50 ± 2°C for a period of 15 days. The samples were taken periodically to analyze drug content for SLN capsule.
Analysis of Release Mechanism
In vitro release kinetics of Roxatidine Acetate from SLN Capsule was analyzed by mathematical modeling. The in vitro drug release data obtained were fitted to various release kinetics models, first- order, Higuchi, Hixson-Crowell cube root, Korsemeyer-Peppas and zero-order mathematical models. Selection of a suitable release model was based on values of r2 (correlation coefficient), k (release constant) and n (diffusion exponent) obtained from curve fitting of release data. In various kinetic models with their equations are depicted.
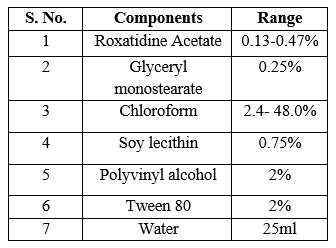
Table No. 4. Preparation of solid lipid nanoparticles by solvent evaporation technique
RESULTS AND DISCUSSION
Identification and characterization of itraconazole
Physical description
The sample of Roxatidine Acetate was identified and characterized results are shown in table 5.
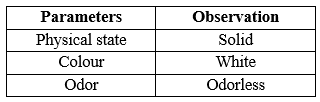
Table No. 5 Identification and characterization of Roxatidine Acetate
Melting point analysis
The observed experimental melting point by capillary method reported melting point was 145?C.
Identification of the drug Roxatidine Acetate by FTIR spectra
Fig. 1 Structure of Roxatidine Acetate
Determination of absorption maxima (?max) of Roxatidine Acetate
The ?max of Roxatidine Acetate was found to be 277 nm in 0.5% w/v Iodine solution in DCM. The scanning of the drug was done in the range (200-600 nm) as shown in the Fig. 2.
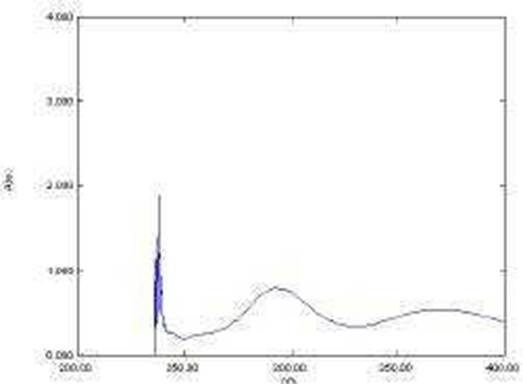
Fig. 2 Scan of Roxatidine Acetate in 0.5% w/v Iodine solution in DCM when scanned between 200- 600 nm.
Calibration curve of Roxatidine Acetate in 0.5% w/v Iodine solution in DCM
The calibration plot of Roxatidine Acetate was prepared by taking 0.4, 0.8, 1.2, 1.6, 2.0 ?g/ml concentrations of Roxatidine Acetate in 0.5% w/v Iodine solution in DCM as shown. The experiments were performed in triplicate to find the standard deviation and percentage relative standard deviation. Absorbance range was found to be 0.147 - 0.745.
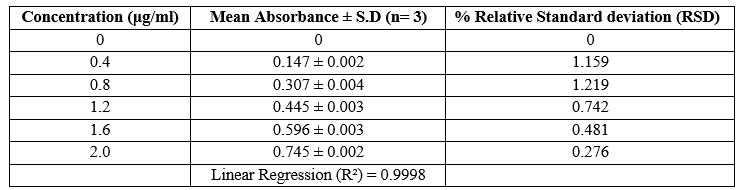
Table No. 6. Absorbance of Roxatidine Acetate in 0.5% w/v Iodine solution in DCM at nm.
