The sunlight consists of harmful radiations which affects the skin. The Ultraviolet radiations are of types Ultraviolet A, Ultraviolet B and Ultraviolet C. This article gives a detailed review on different types of Ultraviolet radiation. To protect our skin from Ultraviolet radiation sunscreen formulations are used which either absorbs scatters or reflects the radiation. The harmful effects on skin like photo aging, skin cancer, DNA damage are explained. This study aimed to formulate, characterize, and evaluate an innovative sunscreen cream enriched with from Juglans regia and carotenoids. The formulation process involved the incorporation of Juglans regia and carotenoids into a base cream matrix optimized for sun protection efficacy. The cream was characterized for its physical properties, including colour, odour, pH, viscosity, spreadability, and stability. UV absorption spectra were obtained to assess the sunscreen activity of the formulation. In-vitro evaluation was conducted to determine the sun protection factor (SPF) and broad-spectrum coverage against UVA and UVB radiation. The results demonstrated that the incorporation of Juglans regia and carotenoids enhanced the SPF and broad-spectrum coverage of the sunscreen cream. Additionally, the formulation exhibited desirable physical properties and stability over the study period. These findings suggest the potential of utilizing natural from Juglans regia and carotenoids as effective ingredients in sunscreen formulations, offering protection against harmful solar radiation while providing skin-nourishing benefits
Sunscreen cream, Skin burn, SPF, Juglans Regia, Carotenoids.
Skin is the largest and outer part of the body, which is directly exposed to sun may leads to photo damage of skin which is most [1] The major effect of solar radiation is caused by the ultraviolet region of the electromagnetic spectrum. It may cause effects to the eye, skin, and Immune system. Extend exposure of UV radiations may initiate the production of reactive oxygen species, may causes oxidative injury and impairment of the antioxidant system[2]. These may lead to impair the metabolic pathways of the skin, which leads to photo aging, erythematic, edema sunburn, lines and wrinkles, photosensitivity, immunosuppressant, DNA damage and skin cancer in most severe conditions.[3] These sunscreens are incorporated in many cosmetic formulations like creams, lotion and moisturizer and other skin care products.[5] The main use of sunscreen is to preserve the skin against UVA and UVB rays and to spread the moisture content of skin and its own natural oils, which may be lost during the expose to solar radiation.[6] The sunscreen should be safeguard, chemically inert, non-irritating, nontoxic and photo stable.[7] The skin’s natural sunscreens such as squalane, proteins, absorbing lipids and nucleotides have been used from many years.[8]
Sunlight reaching the surface of the earth contains: Visible rays, Ultra-violet rays, Infra-red rays UV Rays (particularly wavelength below 320 mµ) are responsible for most of the therapeutic as well as noxious effects that we attribute to sun light. The UV Spectrum is broken in to three parts:
Ultraviolet is categorized in three ranges: [9]
- UVA is radiation in the 320-400 nm range
- UVB is radiation in the 290-320 nm range
- UVC is radiation in the 100-290 nm range
SELECTION OF SUNSCREEN CREAM BASED ON SKIN TYPES[10]
There are 4 types of skin explained below.
Normal Skin:
If your skin shows no oil or no flaking and it feels smooth and supple, then hooray! You have a normal skin type.
Oily Skin: If there is lots of grease on the tissue paper, then you have an oily skin type. It is common that you might have a shine and large pores.
Dry Skin:
If the tissue paper is accompanied by lots of flakes and dead skin, then your skin is dry. You need to consider moisturizing your skin.
Combination: Any combination of the abovementioned skin types is a combination skin type. This is very common and most of you might as well have this skin type. Your skin is generally oily in the forehead and nose area and dry elsewhere.[11]
CLASSIFICATION OF SUNSCREEN [12]
Sunscreens can be classified as follows [13]
- Based on the mode of action they can be classified as
- Physical sunscreen:
Reflect harmful rays away from skin. Eg: zinc oxide and titanium dioxide.
- Chemical sunscreen:
Absorbs UV rays Eg: microfine titanium dioxide, avobenzone and oxybenzone. The combination of both physical and chemical active ingredients is considered to be a best sunblock. Physical sun blocks are having scattering affect thereby results in whitening phenomenon while majority of organic chemicals used in sunscreen formulations have not been established as safe.
- Based on application
- Topical:
They either absorb or reflect radiation to protect from harmful radiation
- Oral:
These are consumed orally to avoid skin damage.
