Abstract
Diabetes mellitus (DM) is among the most severe and fatal diseases which cannot be transmitted. Insulin is commonly administered in the management of diabetes. Hyperglycemia, or elevated blood sugar, is a complication of diabetes mellitus, a chronic illness that is complicated and results from insufficiencies in the production, functioning, or combination. Many biodegradable and non-biodegradable polymers are currently being studied; however, non-biodegradable polymers have drawbacks such as toxicity, evacuation challenges, and inability to produce persistent insulin release over time. The majority of naturally produced polymers are currently used just like excipients in pharmaceutical compositions since they are often thought to be relatively safe in vivo. Multiple natural polymers, including proteins and polysaccharides, have recently been extensively researched as potential insulin mediums. The study highlights a wide range of naturally occurring polymers, including chitosan, alginate, gelatin, casein, pectin, cyclodextrin, dextran, and starch, demonstrating great potential towards treatment for diabetes-related problems. The natural polymers used to treat diabetes mellitus have been the subject of the present investigation, which has been successful in displaying a wide range of benefits, including enhanced encapsulation performance, blood glucose optimization, more persistent drug delivery, and patient acceptability. Additionally, a number of benefits like affordability, sustainability, safety, and accessibility to everyone support the continual improvement of a potential polymer incorporated insulin delivery system. In this review article an attempt has been made to demonstrate the use of natural polymers in improving the effectiveness of anti-diabetic formulation.
Keywords
Nature, Polymers, Diabetes, Mellitus, DM
Introduction
Diabetes mellitus (DM) has been recognized by man for over 2000 years1. Diabetes mellitus has been roughly pretentious to 451 million up until the present time.2 Diabetes mellitus, a chronic lifelong metabolic condition, has reached frightening proportions as a major global health issue.3 According to the most recent International Diabetes Federation figures, The most current data from the International Diabetes Federation indicate that if appropriate attempts are not made to stop the epidemic, almost 5,78,000 individuals would be negatively impacted by twenty-third century. The count of individuals will have increased to an unbelievable seven hundred million by 2045.4 Diabetes mellitus is classified into four categories: Type 1 diabetes T1D, often called dependent upon insulin diabetes, is a chronic condition; Type 2 diabetes, more commonly referred to as non-insulin-dependent diabetic mellitus T2DM, Gestational diabetes & diabetes caused by genetic modification.5 T1D is caused by an insufficient amount of insulin and is associated with an autoimmune response. This illness was originally referred to as insulin-dependent diabetic mellitus IDDM until being reclassified based on etiopathology.6 T2DM is primarily caused by inadequate insulin production from cells in the context of insulin resistance. Insulin resistance occurs when insulin cannot be efficiently used by cells after it is generated by the pancreas.7 Gestational diabetes is described as any degree of glucose intolerance that is initially identified during pregnancy, resulting in hyperglycemia of variable severity.8 Diabetes caused by genetic mutations can induce diabetes mellitus, just as mutations in a single gene can cause monogenic diabetes. The most common kind of monogenic diabetes is neonatal diabetes.9
These are certain severe issues that are been driven on by worsening diabetes mellitus. This Analysis reveals that the sickness raises the likelihood of acquiring additional serious conditions such as impaired kidney function, cardiac arrest, stroke, loss of vision, and amputation of the leg below the knee. As you can see, people with diabetes often have other illnesses that are extremely serious on their own. Therefore, in order to keep diabetes from taking control of one's ability to maintain a stable quality of life, several factors that promote health must be understood.10 Individuals with type 1 diabetes require insulin throughout their lives. Insulin is not a treatment for diabetes, which puts individuals at risk for catastrophic consequences such as heart and kidney damage, as well as blindness.11 The treatments for type 2 diabetes are exogenous production sources of substitute ?-cells, such as liver cells, stem cells that are pluripotent, donated mammalian pancreases, and fetus pancreatic tissue, are used as an additional therapy for type 2 diabetes. While the majority of exogenous supplies are heterologous with respect to the recipient, several include naturally occurring, for example stem cells that are derived from fibroblasts in the skin and blood from the umbilical cord.12 There are now several oral therapies for diabetes of the second type that are not insulin-based. SGLT2 inhibiting agents, amylin antagonists, incretin mimetics, biguanides, insulin sensitizers, and insulin secretagogues. Recently, incretin mimetics like DPP-IV inhibitors and GLP-1 antagonists, SGLT2 antagonists/inhibitors, amylin agonists, and alpha glucosidase blockers are the most recent medication classes utilized to treat type 2 diabetes.13 While several novel drug deliveries are also denoted by NDDSs are being studied to treat different illnesses, only a fewer number have been found to treat type 2 diabetes. The two elements of the Particulate structure are the Microparticulate and Nanoparticulate systems, as well as liposomes and niosomes in the vesicles systems and other therapies such as self-nano-emulsifying is also called SNEDDS.14 In additionally the antidiabetic formulation can be developed by encapsulating the active ingredient in ethosomes and phytosomes.
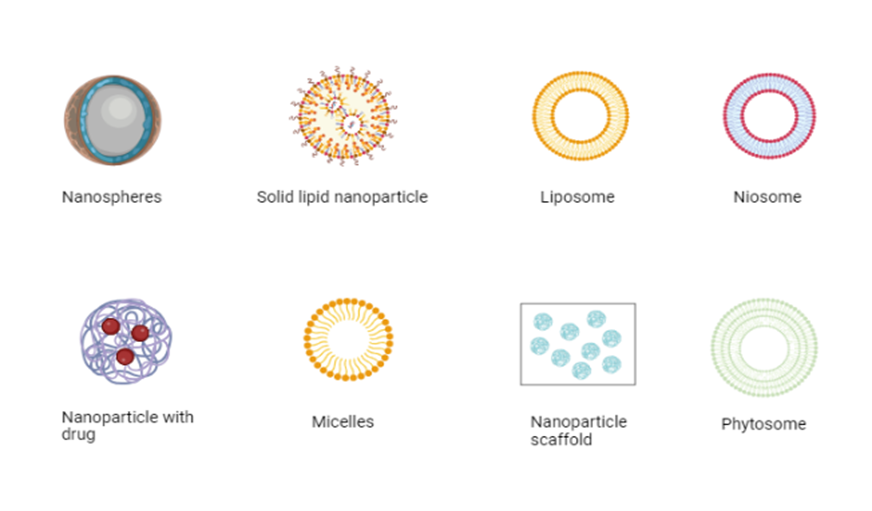
Figure 1: Current insight on anti-diabetic therapy: novel technologies (created with Biorender.com)
The life-style measures that can help in controlling blood-glucose levels: This information is based on several scientific investigations that shows modifying a person's routine can prevent or postpone the beginning of diabetes at a reduced cost, with a 58 percent decrease in threat after three years.15 The studies have shown significant improvement in controlling glycemic by preforming exercises and showed it can improve patients' general health and reduce the hemoglobin A1C significantly 0.66%, regardless of whether or not a significant decrease in body mass index is made.16 Moderate alcohol consumption like ?2 drinks for men, ?1 drink for women and intake of sodium to be reduced are other lifestyle measures that should be taken into account in the treatment plan for patients with diabetes, particularly in those who also have comorbid conditions like hypertension, habitual tobacco use, and a lack of immunizations like pneumococcal, hepatitis B, influenza, diphtheria, pertussis, tetanus, and tetanus.17 This review article highlights how the use of natural polymers enhances the anti-diabetic pharmaceuticals' effectiveness for delivery.