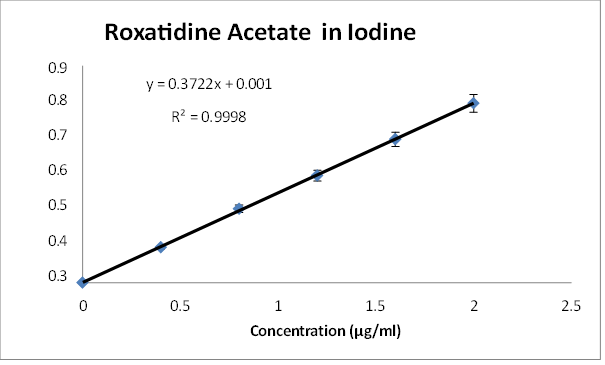
Fig. 3. Calibration curve of Roxatidine Acetate in 0.5% w/v Iodine solution in DCM at 368 nm
Accuracy
Accuracy results as shown in table 6.4 displayed good reproducibility with RSD value below
2. The method was found to be accurate as percentage recovery was found to be within the range of 97.9 – 99.2. These results proved that the method is accurate.
Formulation development trials
Optimization of solid lipid nanoparticles by central composite design
The design of optimization contained four independent variables (X1, X2, X3, X4) and two dependent variables (Y1 and Y2). The X variables were drug (% w/v), organic solvent (% v/v) Homogenization time and Sonication time respectively, whereas, the Y variables were percentage entrapment efficiency and in-vitro % drug release. According to the design, 17 formulations were suggested.
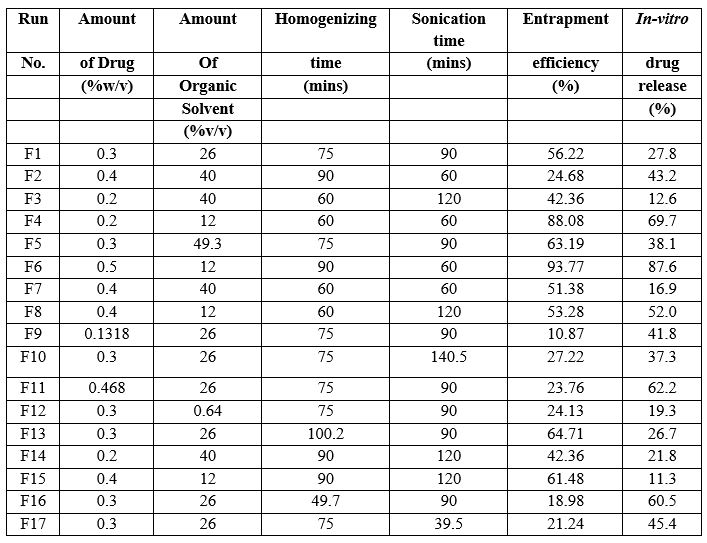
Table No. 7. Factor combination and responses
Characterization and Evaluation of SLNs
Transmission electron microscopy (TEM)
TEM photomicrographs of the representable solid lipid nanoparticles dispersion are shown in Fig 4. The grid containing the sample was observed under the transmission electron microscope with an accelerating voltage of 120 kV with magnification between 190000 X –290000 X.
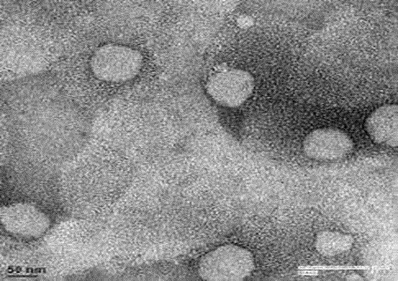
Fig. 4. Transmission electron micrograph of SLN formulation F6 with the magnification of
Entrapment efficiency
The entrapment efficiency of all the 17 formulations. From the entrapment data, it was observed that the ratio of the components within an optimum range offered good entrapment efficiency. The effect of the homogenization and sonication time was also determined from the study.
