Abstract
Hydrogels have long been recognized for their versatility and biocompatibility, making them valuable in biomedical applications such as tissue engineering and drug delivery. However, their inherent mechanical weaknesses, including low tensile strength and brittleness, limit their use in load-bearing and high-stress environments. To address these limitations, this article explores alternative materials that offer enhanced mechanical and functional performance compared to traditional hydrogels. We review aerogels, alginate-based gels, and superabsorbent polymers (SAPs), highlighting their unique properties, applications, and advantages. Aerogels, with their low density and high porosity, excel in applications requiring thermal insulation and gas absorption. Alginate-based gels, derived from seaweed, provide superior biodegradability and biocompatibility, making them suitable for medical and pharmaceutical uses. SAPs, known for their high water absorption capacity, are cost-effective for large-scale applications but may lack the necessary biocompatibility for certain biomedical uses. This comparative analysis underscores the potential of these alternatives to overcome the mechanical and functional constraints of hydrogels, offering new avenues for industrial and medical applications.
Keywords
Hydrogels, Aerogels, Alginate-Based Gels, Superabsorbent Polymers (SAPs), Mechanical Properties, Biomedical Applications.
Introduction
Hydrogels are three-dimensional polymer networks capable of absorbing significant amounts of water while maintaining their structural integrity. Their unique properties, including high water content, flexibility, and biocompatibility, have made them invaluable in a range of biomedical applications such as tissue engineering, drug delivery systems, and wound dressings. Despite their advantages, hydrogels face inherent limitations related to their mechanical properties. Specifically, their high water content often results in low tensile strength, reduced durability, and increased brittleness, which can restrict their application in environments that require robust mechanical performance [1-3]

Fig. 1. Hydrogels
To address these challenges and expand the functional potential of hydrogels, researchers have turned their attention to alternative materials that offer enhanced mechanical and functional properties. Aerogels, for instance, are highly porous and lightweight materials known for their excellent thermal insulation and low density [4, 5]. Their ability to absorb gases and liquids makes them suitable for environmental and aerospace applications where hydrogels may fall short due to their high-water content. Similarly, alginate-based gels, derived from natural sources such as seaweed, present a compelling alternative. These biopolymers are prized for their biodegradability and biocompatibility, making them particularly useful in medical and pharmaceutical applications. Alginate gels offer a favorable balance of mechanical strength and biocompatibility, addressing some of the shortcomings of synthetic hydrogels in medical contexts. Superabsorbent polymers (SAPs) represent another viable alternative, with their capacity to absorb and retain large volumes of water [6-9]. While SAPs are widely used in consumer products such as diapers and agricultural soil conditioners, they offer different mechanical and cost advantages compared to hydrogels, particularly in large-scale industrial applications [10-12].
This article aims to provide a comprehensive evaluation of these hydrogel alternatives, focusing on their composition, properties, and specific applications. By comparing these materials to traditional hydrogels, we seek to highlight their potential to overcome the limitations of hydrogels and address diverse needs in both industrial and biomedical fields.
Beyond Weakness: Enhancing Hydrogel Mechanics
Hydrogels, characterized by their high-water content and versatile properties, have emerged as promising materials for various biomedical applications. Their biocompatibility and flexibility make them ideal for tissue engineering, drug delivery, and other medical devices. However, a significant limitation hindering their wider adoption is their inherent mechanical weakness [13-15]. The loosely crosslinked polymer networks that constitute hydrogels, coupled with their high-water content, result in low tensile strength, brittleness, and limited durability. This restricts their use in applications requiring structural integrity, such as load-bearing implants or soft robotics [16, 17]. To address these challenges and expand the potential applications of hydrogels, researchers have been exploring various strategies to enhance their mechanical properties. One approach involves the creation of composite hydrogels, where inorganic or organic materials are incorporated into the hydrogel matrix to provide additional structural support. These composite materials can significantly improve the tensile strength, stiffness, and fatigue resistance of hydrogels, making them more suitable for demanding applications [18 - 21].
Another strategy is the development of double-network hydrogels. These hydrogels consist of two interpenetrating polymer networks with distinct mechanical properties. By combining a strong, brittle network with a tough, deformable network, double-network hydrogels can exhibit both high strength and toughness, overcoming the limitations of traditional hydrogels. Furthermore, hybrid hydrogels that combine hydrogels with other materials, such as elastomers or shape-memory polymers, can offer unique mechanical properties and functionalities. These hybrid materials can be tailored to specific applications, such as soft robotics or implantable devices, where both flexibility and strength are required [22 -25].