Eg: Carotenoids Topical sunscreens are divided into two classes based on their mechanism of protection
- Organic sunscreen
- Inorganic sunscreen
- Organic Sunscreen:
Organic sunscreen works by absorbing into skin and converting UV rays into heat .it is thin and ideal for everyday use allow for skincare ingredients to be added easily. Organic sunscreen actives chemical carbon-based compound .it contains non mineral active ingredient.
These are particles that scatter and reflect uv rays back to the environment they act as physical barrier to indent ultraviolet and uv light. They are considered broad spectrum as they cover entire ultraviolet spectrum. the Inorganic sunscreen is also referred to as sunblock.
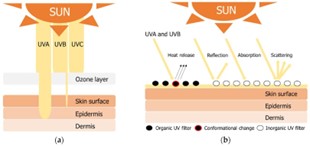
Fig 1. Mechanism of photoprotection
MATERIALS AND METHODS:
Collection of oils: -
The collection of Walnut oil (Juglans Regia) is purchased from Essential Extracts, Lajpat nagar -2 New Delhi-110024 From www.flipkart.com
The collection of Carrot seed oil (Carotenoids) is purchased form Turfy Private Limited, Varthur Hobli, outer ring road, Bengluru, Karnataka, 560103
- WALNUTS OIL
Kingdom- Plante
Class- Magnoliopsida
Family-Juglandaceae
Uses-Self-tanning sunscreen agent
Genus- Juglans
Chemical constituents- Oleic acid, Linoleic acid


Fig No 2 WALNUTS OIL
- CAROTENOIDS OIL[15]
Kingdom- Plante
Biological name- Zeaxanthin
Uses-Provide a sun protective effect cause up to SPF
Chemical constituents-Alpha- carotene, Beta-


Fig 3. CAROTENOIDS OIL
Preparation [16]:-
Step -1. Water Phase
Water phase was prepared by collecting deionised water and make up final volume and add water soluble components Disodium EDTA, Methyl paraben, Triethylamine were dissolved in deionised water and add stearic acid, Cetyl alcohol, Cetosteryl alcohol and Carbopol was allowed to swell using a Homogenizer and heated up to 80-degree c. and quantity will be taken according to the batch preparation (F1,F2,F3).

Step-2. Oil Phase
Oil phase was prepared by heating oil containing Juglans Regia (walnut oil) and carrot seed oil at 80-degree c, was inculpated separately for each cream and quantity of oil was take according to Batch formulation.

Step-3.
Oil phase was added in water phase at 80-degree c, with continuous stirring for 20-25 min and then it was homogenized till uniform emulsion is formed. And semisolid consistency. It was then poured. Then width mouth container and stored at temperature not exceeding 37-degree c.

Final product (F1,F2,F3)
FORMULATION OF SUNSCREEN CREAM:
Table no 1 Ingredient
EVALUATION PARAMETER:
- Physical parameters:
Colour:
The colour of formulation was checked manually and observed.
Odour:
The smell of Formulation was checked by applying preparation on hand and feel the fragrance.
Appearance:
Visually checked the appearance of the formulation.
- Consistency:
The consistency was evaluated by applied on the skin.
- Determination of pH:[17]
The Ph of sunscreens was determined using a digital pH meter. pH was measured after 1g of the formulation was dissolved in 100ml of newly prepared distilled water for 2 hours. The purpose of this study was to guarantee that the pH of the produced sunscreens is similar to the pH of the skin after 24 hours of use. The results were triple-checked, and S.D. was recorded.
- Determination of Viscosity:[18]
The Brookfield viscometer was used to test viscosity, with the proper number of spindles selected. A 50 ml beaker was used to hold 40 gm of preparation until the spindle groove was dipped and rpm was set. Sunscreen viscosity was measured at 5, 10, 20, 50, and 100 rpm. The viscosity was computed using the factor obtained from the reading.
- Spreadability [19]
The spreadability of sunscreens determined by two slides are taken and sample was placed on one slide. Another slide was placed on the first slide. 100g of weight was kept on the slides so that it spreads as a thin layer. Weight was being eliminated much high than the prisons. Next weight of 20g was kept on the upper slide. It was kept on the upper slide. Spreadability was calculated by using following formula,
S=M. L/T
Where,
S- Spreadability
M- Weight tied to the upper slide (20g)
L- Length of the glass (7.04)
T- Time
- Irritancy Test
Mark an area (one sq.cm) on the left hand dorsal surface. The cream was applied to the specified area and time was noted. Irritancy was checked if any for regular interval up 24hrs and reported.