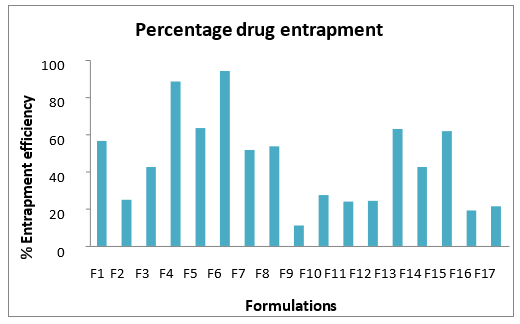
Fig 5. Percentage drug entrapment efficiency of various solid lipid nanoparticle formulations
Percentage in-vitro drug release
In vitro drug release of formulation through diffusion membrane (mol. Size 12000- 14000 Da) at second hour was done which was considered as one of the response in optimization study. The cumulative release of different formulation batches. Fig.6 showed the percentage drug release data at 2nd hour. Drug release was found to be in range of 11.3 – 87.6 %. Generally, the drug release was found to increase within an optimum range of lipid and surfactant which could be due to the fact that the release of the drug through the torturous structure of solid lipid nanoparticles delays the drug release.
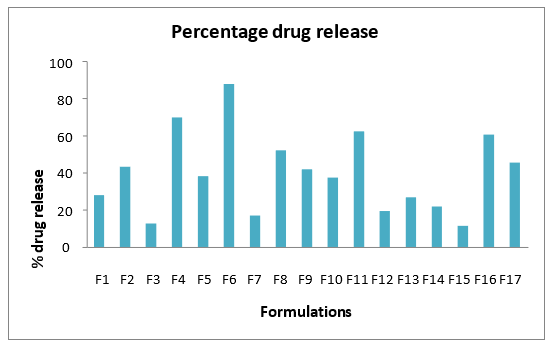
Fig 6. Percentage drug release profile of various solid lipid nanoparDticle formulations
CONCLUSION
The optimized SLN capsules was studied for various evaluation parameters such as in vitro release, SLN stability and release kinetics. The prepared SLN capsule was compared with the marketed Roxatidine Acetate capsule preparation to observe the difference in the performance by carrying in vitro dissolution study of both the capsules, it was observed that the SLN formulation enhanced the drug release 1.1 times as compared to the marketed Roxatidine Acetate capsule. From the different studies which were carried on SLN dispersion and SLN capsule, it could be concluded that the optimized Roxatidine Acetate SLN capsules presents with a promising formulation to treat obesity and may be further studied to convert it into a commercial product.
REFERENCE
-
-
-
- Ahlin P., Kristl J., Kobar S., 1998. Optimization of procedure parameters and physical stability of solid lipid nanoparticles in dispersion. Acta Pharm. 48, 257–67
- Aronne LJ., Segal KR., 2003. Weight gain in the treatment of mood disorders. J Clin Psychiatry. 64(Suppl 8),22–29.
- Baigent C., Keech A., Kearney PM. 2005. Efficacy and safety of cholesterol-lowering treatment: prospective meta-analysis of data from 90,056 participants in 14 randomised trials of statins. Lancet 366, 1267–1278.
- Barbier P, Schneider F., 1987. Syntheses of tetrahydrolipstatin and absolute configuration of tetrahydrolipstatin and lipstatin. Helvetica Chimica Acta. 70 (1), 196–202.
- Bummer PM., 2004.Physical Chemical Considerations of Lipid-Based Oral Drug Delivery—Solid Lipid Nanoparticles. Therapeutic Drug Carrier Systems. Volume 21, Issue 1, 20 pages.
- Casadei MA., Cerreto F., Cesa S., Giannuzzo M., Feeney M., Marianecci C., Paolicelli P., 2006. Solid lipid nanoparticles incorporated in dextran hydrogels: A new drug delivery system for oral formulations. International Journal of Pharmaceutics. 325,140–146.
- Cavalli R., Gasco MR., Chetoni P., Burgalassi S., 2002. Solid lipid nanoparticles (SLN) as ocular delivery system for tobramycin. International Journal Pharm. 238, 241 – 245.


 Jyoti Prajapati*
Jyoti Prajapati*













 10.5281/zenodo.13096779Jyoti Prajapati
10.5281/zenodo.13096779Jyoti Prajapati