In addition to this, while hydrogels offer significant potential for biomedical applications, their inherent mechanical weakness remains a critical challenge. By exploring strategies such as composite formation, double-network hydrogels, and hybrid systems, researchers are working to overcome these limitations and expand the application scope of hydrogels to include more demanding and structurally critical applications [26, 27]. Recent research into the mechanical properties of hydrogels has revealed significant challenges, particularly related to their inherent mechanical weakness. Hydrogels, primarily composed of a water-swollen polymer network, possess limited tensile strength, which makes them unsuitable for applications requiring robust mechanical performance. A notable study conducted in 2021 by Yuk et al. demonstrated that traditional hydrogels, such as polyacrylamide (PAAm) and polyethylene glycol (PEG)-based hydrogels, exhibit poor stress resistance and tend to fracture under cyclic loading due to their low fracture toughness and fatigue resistance. This mechanical fragility arises because of the nature of their polymeric structure, where the hydrated polymer chains are loosely cross-linked, resulting in limited energy dissipation when subjected to mechanical forces [28 - 31]. In another study published in Advanced Materials, researchers investigated the use of hydrogels in load-bearing biomedical applications such as cartilage replacement. They found that the compressive strength of conventional hydrogels was orders of magnitude lower than that of natural tissues like cartilage or bone, often leading to early mechanical failure under physiological conditions. Moreover, the introduction of reinforced or composite hydrogels, while improving some mechanical aspects, still fell short of replicating the mechanical resilience required for prolonged use in vivo. Thus, recent experimental data consistently point toward the mechanical weakness of hydrogels as a critical limitation in their broader application, particularly in fields like tissue engineering and regenerative medicine, where mechanical durability is paramount [32 - 35]. These studies highlight the need for innovative approaches to improve the mechanical robustness of hydrogels, such as double-network hydrogels, nanocomposite reinforcement, or dynamic covalent bonding strategies, which are currently under exploration. Nonetheless, the mechanical weakness remains a significant obstacle in hydrogel development, especially for high-stress environments [36 - 38].
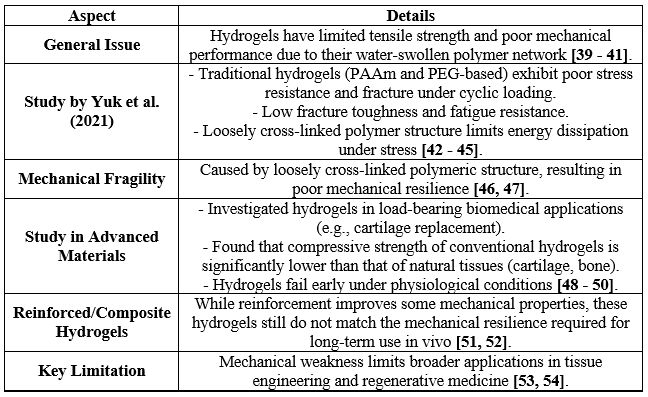
Table-I. Recent Findings on the Mechanical Weakness of Hydrogels
Challenges of Brittleness in Hydrogel Applications
Brittleness is a critical limitation in the functionality of hydrogels, especially when mechanical flexibility and durability are required. Hydrogels, characterized by their water-swollen polymer networks, often exhibit low mechanical strength due to the weak interactions between their polymer chains. This is particularly problematic when the polymer structure is inadequately cross-linked, leading to rigid, fragile networks that fracture under stress rather than deform elastically. In some hydrogels, the covalent cross-linking used to maintain structural stability can inadvertently increase rigidity, making the material prone to cracking or breaking when subjected to mechanical forces [55 - 58]. Environmental conditions like dehydration or freezing can further exacerbate this brittleness by restricting the mobility of the water molecules that contribute to the hydrogel’s flexibility [59, 60]. Consequently, this brittleness limits the use of hydrogels in applications where flexibility, durability, and mechanical strength are essential, such as in tissue scaffolding or soft robotics. To address these challenges, researchers are exploring various strategies to improve hydrogel toughness, including incorporating dynamic cross-linking, hybrid materials, or the inclusion of elastic polymers to enhance flexibility while retaining the hydrogel's beneficial properties [61, 62].