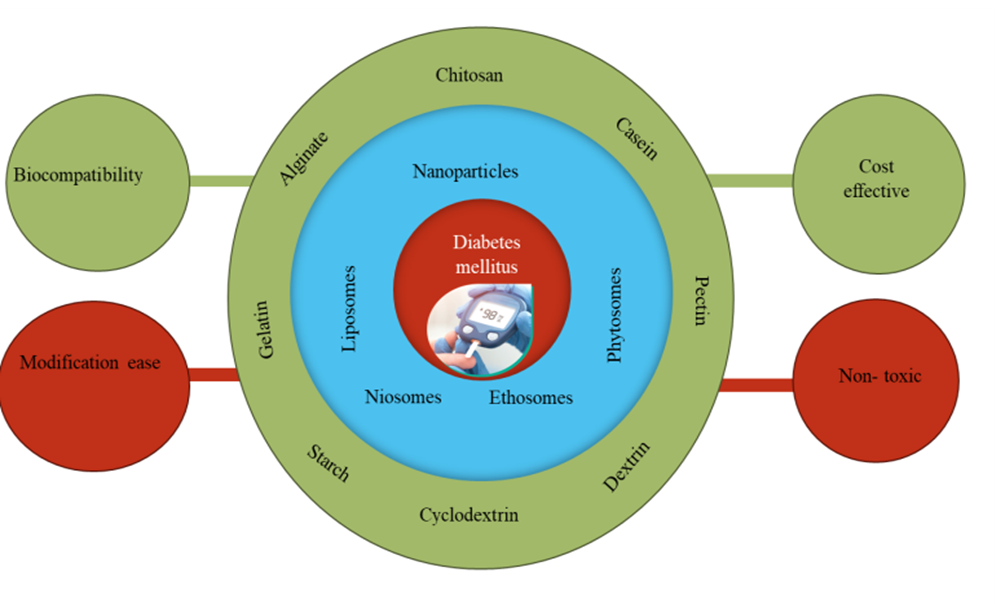
Figure 2: Natural polymers based on different novel deliveries and benefits of natural for the treatment of diabetes mellitus.
POLYMERS:
The importance of polymer science has grown over the past few decades as the potential for structural changes has improved. As polymer components come in numerous diverse forms, it is feasible to alter the physical and chemical features of nanoparticles, including entrapment efficiency, charge and customize them to maintain the stability of insulin, give an effective bioavailability, modulate the release nature, balance systems and modify biological activities.18 A polymeric substance is a large compound composed up of structural components that repeated or reoccur, commonly referred to as a macromolecule. Covalent chemical bonds are typically employed to join these subunits.19 There are two forms of polymers that can be used in employing anti-diabetic drug to improve the effectiveness in delivering the drug. They are natural and synthetic polymer.
Natural polymers:
The majority of natural polymers are now employed as excipients in pharmaceutical formulations as they have been demonstrated to be safe in vivo. Natural materials are preferable than artificial materials in terms of biocompatibility, accessibility, and modification ease. Additionally, as the original natural materials possess reactive groups, other functional groups may potentially be added to, offering the newly developed materials extraordinary functions, or changing their chemical and physical characteristics.20,21 Polymers also offer outstanding characteristics and often serve as nanocarriers for treatments, diagnostics, medication transport, and protection.22 This review article highlights how the use of natural polymers enhances the anti-diabetic pharmaceuticals' effectiveness for delivery.
Classification of natural polymers used for diabetes mellitus
There are 2 kinds of naturally occurring polymers viz: polysaccharide and protein: Polysaccharides Chitosan, Alginate, Dextran, Starch, so the other form is Pectin and Proteins which includes Casein and Gelatin. As polysaccharides are exceptionally durable, secure, environmentally friendly, and have gel-forming attributes, it is possible to modify them chemically and biochemically to make them suitable for consumption during oral protein administration.23 Charged polymers, like alginate as well as chitosan (CS), can electrostatically interact with differently charged components to create polyelectrolyte complexes PECs that produce ion pairing without compromising the fundamental properties of the polymer.24,25 Proteins are known as molecules possessing molecular weights more than 5000 Da, whereas peptides are defined as molecules with molecular weights around 500 and 5000 Da.26 Although being made up of amino acids, they are synthetically distinguished by factors such as molecular weights, spatial conformations, and amino acid units.27
Chitosan:
Chitin, which is sourced from the cuticles of insects, the exoskeletons of crustaceans, and the cell walls of fungus, is alkaline deacetylated to form chitosan, a kind of polycationic polysaccharide.28,29 Chitosan is also well-known carbohydrate polymer, has gained a lot of attention lately due to its biocompatibility, Low toxicity, readily available, and biodegradable.30 Numerous types of chitosan have shown promising effect of improving the poor lipid and glucose metabolism associated with diabetes mellitus. However, chitosan has also produced a number of innovative drug carriers that can be used to transport antidiabetic medications to their intended locations. The present research emphasizes the rising significance of chitosan as polymer-based formulations for the administration of antidiabetic medications to achieve improved control of hyperglycemia and summarizes the possible actions of chitosan in modulating impaired blood sugar and fatty acid metabolism correlated with diabetes mellitus.31 Chitosan can be produced into powders or beads. It typically appears as white, yellowish flakes. Additionally, the Deacetylation (DD) plays a vital role to the molecular chitosan weight. Specifically, a lower DD corresponds to a larger molecular weight, resulting in increased chemical stability and mechanical strength. Chitosan has an average molecular weight of around 1.2 × 105 g mol-1.32
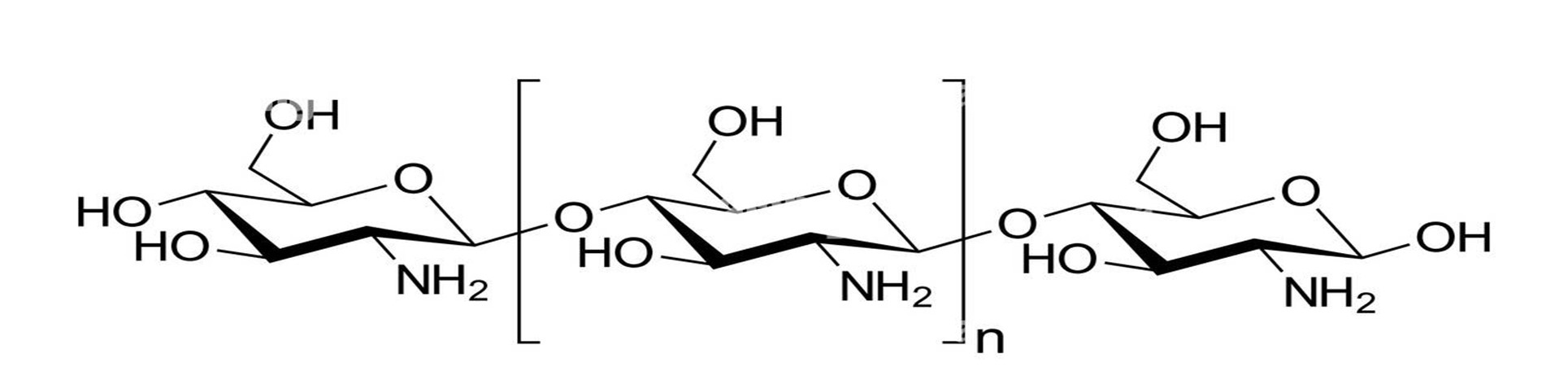
Figure 3: Structure Of Chitosan
Chitosan is cationic in nature as a result of containing amino and hydroxyl groups. Chitosan is capable of being modified chemically and physically by a variety of techniques including as grafting, complexation, crosslinking, and blending. Hydrogen bonds exist in the molecular structure of chitosan, resulting it to be a rigid polymer.33
Researcher named Florentina Geanina Lupascu et.al 2015 designed xanthine analogues to enhance the chitosan-based scaffold's biological and pharmacokinetic attributes. It was observed that the experiments showed a decrease in the blood glucose level with 59.30% and 4.53% of glycosylated hemoglobin. The formulation containing chitosan formulation (CS-6) displayed lower blood glucose level (114.5 mg/dl) than the one induced by pioglitazone which was was 148.5 mg/dl while taken alongside a regular diabetes medication.34