- Homogeneity [20]
The formulation was tested for the homogeneity by visual appearance and touch.
- Rancidity [21]
Rancidification is the process of complete or incomplete oxidation or hydrolysis of fats and oils when exposed to air, light, or moisture or by bacterial action, resulting in an unpleasant taste and odor. Rancidity is performed by using the Phloroglucinol solution. The rancidity is due to the oxidation of the fats and oils; during oxidation free fatty acids are liberated. These free fatty acids react with the Phloroglucinol solution and give pink color indicating the rancidity of the product. 10 ml of cream was taken then added 10 ml of concentrated Hydrochloric acid and 10 ml of Phloroglucinol solution and shaken for one minute. The cream should have passed the test if no pink color develops.
- Solubility of sunscreen cream [22]
To obtain the best solubility of the prepared samples, a different mixture of ethanol (polar solvent) and hexane (non-polar solvent) were used as shown in Table 6 (entries 1-4). In brief, the best solubility of sunscreen cream is obtained using equal proportions of hexane and ethanol (entry 3).
- Stability Testing[23]
Stability tests were achieved at different conditions for emulsions to explore the effect of these conditions on the storage of emulsions. These tests were performed on samples kept at 8 C±2 C, 25 C±2 C and 40 C±2 C. Color, phase separation and liquefaction of emulsions were observed at various time intervals during 28 days.
- Determination of SPF [24]
Weigh 1 g of all samples, transfer to a 100 mL volumetric flask, dilute to volume with ethanol, followed by ultrasonication for 5 min and then filter through cotton, rejecting the first ten ml. Transfer a 5.0 mL aliquot to 50 mL volumetric flask and dilute to volume with ethanol. Then transfer a 5.0 mL aliquot to a 25 mL volumetric flask and complete the volume with ethanol. Measure the absorptions of samples in solution in the range of 290 to 450 nm with every 5 nm increment using 1 cm quartz cell, and ethanol as a blank. Calculate average of three determinations and calculate SPF by Masur equation. EE* I value is constant and given in 320 spectrophootometric 290
SPF= CF? EE (? ) I (? ) Abs (? )
Where: EE (I)–erythemal effect spectrum; I (l)–solar intensity spectrum; Abs (l)–absorbance of sunscreen product; CF-correction factor (=10).
- Antimicrobial Activity [25]
Cup plate of cylinder plate method
This method relies on the diffusion of an antibiotic from a vertical cavity or a cylinder through the solidified agar layer in a petri plate. The growth of test microorganism in observe to be inhibited in a circular area or zone around the cavity containing antibiotic solution.
Steps involved in cup plate method are given below,
-
- A liquefied assay medium (43–45-degree c.) is inoculated by the suspension of test microorganism.
- This inoculated test culture medium poured and spread on sterile petri or preprepared agar plates.
- Standard and test antibiotic solution of known concentration are prepared in appropriate solutions, which are then added to sterile cavities prepared on solid medium.
- Uniform volume of sodium should be added to each cavity to fill them sufficiently if paper discs are used, they should sterilize first, then dipped in standard or test Cup solution and finally placed on medium surface.
- The plates are allowed to stand at room temperature or at 4degree c. for 1-2 hours. This is the period of Pre Incubation diffusion which minimises the effect of variation time between the applications of different solutions.
- All plates are then incubated at temperature 32–35-degree c. for 18-24 hours.