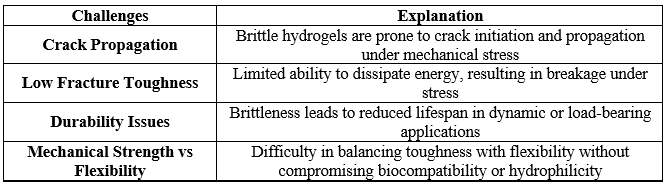
Table-II. Challenges in Enhancing the Mechanical Properties of Hydrogels
Challenges of the Load-Bearing Capacity of Hydrogels
Hydrogels, due to their high-water content and soft nature, exhibit limited load-bearing capacity, which poses a significant challenge for their use in applications requiring mechanical robustness, such as tissue engineering, soft robotics, and drug delivery systems [63, 64]. The intrinsic structural weaknesses of hydrogels arise from their polymer network, which is prone to deformation and rupture under mechanical stress. This limitation restricts their use in dynamic environments where durability and strength are critical. One of the main factors contributing to this weakness is the low fracture toughness, meaning the hydrogel has a reduced ability to dissipate mechanical energy, leading to crack initiation and propagation under stress. To address this, researchers have explored multiple strategies to enhance the mechanical properties of hydrogels, focusing on reinforcing their structure without sacrificing their biocompatibility, flexibility, or hydrophilicity [65, 66]. Incorporating nanomaterials such as graphene oxide, silica nanoparticles, or cellulose nanocrystals into the hydrogel matrix has shown promise in improving the mechanical strength and toughness of hydrogels [67, 68]. The design of hybrid hydrogels—combining natural and synthetic polymers—also enables better load distribution, enhancing durability under stress [69, 70]. Another widely researched approach is the development of double-network hydrogels, where a soft, stretchable network is combined with a rigid, brittle network, allowing the material to dissipate energy effectively while maintaining flexibility [71, 72]. Furthermore, innovative crosslinking techniques and the introduction of sacrificial bonds that can break and reform under stress provide avenues to improve the toughness of hydrogels, making them more suitable for load-bearing applications [73, 74].
Slow Response of Hydrogels to Environmental Changes
Hydrogels are highly sensitive materials capable of responding to various environmental stimuli, such as changes in temperature, pH, ionic strength, or light. However, one significant drawback of many hydrogels is their slow response time to these changes, which limits their effectiveness in time-sensitive applications like drug delivery, biosensors, and tissue engineering [75, 76]. This sluggish response is largely due to the internal structure of the hydrogel, where water-filled polymer networks hinder rapid diffusion of molecules or ions. The rate at which these hydrogels swell or shrink, for example, is often limited by the slow diffusion of water into or out of the network [77]. Additionally, hydrogels with densely crosslinked polymer structures may take even longer to respond due to the reduced space available for the movement of solvent molecules [78, 79]. To address this issue, researchers have focused on several strategies to enhance the responsiveness of hydrogels to environmental changes. One approach is to reduce the size of the hydrogel, creating micro- or nano-hydrogels that have a larger surface area-to-volume ratio, allowing for quicker interactions with environmental stimuli [80]. Another method is to modify the polymer composition, incorporating more hydrophilic groups that facilitate faster water transport within the hydrogel matrix. The use of porous hydrogels with interconnected channels is also being explored, as these structures enable rapid diffusion of molecules and ions, speeding up the response. Additionally, the incorporation of smart materials, such as shape-memory polymers or responsive nanoparticles, into the hydrogel matrix can enhance its ability to react quickly to external stimuli [81, 82].
Hydrogels, while highly versatile and widely used in medical, pharmaceutical, and cosmetic applications, can pose certain side effects, depending on their composition, usage, and degradation products. One of the primary concerns is the potential for toxicity, particularly when synthetic polymers or chemical crosslinkers are used. Some hydrogels incorporate materials like acrylamide, which can release toxic byproducts during degradation, leading to inflammatory responses or tissue irritation [83, 84]. In medical applications, especially when used as drug delivery systems or wound dressings, these byproducts may cause local or systemic side effects, including allergic reactions, rashes, or chronic inflammation. There is also evidence from studies suggesting that some hydrogel formulations, particularly those not fully biocompatible, can lead to delayed healing or adverse immune responses [85].