Jubril Olayinka Akolade et.al 2017 specified the use of chitosan alginate beads polymeric complex with curcumin as therapeutic agent for diabetes mellitus. The studies revealed an improved encapsulation efficiency was (64–76%), loading capacity was (20–26%) and yield was (50–72%). Moreover, curcumin's biological properties, retaining status, and chemotherapeutic functionality were all substantially improved due to its nanoencapsulation in chitosan based polyelectrolyte complex. Further, the complex reduced loss of curcumin by 20% & also extended mean release time by 40 minutes in simulated gastric fluid.35 This case report is on chitosan being an effective polymer for formulating antidiabetic formulation reported by E. Jaisankar et.al 2020. They developed chitosan co-polymer membranes fabricated from thiourea, phenyl-hydrazine & formaldehyde via polycondensation method loaded with metformin as an anti-diabetic agent. The metformin loaded tablet formulation were developed. The results showed improved antidiabetic effect by inducing a sustained release of the formulation. Additionally, the developed nanocomposite displayed antimicrobial development properties providing its potential to recover from wounds in diabetic individual.36
Alginate:
Alginate is a renewable polysaccharide comprised of two monomers: mannuronic and guluronic acid. Seaweed develops a lengthy chain of alginic acid and salts. Natural alginate is biocompatible but not biodegradable under physiological conditions. Therefore, it is dissolved in divalent ions like calcium and utilized for wound dressing, scaffolding, and hemostats.37,38,39 Alginate originates from brown algae called Phaeophyceae comprising Laminaria hyperborea, Laminaria digitata, Laminaria japonica, Ascophyllum nodosum, and Macrocystis pyrifera, employing water-based alkaline solutions, usually NaOH.40 Alginate is a negatively charged polymer. Therefore, it is widely studied and is also been used in biomedical fields because of its relatively safe, harmless gelation with divalent cations like calcium cation, and biological suitability.41 Alginate is a remarkable polymer which offers numerous benefits and has recently been widely used in the development of controlled-release systems that deliver medications.42 The molecular weights of commercial sodium alginates vary between 32,000 to 400,000 g/mol.43 Maximizing the molecular weight of alginate upgrades the physical characteristics of the gels. Highly molecular weighted polymeric alginate solutions can be extremely viscous, making them unfavorable for the processing step.44 Alginate constitutes a straight copolymer consisting of d-mannuronate and l-guluronate residues connected by a 1,4 bond. The Blocks consist of alternate M and G residues, consecutive G residues, and consecutive M residues. Different sources of alginates have various degrees of concentration of M and G.45
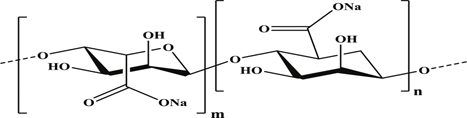
Figure 4: Structure Of Alginate
In relevance to the following the polymer alginate and the antidiabetic agent was been formulated by S. K. Bajpai et.al 2017 and the formulation included calcium alginate beads containing gliclazide as the antidiabetic agent. This formulation provided an extended release of Ca (II)-ions crosslinked alginate beads and keeping it stable for more than 48h, in the gliclazide the physiological fluid of pH 7.4, while ions crosslinked alginate beads not only enhance the stability of the composite beads but also improved retention time of anti-diabetic drug gliclazide. Additionally, the enhanced stability and prolonged release were confirmed by an in-vivo study on Albino Wistar rats.46
In another research Mansi Butola et.al 2023 had an objective to demonstrate the synthesis of compressed tablets incorporating sodium & pectin alginate with metformin HCL. The studies revealed the developed tablets showed sustained drug release pattern. It was found that with increase in polymer concentration the drug release was decreased.47
Dilipkumar Pal et.al 2011 developed & optimized alginate methyl-cellulose mucoadhesive microcapsules of gliclazide by central composite design. The developed microcapsules exhibited good mucoadhesive property & showed controlled drug release. In-vivo studies revealed that blood glucose was lowered after administration of optimized gliclazide containing mucoadhesive microcapsules to prolong the systemic absorption and also improved patient compliance.48
Cyclodextrin:
Cyclodextrins (CDs) are oligosaccharide and are widely employed in the pharmaceutical sector which are capable of forming inclusion complexes through interactions with guest molecules.49 Their cylindrical structures, with cavities close to 0.7 nm deep and 0.5-0.8 nm interior diameter, exhibit remarkable features.50
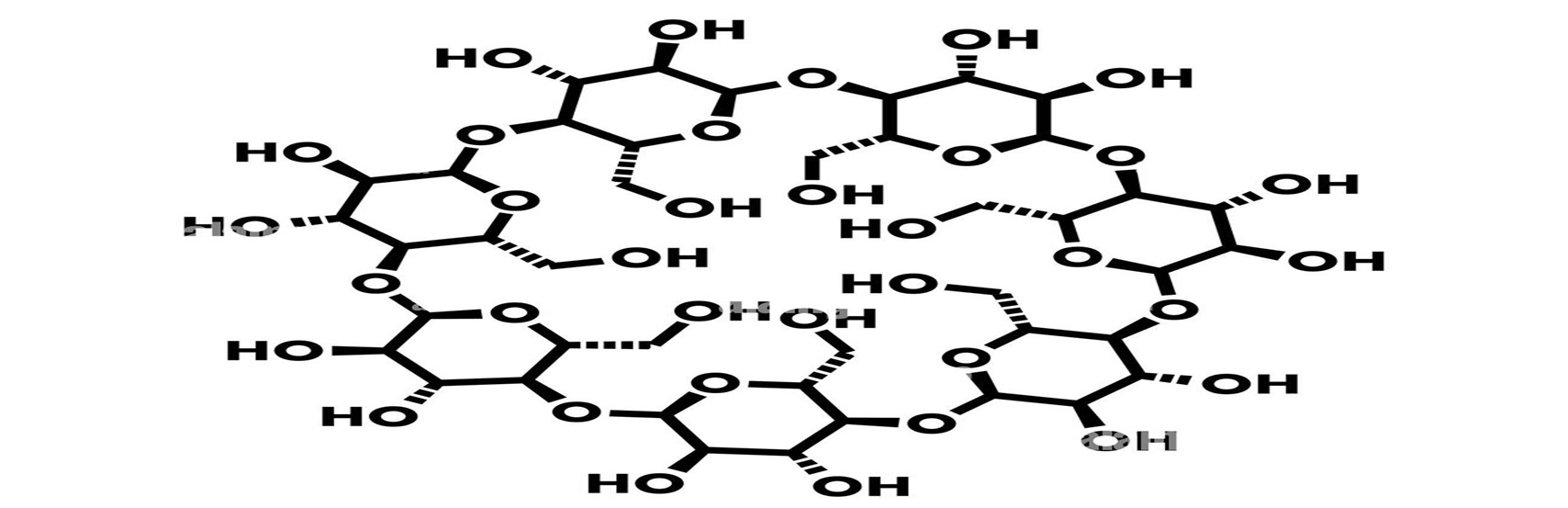
Figure 5: Structure Of Cyclodextrin
Kuljit Kaur et.al 2019 developed an inclusion complex of metformin hydrochloride (MF) and beta-cyclodextrin (?-CD) microwave irradiation method with the aim to improve sustained release, dissolution and oral bio-availability of metformin. Complexation with ?-CD was prepared by 4 using ways physical mixture, kneading method, co-precipitation method, microwave method.51
Dextran:
Dextran-based delivery methods have been widely explored over the past decade, with applications in food science, nutraceuticals, pharmaceuticals, and biomedicine.52 Dextran possess a molecular weight of up to 440 MDa. They are categorized into two categories based on chain length: those with a weight of molecule more than 40 kDa are simply termed dextran, whereas those with Oligodextrans are molecules with a weight less than 40 kDa.53,54,55 Dextran is a neutral complex that is branched glucan and is made up of ?-1,6 glycosidic connections between glucose monomers, with branches from ?-1, 2, ?-1, 3, and ?-1, 4 links.56