- The diameters or areas of circular inhibition zone produce by standard and antibiotic solution are accurately measured
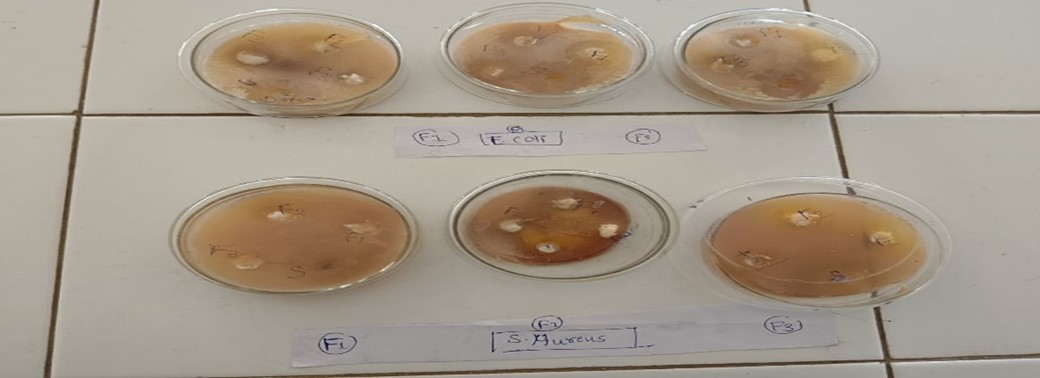
Fig 7 Antimicrobial testing (Cup plate method)
RESULT AND DISCUSSION:
Table no 2 Determination of physical Parameters
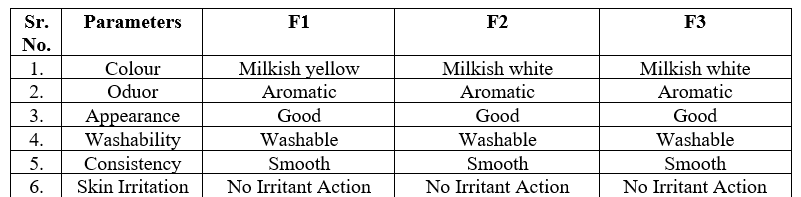
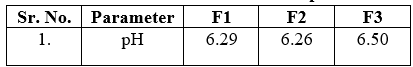
Table no 3 Determination of pH
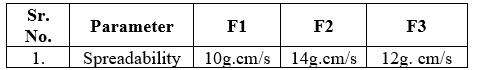
Table no 4 Determination of Spreadability
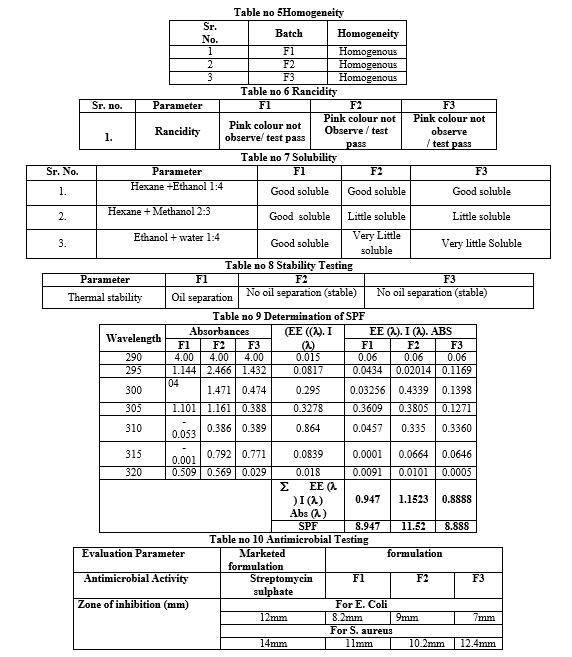
Fig no 8 Antimicrobial testing (Cup plate method)
CONCLUSION:
Thus, it can be concluded that there is great market potential for sunscreen chemicals either synthetic or natural or in combination due to awareness of protection from hazardous UVA as well as UVB rays. Photo-stable, uniform UVA/UVB protective sunscreen product with high SPF can be minimum ideal requirement but natural chemicals like polyphenols (flavonoids, tannins), carotenoids, anthocyanidins, few vitamins, fixed oils and volatile oils from vegetables, fruits, medicinal plant parts (leaves, flowers, fruits, berries), algae and lichens are more effective due to their long-term beneficial effects especially against free radical generated skin damages along with UV-rays blocking. These natural chemicals incor- porated sunscreens might provide cost effective, truly broad spectrum sunscreen products with antioxidant, wound healing, anti-inflammatory and many more skin protective effects.
ACKNOWLEDGEMENT:
The success and final outcome of this project required a lot of guidance and assistance from many people and I am extremely privileged to have got this all along with the completion of my project. All that I have done is only due to such supervision and assistance and I would not forget to thank them. I am highly thankful to the department of pharmacy, MANOHARBHAI PATEL INSTITUTE OF B. PHARMACY KUDWA GONDIA (M.S) for providing various facilities during experiments.
I delightedly wish to express my respect and gratitude to the Principal, Mrs. Manisha Mishra for her support and encouragement and for leading to all the facilities required to proceed with our study.