Side Effects and Biocompatibility Concerns in Hydrogel Applications
Another significant issue arises from the mechanical failure of hydrogels, particularly in load-bearing applications. Poor mechanical strength can lead to the breakdown of the material under stress, releasing fragments that can trigger foreign body reactions or hinder the healing process [86, 87]. In cases where hydrogels are used in implants or tissue scaffolds, these structural failures may result in localized infections or implant rejection. Furthermore, the slow degradation of non-biodegradable hydrogels can lead to long-term complications, as the material may persist in the body, causing chronic inflammation or fibrosis. For example, polyacrylate-based hydrogels, often used in wound care and contact lenses, have been associated with eye irritation and corneal damage due to prolonged wear [88, 89]. While biodegradable and biocompatible hydrogels are being developed to address these issues, the challenge remains to balance functionality with safety. Research continues to focus on improving the biocompatibility of hydrogels through the use of natural polymers, such as alginate, gelatin, or chitosan, which are generally well-tolerated by the body. However, even natural hydrogels can occasionally cause immune responses, emphasizing the need for thorough biocompatibility testing before widespread clinical use [90, 91].
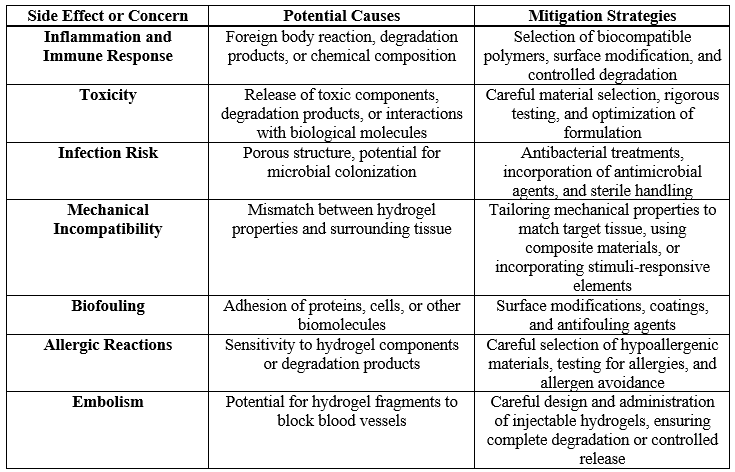
Table-III. Side Effects and Biocompatibility Concerns in Hydrogel Applications
Exploring Alternatives to Hydrogels: Aerogels, Alginate-Based Gels, and Superabsorbent Polymers for Diverse Applications
Alternatives to hydrogels include materials such as aerogels, alginate-based gels, and superabsorbent polymers (SAPs), each offering distinct advantages based on specific applications. Aerogels, which are lightweight and highly porous, are made from materials like silica, carbon, or organic polymers. Unlike hydrogels, which contain significant amounts of water, aerogels are low-density materials with excellent insulation capabilities. Their porous structure makes them ideal for absorbing gases or liquids, particularly in environmental clean-up or aerospace insulation applications, where hydrogels are less effective due to their high water content. Alginate-based gels, derived from seaweed, represent another alternative. These natural biopolymers are extensively used in wound healing and drug delivery systems due to their biodegradability and biocompatibility, making them preferable to synthetic hydrogels in many medical and pharmaceutical applications [92 - 95].
Superabsorbent polymers (SAPs), on the other hand, are cross-linked polymers that, like hydrogels, can absorb and retain large amounts of water. SAPs are commonly employed in products such as diapers, agricultural soil conditioners, and water retention aids. Although they share the water absorption properties of hydrogels, SAPs are often more cost-effective in large-scale industrial applications but may lack the tunability and biocompatibility required for biomedical uses. The choice of these alternatives over hydrogels is often driven by the specific needs of an application. For example, aerogels excel in situations requiring thermal insulation or low weight, whereas SAPs are more efficient in absorbing water in commercial products. In medical applications or environmentally sensitive areas, natural options like alginate gels are favored due to their lower environmental impact and superior biocompatibility. Moreover, alternatives such as aerogels or SAPs may offer enhanced mechanical strength, thermal stability, or chemical resistance, making them more suitable than hydrogels for industrial or engineering purposes. Ultimately, the selection of an alternative to hydrogels depends on factors such as performance requirements, environmental sustainability, and cost-effectiveness in specific contexts [96 - 101].