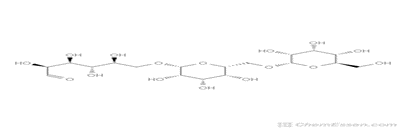
Figure 6: Structure Of Dextran
An attempt was made in this study by Ning-Hui Lu et.al; 2018 to show dextran as effective polymer and developed a formation with dextran as polymer with modified magheminte of nanoparticles to the human insulin. The aim of this work is to demonstrate the impact of nanoparticles on human insulin's in vitro amyloid fibrillogenesis. Insulin fibril formation was reduced when modified dextran polymer consisting of maghemite nanoparticles that were added to human insulin. The outcome as well as the nanoparticle' size and concentration were strongly connected.57 S. K. Bajpai et.al 2016 designed dextran-based polymer-coated nanoparticles formulation consisting of the dextran hydrogel with the gliclazide as an antidiabetic agent. When compared to the ordinary drug, the drug-loaded hydrogel was found to be reasonably effective in drastically reducing the glucose level at reduced injection repetitions.58
Starch:
The 2nd most prevalent organic bio-polymer, is an inexpensive, adaptable, inexhaustible agricultural commodity with a variety of industrial and therapeutic applications.59 Starch molecule have structure consists of two forms amylose and amylopectin.60 Amylose is a polymer composed of ? (1, 4) glucopyranose and has a mild branching pattern. Between 105 and 107 g/mol are responsible for its molecular mass, and it has a degree of polymerization of 6000. A densely branched polymer with approximately two million polymerization degrees, amylopectin is made up of ? (1, 4) glucopyranose molecules connected by ? (1, 6) links. The molecular mass of amylopectin ranges between 107 and 109 g/mol.61 Starch is primarily made up of two D-glucose homopolymers [8]: amylase, a linear ? D (1, 4')-glucan, and branched amylopectin, which has the corresponding constitution to that of amylose but has more ?-1, 6'-linked branches.62
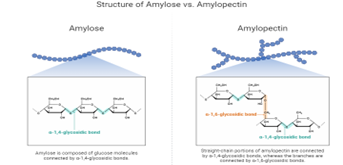
Figure 7: Structure Of Two Forms Starch
Formulation of glipizide as controlled release matrix tablets by employing starch acetate as a polymer to enhance the effectiveness of the anti-diabetic formulation was developed by P. Seenivasan et.al 2013. It was concluded that the use of starch acetate has improved the effect of formulation by providing a controlled release for 24h.63 Dioscorea oppositifolia starch has been tested as a polymer for the formation of floating gastro-retentive beads enabling the carefully controlled administration of metformin hydrochloride in a diabetic medication created by A. Okunlola et.al 2010. The ionotropic gelation approach was used by the team of scientists to create floating micro beads. In contrast to the starch to alginate ratio, releasing of metformin and the starch mixture from floating micro beads in a regulated manner.64 Ying Zhou et.al 2014 prepared a formulation containing the natural polymer indica rice starch with glimepiride as an antidiabetic agent. This research revealed that dual modification revolutionized the structure of indica rice starch, impacting both the diabetic mice's blood glucose levels and the starch's digestibility.65
Pectin:
Pectin is a biocompatible polysaccharide with intrinsic biological activity that may demonstrate different structural features depending on its source or extraction method.66 Pectin can be encountered in all terrestrial plant organs, including meristematic and parenchymal tissues. Pectin originates in plant cells in the cell wall and middle lamella section. However, the quality and quantity of pectin varies depending on the species.67 Pectin is soluble in water, anionic polysaccharide having linear chains of a-(1, 4)-D-galacturonic acid, 1,2 D-rhamnose, and side chains of D-galactose and D-arabinos.68
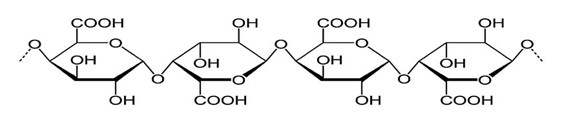
Figure 8: Structure Of Pectin
Researcher Santhosh Kumar Chinnaiyan et.al 2018 developed an antidiabetic formulation consisting of pectin as a polymer in metformin loaded nanoparticles. The method used for preparation of nanoparticles was ionic gelation method. This study depicted slow and sustained release of formulation at pH 6.8 and eventually resulting in increased retention duration in blood circulation.69
Casein:
Milk contains casein, which naturally transfers nutrients from the mother to the baby. It is affordable, safe, and easily absorbed.70 These molecules have molecule weights which range from 19 to 25 kDa with typical isoelectric point pI of 4.6 percent to 4.8 per cent. All the 4 caseins are amphiphilic in nature, with undefined structures.71 Caseins are proteins comprises of amphiphilic proteins that form stable micellar structures in water liquids. Casein micelles are structures made primarily of 4 phosphoproteins bound to one another through hydrophobic forces and calcium phosphate nanoclusters that (CCP) associated through the adjacent chain casein's phosphorylation serine residue.72
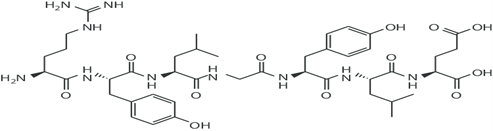
Figure 9: Structure Of Casein
Scientist Janardhan raj et.al 2015 demonstrated the use of casein micelles incorporated with an antidiabetic agent of metformin. The results showed that the use of this formulation lead to the release of metformin loaded casein micelles to provide a controlled release and the micelles also depicted a stronger stability.73
Gelatin:
Gelatin is a naturally occurring protein that is exceptionally biocompatible and biodegradable in physiological circumstance. It is obtained from the hydrolysis of collagen.74 Collagen and gelatin molecules have repeated Gly-X-Y triplets, including proline (Pro) and hydroxyproline (Hypro) amino acids.75 Gelatin is an effective medication delivering medium owing to its ability to carry charged biomolecules. It is true that the gelatin isoelectric point (IEP) can be adjusted to optimize drug loading performance based on the electrostatic characteristics of the target compound by choosing an alkaline or acidic preparation.76
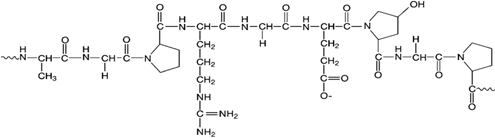
Figure 10: STRUCTURE OF GELATIN
The research study performed by Ying-Zheng Zhao et.al 2021 on modified gelatin which was incorporated with the insulin and thus resulting it to their feasibility as insulin pulmonary administration system. Pharmacodynamic research studies determined that the bioavailability was improved and the duration of hypoglycemic effects was also extended. Sustained release in lung tissue represents a particular benefit that polymeric nanoparticles are capable of providing, which can lower the dosage frequency and increase patient compliance. In additionally it also resulted in providing affordable price, excellent physical characterization, high bioavailability, and quick and consistent hypoglycemic impact.77
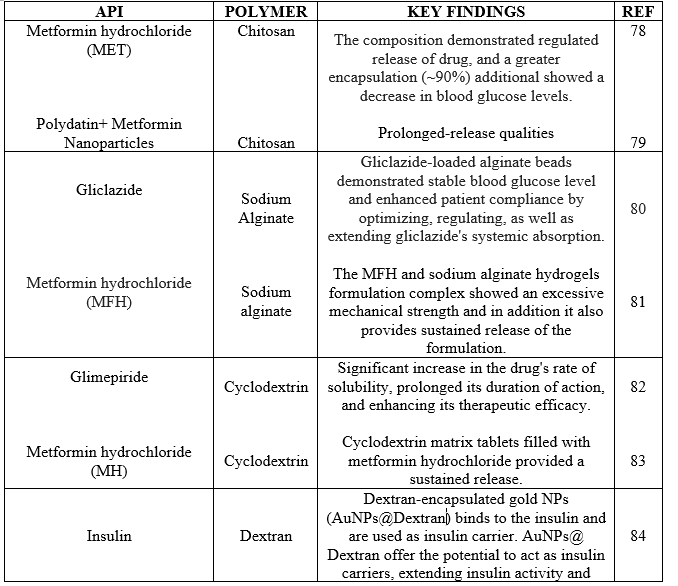
Table 1: Polymer And Key Findings In Anti-Diabetic Drugs
FUTURE ASPECTS:
The emphasis has centered on analyzing and presenting multiple polymeric carriers that are capable of a tendency to boost insulin intestinal absorption in a considerably greater range than an oral insulin solution. Although the conclusions reached are considerably below the hypoglycemic impact obtainable by utilizing subcutaneous insulin, beneficial effects have been observed.89
It is possible to combine many biopolymers into one carrier to suit all of the properties which are been covered in this review article. Though the results are not nearly as hypoglycemic compared to those obtained with injection-based insulin, there do appear to be some positive and encouraging results. Even yet, a larger quantity of insulin is needed in the formulation for oral delivery methods than for injectable drugs. This is an important problem since the entrapment efficiency of the carriers counts when considering cost-effectiveness. Development of oral insulin carriers utilizing naturally occurring polymers that function more adequately or at least resemble the features of the injection through the skin thus served as the primary objective of study. In order to maximize protection for users, investigations into the long-run toxicities associated with the carrier molecules is further recommended.90
CONCLUSION:
Globally, the occurrence of diabetes is consistently rising, while over the following fifty years, this upward trajectory is anticipated to persist. Since, insulin therapy was introduced 88 years ago. The vast majority of naturally occurring biopolymers across the habitat are polysaccharides, which can be found in a variety of biosphere constituents such as microbes, plants, and animals, aquatic life, etc. Polymeric nanoparticle offers numerous benefits, including easy preparatory work, specific distribution, minimal dosage, and excellent medicinal effectiveness. It was determined in this assessment that one of the most advanced techniques using nanoparticles made of natural polymeric material for the diabetes mellitus therapy. Polysaccharide nanoparticles have shown themselves as the highly significant biological nanocarriers of the future. Despite this, the majority of the investigation on polysaccharide-based nanocarriers was limited to preclinical settings, necessitating additional study of these polysaccharide NPs with potential for clinical application. The considerable amount of research demonstrating the exceptional biological and physiochemical properties of polysaccharides makes it seem likely that such compounds will find application as fascinating biomaterials in coming years. This review article investigates on the natural polymers used for diabetes mellitus has succeeded in demonstrating many different advantages like improvement in encapsulation efficacy, optimized blood glucose level, increased drug's rate of solubility, sustained release and compliance among patients. In this review it was also concluded that the usage of polymeric compounds is the advanced method to enhance treatment of diabetes mellitus.
REFERENCE
- Deopa Deepika, Sharma Kumar Satish and Singh Lalit. (2013); Current updates on Anti-Diabetic Therapy; JDDT; Vol. 3 No. 6; 121-126.
- Cho, N.; Shaw, J.; Karuranga, S.; Huang, Y.; Fernandes, J.D.R.; Ohlrogge, A.; Malanda, B. IDF Diabetes Atlas: Global estimates of diabetes prevalence for 2017 and projections for 2045. Diabetes Res. Clin. Pract. 2018, 138, 271–281. [CrossRef] [PubMed]
- Fangueiro JF, Silva AM, Garcia ML, Souto EB. Current nanotechnol ogy approaches for the treatment and management of diabetic retinopathy. Eur J Pharm Biopharm. 2015;95(Pt B):307–322. doi:10.1016/j.ejpb.2014.12.023
- Uppal S, Italiya KS, Chitkara D, Mittal A. Nanoparticulate-based drug delivery systems for small molecule anti-diabetic drugs: an emerging paradigm for effective therapy. Acta Biomaterialia. 2018;81:20–42. doi:10.1016/j.actbio.2018.09.049
- Gopalasatheeskumar K, Komala S, Mahalakshmi M. An overview on polymeric nanoparticles used in the treatment of diabetes mellitus. Pharmatutor. 2017 Dec 1;5(12):40-6.