I own my deep gratitude to our project guide, Mr. Rahul G. Chaudhari, who took a key interest in our project work and guided us all along, till the completion of our project work by providing all necessary information for a good system. I also want to thank to Mr. Uikey sir, Mr. Meshram sir, Mr. Vinit Patel Sir, Mr. Naveen Tank Sir, for their support throughout the project. Last but not least, I express a deep sense of gratitude to my parents, my family, my friends, for their kindness and valuable support.
REFERENCES
- Wells FV, “Cosmetics and the skin”. Reinhold Book Corporation, London. 1969; Page No.8.
- Balogh TS, et al., A Bras Dermatol. 2011: Page No.732-742.
- Butler H, “Textbook of Poachers, Perfumes, Cosmetics and soaps”. 2000; Edition 10, Page No. 468
- Radice M, Manfredini S, Ziosi P, et al., Fitoterapia. 2016, 114; Page No. 144-62.
- https://www.fda.gov/radiation-emitting-products/tanning/ultraviolet-uv-radiation
- Ankita Thakur, “Comparative life cycle assessment chemical versus Nano-titanium dioxide as UV blocker” (2014); Page no 67-70.
- Kaimal S., Abraham A. Sunscreens; Indian J. of Dermatology, Venereology & Leprology, (2011) vol.77,2.
- 8.Harborne JB, Phytochemical methods, A guide to modern techniques of plant analysis; Springer publication. New Delhi 2005,3rd edition, Page no 138-144.
- Postaire E, Jungmann H, Bejot M et al. Biochem Mol Biol Int 1997: 42: 1023–1033.
- Geoffrey K., Mwangi AN., “Sunscreen products: Rationale for use, formulation development & regulatory consideration” Saudi Pharmaceutical Journal, (2019), Page No. 56.
- Fakhri, N.A.; Qadir, K. J. Envir. Sci. Eng.2011, 5, 1-12.
- Fakhri, N.A.; Qadir, K. J. Envir. Sci. Eng.2011, 5, 1-12.
- Ali, M.; Ullah, A.; Ullah, H.; Khan, F.; Ibrahim, S.M.; Ali, L.; Ahmad, S. Pak. J. Nut. 2010, 9, 240-244.
- International Journal of Recent Development in Engineering and Technology Website: www.ijrdet.com (ISSN 2347-6435(Online) Volume 4, Issue 1, January 2015)
- Abdulrasheed A.1, Aroke U.O.2, Sani I. M.3Department of Chemical Engineering, Abubakar Tafawa Balewa University. P.M.B. 0248, Bauchi. Nigeria.
- 16.Diffey BL and Robson JJ, soc comet chem. 1989; page no: 127-33.
- Geeta vaman bhople, A.S. and Dr. Prachi udapurkar, Kishori college of pharmacy, Beed Dr. Babasaheb Ambedkar technological university, lonere volume 11 issue date 6 june 2023.
- Khoo HE, Azlan A, Tang ST, et al., J Food Nutri Res. 2017, 61(1): p. 1-21.[2] Balogh
- Sumeet D, Shailesh G. Acta Chim Pharm Indica, 2012, 2(1): p. 54-59.
- Mark R. Eur J Dermatol,1999, 9(5): p. 406-412.
- Mishra AK, Chattopadhyay P, “Herbal Cosmeceuticals for Photoprotection from Ultraviolet B Radiation”: A Review. Tropical Journal of Pharmaceutical Research. 2011; 10 (3): 351-360.
- Nash, J. F. & Tanner, P. R. (2009) Sunscreens. In Z. D. Draelos & L. A. Thaman (Eds), “Cosmetic Formulation of Skin Care Products “(pp. 3-14). Taylor & Francis Group, 270 Madison Avenuem, New York, US.
- 23.Silm Smaoui, hater ben hlima, ines ben chobba, adel Kadri department of life science BP 117.Date 27 February 2013
- Mukund manikrao donglikar, sharada laxman deore, “development and evaluation of herbal sunscreen”, pharmacognosy journal 2017; 9(1):83-97.
- Hoppe VU, Koppiow HJ, Wiseman, “A Statistical evaluation of light protection factors”, Arzneimittelforschung. 1975;(5):817-25, German.


 Rahul G. Chaudhari*
Rahul G. Chaudhari*
 Akash R. Lilhare,
Akash R. Lilhare,
 Ankush.s sharnagat
Ankush.s sharnagat













 10.5281/zenodo.10974800
10.5281/zenodo.10974800