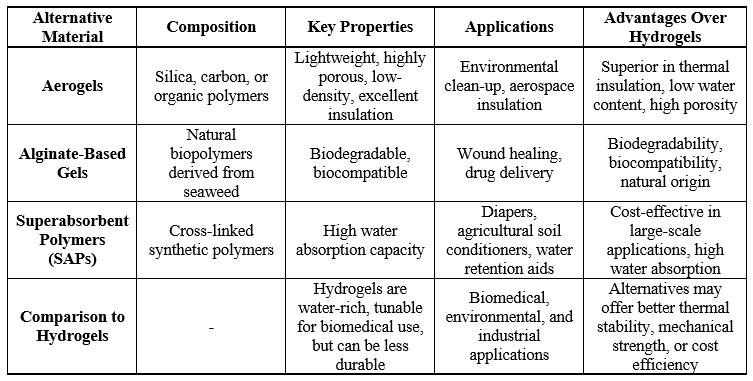
Table-IV. Comparative Analysis of Hydrogel Alternatives: Composition, Properties, and Application Potential

Fig. 2. Aerogels

Fig. 3. Alginate- Based Gels

Fig. 4. Super Absorbent Polymers
CONCLUSION:
In summary, while hydrogels have demonstrated substantial utility in various biomedical applications due to their high water content and biocompatibility, their mechanical limitations—such as low tensile strength and brittleness—pose significant challenges for their broader use in demanding environments. This review highlights that alternative materials such as aerogels, alginate-based gels, and superabsorbent polymers (SAPs) offer promising solutions to these limitations. Aerogels stand out for their exceptional thermal insulation and low density, making them suitable for applications requiring minimal weight and superior insulating properties. Their ability to absorb gases and liquids efficiently positions them as valuable materials in environmental and aerospace contexts where hydrogels' high water content is less advantageous.
Alginate-based gels provide an alternative that combines biodegradability and biocompatibility with improved mechanical strength, making them particularly advantageous in medical and pharmaceutical applications. Their natural origin and favorable interaction with biological systems offer benefits over synthetic hydrogels in contexts requiring both performance and environmental consideration. Superabsorbent polymers (SAPs) offer high water absorption capabilities at a lower cost, which is beneficial for large-scale industrial applications. However, their limited biocompatibility makes them less suitable for certain biomedical uses compared to hydrogels and alginate-based gels. Ultimately, the selection of an alternative material depends on the specific requirements of the application, including mechanical performance, environmental sustainability, and cost considerations. By addressing the mechanical weaknesses of traditional hydrogels with these advanced alternatives, researchers and engineers can enhance the functional performance and application scope of hydrogel-like materials, paving the way for innovations across a variety of fields.
REFERENCES
- Peppas NA, Hilt JZ, Khademhosseini A, Langer R. Hydrogels in biology and medicine: From molecular principles to bionanotechnology. Adv Mater. 2006;18(11):1345-1360. doi:10.1002/adma.200501612.
- Hoffman AS. Hydrogels for biomedical applications. Adv Drug Deliv Rev. 2012;64:18-23. doi:10.1016/j.addr.2012.09.010.
- Lee KY, Mooney DJ. Hydrogels for tissue engineering. Chem Rev. 2001;101(7):1869-1879. doi:10.1021/cr000108x.
- Balamurugan S, Naresh N, Prakash I, Satyanarayana N. Capacity fading mechanism of Li?O loaded NiFe?O?/SiO? aerogel anode for lithium-ion battery: ex-situ XPS analysis. Appl Surf Sci. 2021;535:147677. https://doi.org/10.1016/j.apsusc.2020.147677
- Bhagat SD, Kim YH, Suh KH, Ahn YC, Yeo JG. Superhydrophobic silica aerogel powders with simultaneous surface modification, solvent exchange, and sodium ion removal from hydrogels. Microporous Mesoporous Mater. 2008;112(1-3):504-9. https://doi.org/10.1016/j.micromeso.2007.10.030
- Lee KY, Mooney DJ. Alginate: properties and biomedical applications. Prog Polym Sci. 2012;37(1):106-126.
- Draget KI, Taylor C. Alginate gels: formation and biomedical and pharmaceutical applications. In: Handbook of Hydrocolloids. 2nd ed. Woodhead Publishing; 2011. p. 807-828.
- Filipovi? VV, Nikodinovic-Runic J, Vukomanovi? M. Alginate-based hydrogels and scaffolds for biomedical applications. Mar Drugs. 2023;21(3):177.
- Freeman I, Kedem A, Cohen S. The effect of sulfation of alginate hydrogels on the specific binding and controlled release of heparin-binding proteins. Biomaterials. 2008;29(23):3260-3268.
- Buchholz FL, Graham AT. Modern superabsorbent polymer technology. New York: Wiley; 1998.
- Annabi N, Tamayol A, Uquillas JA, Akbari M, Bertassoni LE, Cha C, et al. 25th anniversary article: Rational design and applications of hydrogels in regenerative medicine. Adv Mater. 2014;26(1):85-124.