- Katsarou, A.; Gudbjörnsdottir, S.; Rawshani, A.; Dabelea, D.; Bonifacio, E.; Anderson, B.J.; Jacobsen, L.M.; Sch iatz, D.A.; Lernmark, Å. Type 1 diabetes mellitus. Nat. Rev. Dis. Primers 2017, 3, 17016. [CrossRef] [PubMed]
- Sun Z, Sun X, Li J, et al. Using probiotics for type 2 diabetes mellitus intervention: advances, questions, and potential. Crit Rev Food Sci Nutr. 2020;60(4):670–683.
- Metzger BE, Couston DR. Summary and recommendations of the fourth international workshop conference on gestational diabetes mellitus. Diabetes Care 1998;21(2):B161–7.
- X. Sun, W. Yu, C. Hu, Genetics of Type 2 Diabetes: Insights into the Pathogenesis and Its Clinical Application, BioMed Res. Int. 2014 (2014), 926713, https://doi. org/10.1155/2014/926713, 15.
- Wilson, E. B. (1990). An introduction to scientific research. Courier Corporation. Zozulinska, D., & Wierusz-Wysocka, B. (2006). Type 2 diabetes mellitus as inflammatory disease. Diabetes Research and Clinical Practice, 74(2), S12-S16. Lune, B. B. H. (2004). Qualitative research methods for the social sciences Vol. 5. McDonough, J., & Shaw, C. (2012). Materials and Methods in ELT. John Wiley & Sons.
- Morales A. A better future for children with type 1 diabetes: Review of the conclusions from the Diabetes Control and Complications Trial and the Epidemiology of Diabetes Interventions and Complications study. J Ark Med Soc. 2009;106:90– 3.
- Couri CE, Voltarelli JC. Autologous stem cell transplantation for early type 1 diabetes mellitus. Autoimmunity. 2008;41:666–72.
- Padhi S, Nayak AK, Behera A. Type II diabetes mellitus: a review on recent drug based therapeutics. Biomedicine & Pharmacotherapy. 2020 Nov 1;131:110708.
- V. Rai, N. Mishra, A. Agrawal, S. Jain, N. Yadav, Novel drug delivery system: an immense hope for diabetics, Drug. Deliv. 23 (2016) 2371–2390, https://doi.org/ 10.3109/10717544.2014.991001. Padhi S, Nayak AK, Behera A. Type II diabetes mellitus: a review on recent drug based therapeutics. Biomedicine & Pharmacotherapy. 2020 Nov 1;131:110708.
- Tuso P. Prediabetes and lifestyle modification: time to prevent a preventable disease. Perm J (2014) 18(3):88–93. doi:10.7812/TPP/14-002.
- Umpierre D, Ribeiro PA, Kramer CK, Leitão CB, Zucatti AT, Azevedo MJ, et al. Physical activity advice only or structured exercise training and association with HbA1c levels in type 2 diabetes: a systematic review and meta-analysis. JAMA (2011) 305(17):1790–9.
- Pietraszek A, Gregersen S, Hermansen K. Alcohol and type 2 diabetes. A review. Nutr Metab Cardiovasc Dis (2010) 20(5):366–75. doi:10.1016/j.numecd.2010.05.001.
- Chaudhury A, Das S. Recent advancement of chitosan-based nanoparticles for oral controlled delivery of insulin and other therapeutic agents. AAPS PharmSciTech. 2011;12:10-20.
- Satturwar P.M., Fulzele S.V., Dorle A.K., Biodegradation and in vivo biocompatibility of rosin: A natural film-forming polymer, AAPS Pharm. Sci. Tech. 2003; 4 : 1-6.
- X. Qi, L. Lin, L. Shen, Z. Li, T. Qin, Y. Qian, X. Wu, X. Wei, Q. Gong, J. Shen, Efficient Decontamination of Lead Ions from Wastewater by Salecan Polysaccharide-Based Hydrogels, ACS Sustain. Chem. Eng. 7(12) (2019) 11014– 11023. https://doi.org/10.1021/acssuschemeng.9b02139.
- X. Qi, W. Wei, J. Li, Y. Liu, X. Hu, J. Zhang, L. Bi, W. Dong, Fabrication and Characterization of a Novel Anticancer Drug Delivery System: Salecan/Poly(methacrylic acid) Semi-interpenetrating Polymer Network Hydrogel, ACS Biomater. Sci. Eng. 1(12) (2015) 1287–1299. https://doi.org/10.1021/acsbiomaterials.5b00346.
- Hamid Akash MS, Rehman K, Chen S. Natural and synthetic polymers as drug carriers for delivery of therapeutic proteins. Polymer Reviews. 2015 Jul 3;55(3):371-406.
- Zheng, Z.; Pan, X.; Luo, L.; Zhang, Q.; Huang, X.; Liu, Y.; Wang, K.; Zhang, Y. Advances in oral absorption of polysaccharides: Mechanism, affecting factors, and improvement strategies. Carbohyd. Polym. 2022, 282, 119110. [CrossRef]
- 5 Halder, A. et al. (2005) Entrapment efficiency and release characteristics of polyethyleneimine-treated or -untreated calcium alginate beads loaded with propranolol–resin complex. Int. J. Pharm. 302, 84–94.
- Sonia TA, Sharma CP. An overview of natural polymers for oral insulin delivery. Drug discovery today. 2012 Jul 1;17(13-14):784-92.
- Wang L, Wang N, Zhang W, Cheng X, Yan Z, Shao G, Wang X, Wang R, Fu C. Therapeutic peptides: current applications and future directions. Signal Transduction and Targeted Therapy. 2022 Feb 14;7(1):48.
- Craik DJ, Fairlie DP, Liras S, Price D. The future of peptide?based drugs. Chemical biology & drug design. 2013 Jan;81(1):136-47.
- Sami El-banna F, Mahfouz ME, Leporatti S, El-Kemary M, Hanafy AN. N. Chitosan as a Natural Copolymer with Unique Properties for the Development of Hydrogels. Applied Sciences. 2019;9(11):11. doi:10.3390/app9112193
- Rizeq BR, Younes NN, Rasool K, Nasrallah GK. Synthesis, Bioapplications, and Toxicity Evaluation of Chitosan-Based Nanoparticles. Int J Mol Sci. 2019;20(22):22. doi:10.3390/ijms2025776.
- Chinnaiyan SK, Deivasigamani K, Gadela VR. Combined synergetic potential of metformin loaded pectin-chitosan biohybrids nanoparticle for NIDDM. International journal of biological macromolecules. 2019 Mar 15;125:278-89.
- Sarkar S, Das D, Dutta P, Kalita J, Wann SB, Manna P. Chitosan: A promising therapeutic agent and effective drug delivery system in managing diabetes mellitus. Carbohydrate polymers. 2020 Nov 1;247:116594.
- Nagasawa K, Tohira Y, Inoue Y, Tanoura N. Reaction between carbohydrates and sulfuric acid: Part I. Depolymerization and sulfation of polysaccharides by sulfuric acid. Carbohydrate Research. 1971 May 1;18(1):95-102.