- Peppas NA, Bures P, Leobandung W, Ichikawa H. Hydrogels in pharmaceutical formulations. European Journal of Pharmaceutics and Biopharmaceutics. 2000;50(1):27-46.
- Zhang YS, Khademhosseini A. Advances in engineering hydrogels. Science. 2017;356(6337)
- Li J, Mooney DJ. Designing hydrogels for controlled drug delivery. Nat Rev Mater. 2016;1(12):16071.
- Caló E, Khutoryanskiy VV. Biomedical applications of hydrogels: A review of patents and commercial products. Eur Polym J. 2015;65:252-67.
- Wang XQ, Xie AQ, Cao P, Yang J, Ong WL, Zhang KQ, Ho GW. Structuring and Shaping of Mechanically Robust and Functional Hydrogels toward Wearable and Implantable Applications. Advanced Materials. 2024 Feb 23:2309952.
- Banerjee H, Suhail M, Ren H. Hydrogel actuators and sensors for biomedical soft robots: brief overview with impending challenges. Biomimetics. 2018 Jul 10;3(3):15.
- Lin X, Zhao X, Xu C, Wang L, Xia Y. Progress in the mechanical enhancement of hydrogels: Fabrication strategies and underlying mechanisms. Journal of polymer science. 2022 Sep 1;60(17):2525-42.
- Dhand AP, Galarraga JH, Burdick JA. Enhancing biopolymer hydrogel functionality through interpenetrating networks. Trends in Biotechnology. 2021 May 1;39(5):519-38.
- Kim S, Park MR, Choi C, Kim JB, Cha C. Synergistic control of mechanics and microarchitecture of 3D bioactive hydrogel platform to promote the regenerative potential of engineered hepatic tissue. Biomaterials. 2021 Mar 1;270:120688.
- Ahearne M. Introduction to cell–hydrogel mechanosensing. Interface focus. 2014 Apr 6;4(2):20130038.
- Zhang J, Yang H, Abali BE, Li M, Xia Y, Haag R. Dynamic mechanics?modulated hydrogels to regulate the differentiation of stem?cell spheroids in soft microniches and modeling of the nonlinear behavior. Small. 2019 Jul;15(30):1901920.
- Zhuang Y, Yu F, Chen H, Zheng J, Ma J, Chen J. Alginate/graphene double-network nanocomposite hydrogel beads with low-swelling, enhanced mechanical properties, and enhanced adsorption capacity. Journal of Materials Chemistry A. 2016;4(28):10885-92.
- Lee C, Shin J, Lee JS, Byun E, Ryu JH, Um SH, Kim DI, Lee H, Cho SW. Bioinspired, calcium-free alginate hydrogels with tunable physical and mechanical properties and improved biocompatibility. Biomacromolecules. 2013 Jun 10;14(6):2004-13.
- Wang X, Guo C, Pi M, Li M, Yang X, Lu H, Cui W, Ran R. Significant roles of ions in enhancing and functionalizing anisotropic hydrogels. ACS Applied Materials & Interfaces. 2022 Nov 2;14(45):51318-28.
- Sarrigiannidis SO, Rey JM, Dobre O, González-García C, Dalby MJ, Salmeron-Sanchez M. A tough act to follow: Collagen hydrogel modifications to improve mechanical and growth factor loading capabilities. Materials Today Bio. 2021 Mar 1;10:100098.
- Brandl F, Sommer F, Goepferich A. Rational design of hydrogels for tissue engineering: impact of physical factors on cell behavior. Biomaterials. 2007 Jan 1;28(2):134-46.
- Yuk H, Zhang T, Parada GA, Liu X, Zhao X. Skin-inspired hydrogel–elastomer hybrids with robust interfaces and functional microstructures. Nat Commun. 2021;12(1):1-2.
- Gong JP. Why are double network hydrogels so tough? Soft Matter. 2010;6(12):2583-90.
- Calvert P. Hydrogels for soft machines. Adv Mater. 2009;21(7):743-56.
- Sun JY, Zhao X, Illeperuma WR, Chaudhuri O, Oh KH, Mooney DJ, et al. Highly stretchable and tough hydrogels. Nature. 2012;489(7414):133-6.
- Sokolov VV, de Groot JH, Schakenraad JM. Compressive properties of hydrogels used in load-bearing applications. Adv Mater. 2004;16(13):1122-1126. doi:10.1002/adma.200306454.
- Tsurkan MV, Waller C, Schubert US. Composite hydrogels with reinforced mechanical properties for tissue engineering applications. Biomaterials. 2011;32(31):7943-7954. doi:10.1016/j.biomaterials.2011.06.034.
- Yang J, Han CR, Duan JF, Xu F, Sun RC. Mechanical and viscoelastic properties of cellulose nanocrystals reinforced poly (ethylene glycol) nanocomposite hydrogels. ACS applied materials & interfaces. 2013 Apr 24;5(8):3199-207.
- Fan C, Yang W, Zhang L, Cai H, Zhuang Y, Chen Y, Zhao Y, Dai J. Restoration of spinal cord biophysical microenvironment for enhancing tissue repair by injury-responsive smart hydrogel. Biomaterials. 2022 Sep 1;288:121689.
- Huang X, Li J, Luo J, Gao Q, Mao A, Li J. Research progress on double-network hydrogels. Materials Today Communications. 2021 Dec 1;29:102757.
- Guillet JF, Valdez-Nava Z, Golzio M, Flahaut E. Electrical properties of double-wall carbon nanotubes nanocomposite hydrogels. Carbon. 2019 May 1;146:542-8.
- Nascimento DM, Nunes YL, Figueirêdo MC, de Azeredo HM, Aouada FA, Feitosa JP, Rosa MF, Dufresne A. Nanocellulose nanocomposite hydrogels: technological and environmental issues. Green Chemistry. 2018;20(11):2428-48.
- Yang Y, Wang C, Wiener CG, Hao J, Shatas S, Weiss RA, Vogt BD. Tough stretchable physically-cross-linked electrospun hydrogel fiber mats. ACS Applied Materials & Interfaces. 2016 Sep 7;8(35):22774-9.
- Dehbari N, Tang Y. Water swellable rubber composites: An update review from preparation to properties. Journal of applied polymer science. 2015 Dec 10;132(46).
- Hebeish A, Farag S, Sharaf S, Shaheen TI. Thermal responsive hydrogels based on semi interpenetrating network of poly (NIPAm) and cellulose nanowhiskers. Carbohydrate polymers. 2014 Feb 15;102:159-66.
- Kenny LZ. Intelligent Soft Materials for Bio-Based Applications (Doctoral dissertation, National University of Singapore (Singapore)).
- Nie M, Chen K. Developments and Challenges of Hydrogel Coatings for Long-Term Marine Antifouling Applications. IntecOpen.
- Yilmaz ND. Multicomponent, semi-interpenetrating-polymer-network and interpenetrating-polymer-network hydrogels: smart materials for biomedical applications. Functional Biopolymers. 2018:281-342.
- Xia D, Wang P, Ji X, Khashab NM, Sessler JL, Huang F. Functional supramolecular polymeric networks: the marriage of covalent polymers and macrocycle-based host–guest interactions. Chemical Reviews. 2020 May 19;120(13):6070-123.
- Haq MA, Su Y, Wang D. Mechanical properties of PNIPAM based hydrogels: A review. Materials Science and Engineering: C. 2017 Jan 1;70:842-55.
- Haraguchi K. Nanocomposite hydrogels. Current Opinion in Solid State and Materials Science. 2007 Jun 1;11(3-4):47-54.
- Mehrali M, Thakur A, Pennisi CP, Talebian S, Arpanaei A, Nikkhah M, Dolatshahi?Pirouz A. Nanoreinforced hydrogels for tissue engineering: biomaterials that are compatible with load?bearing and electroactive tissues. Advanced Materials. 2017 Feb;29(8):1603612.
- Zou P, Yao J, Cui YN, Zhao T, Che J, Yang M, Li Z, Gao C. Advances in cellulose-based hydrogels for biomedical engineering: a review summary. Gels. 2022 Jun 8;8(6):364.
- Liu H, Chen T, Dong C, Pan X. Biomedical applications of hemicellulose-based hydrogels. Current Medicinal Chemistry. 2020 Aug 1;27(28):4647-59.
- Martin N, Youssef G. Dynamic properties of hydrogels and fiber-reinforced hydrogels. Journal of the mechanical behavior of biomedical materials. 2018 Sep 1;85:194-200.
- Maranchi JP, Trexler MM, Guo Q, Elisseeff JH. Fibre-reinforced hydrogels with high optical transparency. International materials reviews. 2014 Jun 1;59(5):264-96.
- Imran AB, Seki T, Takeoka Y. Recent advances in hydrogels in terms of fast stimuli responsiveness and superior mechanical performance. Polymer journal. 2010 Nov;42(11):839-51.