- M. E. Gomes, R. L. Reis, Biodegradable polymers and composites in biomedical applications: from catgut to tissue engineering part 1 available systems and their properties, Int. Mater. Review 49(2004) 261-273.
- Lupascu FG, Dash M, Samal SK, Dubruel P, Lupusoru CE, Lupusoru RV, Dragostin O, Profire L. Development, optimization and biological evaluation of chitosan scaffold formulations of new xanthine derivatives for treatment of type-2 diabetes mellitus. European Journal of Pharmaceutical Sciences. 2015 Sep 18;77:122-34.
- Akolade JO, Oloyede HO, Onyenekwe PC. Encapsulation in chitosan-based polyelectrolyte complexes enhances antidiabetic activity of curcumin. Journal of functional foods. 2017 Aug 1;35:584-94.
- Jaisankar E, Pavithra ME, Krishna S, Thirumarimurugan M, Azarudeen RS. Dual property of chitosan blended copolymer membranes: Antidiabetic drug release profile and antimicrobial assay. International journal of biological macromolecules. 2020 Feb 15;145:42-52.
- Y. Le, S. C. Anand, A. R .Horrocks, Recent development in fibres and materials for wound management, Indian J. Fibre Text. Res. 22(1997) 337-347.
- K. Y. Lee, L. Jeong, Y. O. Kang, S. J. Lee, W. H. Park, Electrospinning of polysaccharides for regenerative medicine, Adv. Drug Deliv. Rev. 61(2009) 1020-1032.
- Denuziere A, Ferrier D, Damour O, Domard A. Chitosan–chondroitin sulfate and chitosan–hyaluronate polyelectrolyte complexes: biological properties. Biomaterials. 1998 Jul 1;19(14):1275-85.
- Gombotz WR, Wee SF. Protein release from alginate matrices. Advanced drug delivery reviews. 2012 Dec 1;64:194-205.
- Smidsrød O, Skja G. Alginate as immobilization matrix for cells. Trends in biotechnology. 1990 Jan 1;8:71-8.
- . Tan J, Wang Y, Liu S, Shi Q, Zhou X, Zhou Y, et al. Long-acting metformin vs. metformin immediate release in patients with Type 2 diabetes: A systematic review. Front Pharmacol. 2021;12(12):669814. doi: 10.3389/fphar.2021.669814, PMID 34079464.
- Rinaudo MJ. On the abnormal exponents a ? and a D in Mark Houwink type equations for wormlike chain polysaccharides. Polymer Bulletin. 1992 Jan;27:585-9.
- LeRoux MA, Guilak F, Setton LA. Compressive and shear properties of alginate gel: effects of sodium ions and alginate concentration. Journal of Biomedical Materials Research: An Official Journal of The Society for Biomaterials, The Japanese Society for Biomaterials, and The Australian Society for Biomaterials and the Korean Society for Biomaterials. 1999 Oct;47(1):46-53.
- Tonnesen HH, Karlsen J. Alginate in drug delivery systems. Drug Dev Ind Pharm 2002;28:621–30.
- Bajpai SK, Pathak V, Kirar N. Extended release of Gliclazide from highly stabilized calcium alginate/poly (acrylamide) beads for diabetes management. Journal of Macromolecular Science, Part A. 2017 Jul 3;54(7):472-9.
- Butola M, Bhatt V, Nainwal N, Jakhmola V, Dobhal K, Ale Y. Preparation and Evaluation of Polymer Fused Metformin Hydrochloride Sustained Release Tablet. Ind. J. Pharm. Edu. Res. 2023;57(3):711-7.
- Pal D, Nayak AK. Development, optimization, and anti-diabetic activity of gliclazide-loaded alginate–methyl cellulose mucoadhesive microcapsules. AAPS PharmSciTech. 2011 Dec;12:1431-41.
- K. Uekama, Design and evaluation of cyclodextrin-based drug formulation, Chem. Pharm. Bull. 52 (2004) 900–915.
- Li, J.; Yang, C.; Li, H.; Wang, X.; Goh, S. H.; Ding, J. L.; Wang, D. Y.; Leong, K. W.
- “Cationic supramolecules composed of multiple oligoethylenimine-grafted b-cyclodextrins threaded on a polymer chain for ef?cient gene delivery”, Adv. Mater. 2006, 18, 2969–2974.
- Kaur K, Jindal R, Jindal D. Synthesis, characterization and studies on host-guest interactions of inclusion complexes of metformin hydrochloride with ?–cyclodextrin. Journal of Molecular Liquids. 2019 May 15;282:162-8.
- Easton CJ, Lincoln SF. Modified cyclodextrins: scaffolds and templates for supramolecular chemistry. World Scientific; 1999 Apr 29.
- Liu Z, Ye L, Xi J, Wang J, Feng ZG. Cyclodextrin polymers: Structure, synthesis, and use as drug carriers. Progress in Polymer Science. 2021 Jul 1;118:101408.
- Semyonov D, Ramon O, Shoham Y, Shimoni E. Enzymatically synthesized dextran nanoparticles and their use as carriers for nutraceuticals. Food & function. 2014;5(10):2463-74.
- Zarour, K.; Llamas, M.G.; Prieto, A.; Rúas-Madiedo, P.; Dueñas, M.T.; de Palencia, P.F.; Aznar, R.; Kihal, M.; López, P. Rheology and bioactivity of high molecular weight dextrans synthesised by lactic acid bacteria. Carbohydr. Polym. 2017, 174, 646–657. [CrossRef] [PubMed]
- Heinze, T.; Liebert, T.; Heublein, B.; Hornig, S. Functional polymers based on dextran. In Polysaccharides II; Klemm, D., Ed.; Springer: Berlin/Heidelberg, Germany, 2006; pp. 199–291. ISBN 9783540371021.
- Lu NH, How SC, Lin CY, Tsai SL, Bednarikova Z, Fedunova D, Gazova Z, Wu JW, Wang SS. Examining the effects of dextran-based polymer-coated nanoparticles on amyloid fibrillogenesis of human insulin. Colloids and Surfaces B: Biointerfaces. 2018 Dec 1;172:674-83.
- Bajpai SK, Chand N, Tiwari S, Soni S. Swelling behavior of cross-linked dextran hydrogels and preliminary Gliclazide release behavior. International journal of biological macromolecules. 2016 Dec 1;93:978-87.
- Foster, J.H.; Killen, D.A.; Jolly, P.C.; Kirtley, J.H. Low molecular weight dextran in vascular surgery: Prevention of early thrombosis following arterial recosntruction in 85 cases. Ann. Surg. 1966, 163, 764–770. [CrossRef].
- Heinze T, Liebert T, Heublein B, Hornig S. Functional polymers based on dextran. Polysaccharides Ii. 2006:199-291.
- Lu DR, Xiao CM, Xu SJ. Starch-based completely biodegradable polymer materials. Express polymer letters. 2009 Mar;3(6):366-75.
- Jane JJ. Starch properties, modifications, and applications. Journal of Macromolecular Science, Part A: Pure and Applied Chemistry. 1995 Apr 1;32(4):751-7.
- Seenivasan P, Chowdary KP, Reddy CU, Murthy JS. Design and evaluation of glipizide CR tablets employing starch acetate as rate controlling matrix former. Journal of Pharmacy Research. 2013 Jun 1;6(6):653-5.
- Okunlola A, Patel RP, Odeku OA. Evaluation of freeze-dried pregelatinized Chinese yam (Dioscorea oppositifolia) starch as a polymer in floating gastroretentive metformin microbeads. Journal of drug delivery science and technology. 2010 Jan 1;20(6):457-65.
- Zhou Y, Meng S, Chen D, Zhu X, Yuan H. Structure characterization and hypoglycemic effects of dual modified resistant starch from indica rice starch. Carbohydrate polymers. 2014 Mar 15;103:81-6.
- Kumar A, Chauhan GS. Extraction and characterization of pectin from apple pomace and its evaluation as lipase (steapsin) inhibitor. Carbohydr Polym 2010;82:454-9
- Yabe, T. (2018). New understanding of pectin as a bioactive dietary fiber. Journal of Food 896 Bioactives, 3, 95-100.
- Loth, F. (1993) Industrial Gums: Polysaccharides and Their Derivatives (. In Industrial
- Gums: Polysaccharides and Their Derivatives 3rd edn (Whistler, R.L., . In Industrial Gums: Polysaccharides and Their Derivatives 3rd edn (James, N., BeMiller, J.N., eds), Academic Press
- Chinnaiyan SK, Karthikeyan D, Gadela VR. Development and characterization of metformin loaded pectin nanoparticles for T2 diabetes mellitus. Pharmaceutical nanotechnology. 2018 Dec 1;6(4):253-63.
- Katz Y, Rajuan N, Goldberg M, Cohen A, Heyman E, Leshno M. Avoidance of early regular exposure to cow's milk protein is a major risk factor for development of Ige mediated cow's milk allergy (cma). Journal of Allergy and Clinical Immunology. 2009 Feb 1;123(2):S211.
- Boland M, Singh H, editors. Milk proteins: from expression to food. Academic Press; 2019 Nov 20.
- C.G. De Kruif, C. Holt, Casein micelle structure, functions, and interactions, in: P.F. Fox, P.L.H. McSweeney (Eds.), Advanced Dairy Chemistry: Proteins, Part A, third ed, Kluwer Academic/Plenum, New York, 2003, pp. 233–276.
- Raj J, Uppuluri KB. Metformin loaded casein micelles for sustained delivery: formulation, characterization and in-vitro evaluation. Biomedical and Pharmacology Journal. 2015 Jun 18;8(1):83-9. Zhang F, Xu S, Wang Z. Pre-treatment optimization and properties of gelatin from freshwater fish scales. Food and Bioproducts processing. 2011 Jul 1;89(3):185-93.
- Duan H, Umar S, Xiong R, Chen J (2011) New strategy for expression of recombinant hydroxylated human-derived gelatin in Pichia pastoris KM71. J Agric Food Chem 59:7127–7134.
- Santoro M, Tatara AM, Mikos AG. Gelatin carriers for drug and cell delivery in tissue engineering. Journal of controlled release. 2014 Sep 28;190:210-8.
- Tabata Y, Ikada Y. Protein release from gelatin matrices. Advanced drug delivery reviews. 1998 May 4;31(3):287-301.
- Zhao YZ, Li X, Lu CT, Xu YY, Lv HF, Dai DD, Zhang L, Sun CZ, Yang W, Li XK, Zhao YP. Experiment on the feasibility of using modified gelatin nanoparticles as insulin pulmonary administration system for diabetes therapy. Acta diabetologica. 2012 Aug;49:315-25.
- Lari AS, Zahedi P, Ghourchian H, Khatibi A. Microfluidic-based synthesized carboxymethyl chitosan nanoparticles containing metformin for diabetes therapy: In vitro and in vivo assessments. Carbohydrate Polymers. 2021 Jun 1;261:117889.
- Mostafa F, Abdel-Moneim A, Abdul-Hamid M, Galaly SR, Mohamed HM. Polydatin and polydatin-loaded chitosan nanoparticles attenuate diabetic cardiomyopathy in rats. Journal of Molecular Histology.
- Al-Kassas RS, Al-Gohary OM, Al-Faadhel MM. Controlling of systemic absorption of gliclazide through incorporation into alginate beads. International journal of pharmaceutics. 2007 Aug 16;341(1-2):230-7.
- Mir A, Kumar A, Riaz U. Synthesis and characterization of conducting polymer/alginate composite hydrogels: Effect of conducting polymer loading on the release behaviour of metformin drug Journal of Molecular Liquids. 2023 Feb 15;372:121193.
- Ammar HO, Salama HA, Ghorab M, Mahmoud AA. Formulation and biological evaluation of glimepiride–cyclodextrin–polymer systems. International journal of pharmaceutics. 2006 Feb 17;309(1-2):129-38.
- Giovanna C, Marzia C, Maestrelli F, Mennini N, Mura P. Sustained-release matrix tablets of metformin hydrochloride in combination with triacetyl-b-cyclodextrin European Journal of Pharmaceutics and Biopharmaceutics. 2008;68:303-9.
- Lee KC, Chen WJ, Chen YC. Using Dextran-encapsulated gold nanoparticles as insulin carriers to prolong insulin activity. Nanomedicine. 2017 Aug;12(15):1823-34
- Nayak AK, Pal D. Formulation optimization and evaluation of jackfruit seed starch–alginate mucoadhesive beads of metformin HCl. International journal of biological macromolecules. 2013 Aug 1;59:264-72.
- Nayak AK, Pal D, Santra K. Development of pectinate-ispagula mucilage mucoadhesive beads of metformin HCl by central composite design. International journal of biological macromolecules. 2014 May 1;66:203-11.
- Khodaverdi E, Maftouhian S, Aliabadi A, Hassanzadeh-Khayyat M, Mohammadpour F, Khameneh B, Hadizadeh F. Casein-based hydrogel carrying insulin: preparation, in vitro evaluation and in vivo assessment. Journal of Pharmaceutical Investigation. 2019 Nov;49:635-41.
- Zeng Z, Jiang G, Liu T, Song G, Sun Y, Zhang X, Jing Y, Feng M, Shi Y. Fabrication of gelatin methacryloyl hydrogel microneedles for transdermal delivery of metformin in diabetic rats. Bio-Design and Manufacturing. 2021 Dec;4(4):902-11.
- Fonte P, Araújo F, Silva C, Pereira C, Reis S, Santos HA, Sarmento B. Polymer-based nanoparticles for oral insulin delivery: Revisited approaches. Biotechnology advances. 2015 Nov 1;33(6):1342-54.
- Nur M, Vasiljevic T. Can natural polymers assist in delivering insulin orally?. International journal of biological macromolecules. 2017 Oct 1;103:889-901.


 Dr Vikram T Choudhary*
Dr Vikram T Choudhary*











 10.5281/zenodo.13835088
10.5281/zenodo.13835088