- Dai T, Qing X, Lu Y, Xia Y. Conducting hydrogels with enhanced mechanical strength. Polymer. 2009 Oct 20;50(22):5236-41.
- Hu L, Chee PL, Sugiarto S, Yu Y, Shi C, Yan R, Yao Z, Shi X, Zhi J, Kai D, Yu HD. Hydrogel?based flexible electronics. Advanced Materials. 2023 Apr;35(14):2205326.
- Ding X, Fan L, Wang L, Zhou M, Wang Y, Zhao Y. Designing self-healing hydrogels for biomedical applications. Materials Horizons. 2023.
- Awasthi S, Gaur JK, Bobji MS, Srivastava C. Nanoparticle-reinforced polyacrylamide hydrogel composites for clinical applications: a review. Journal of Materials Science. 2022 May;57(17):8041-63.
- Bao B, Zeng Q, Li K, Wen J, Zhang Y, Zheng Y, Zhou R, Shi C, Chen T, Xiao C, Chen B. Rapid fabrication of physically robust hydrogels. Nature Materials. 2023 Oct;22(10):1253-60.
- Madduma?Bandarage US, Madihally SV. Synthetic hydrogels: Synthesis, novel trends, and applications. Journal of Applied Polymer Science. 2021 May 15;138(19):50376.
- Pan C, Liu L, Gai G. Recent progress of graphene?containing polymer hydrogels: preparations, properties, and applications. Macromolecular Materials and Engineering. 2017 Oct;302(10):1700184.
- Odent J, Wallin TJ, Pan W, Kruemplestaedter K, Shepherd RF, Giannelis EP. Highly elastic, transparent, and conductive 3D?printed ionic composite hydrogels. Advanced Functional Materials. 2017 Sep;27(33):1701807.
- Ahn W, Lee JH, Kim SR, Lee J, Lee EJ. Designed protein-and peptide-based hydrogels for biomedical sciences. Journal of Materials Chemistry B. 2021;9(8):1919-40.
- Yadav A, Rai V, Singh S, Kanojia A. Advancements in Soft Robotic Implants for Long-Term Drug Delivery and Tissue Monitoring. Journal for Research in Applied Sciences and Biotechnology. 2024 Aug 25;3(4):28-39.
- Lima CS, Balogh TS, Varca JP, Varca GH, Lugão AB, A. Camacho-Cruz L, Bucio E, Kadlubowski SS. An updated review of macro, micro, and nanostructured hydrogels for biomedical and pharmaceutical applications. Pharmaceutics. 2020 Oct 15;12(10):970.
- Moser T, Celma C, Lebert A, Charrault E, Brooke R, Murphy PJ, Browne G, Young R, Higgs T, Evans D. Hydrophilic organic electrodes on flexible hydrogels. ACS Applied Materials & Interfaces. 2016 Jan 13;8(1):974-82.
- Xue X, Hu Y, Deng Y, Su J. Recent advances in design of functional biocompatible hydrogels for bone tissue engineering. Advanced Functional Materials. 2021 May;31(19):2009432.
- Kumar A, Rao KM, Han SS. Mechanically viscoelastic nanoreinforced hybrid hydrogels composed of polyacrylamide, sodium carboxymethylcellulose, graphene oxide, and cellulose nanocrystals. Carbohydrate polymers. 2018 Aug 1;193:228-38.
- Shojaeiarani J, Bajwa D, Shirzadifar A. A review on cellulose nanocrystals as promising biocompounds for the synthesis of nanocomposite hydrogels. Carbohydrate polymers. 2019 Jul 15;216:247-59.
- Gyles DA, Castro LD, Silva Jr JO, Ribeiro-Costa RM. A review of the designs and prominent biomedical advances of natural and synthetic hydrogel formulations. European Polymer Journal. 2017 Mar 1;88:373-92.
- Wang C, Stewart RJ, Kope?ek J. Hybrid hydrogels assembled from synthetic polymers and coiled-coil protein domains. Nature. 1999 Feb 4;397(6718):417-20.
- Yin Y, Gu Q, Liu X, Liu F, McClements DJ. Double-network hydrogels: design, fabrication, and application in foods and biomedicines. Advances in Colloid and Interface Science. 2023 Sep 20:102999.
- Chen Q, Chen H, Zhu L, Zheng J. Fundamentals of double network hydrogels. Journal of Materials Chemistry B. 2015;3(18):3654-76.
- Moghadam MN, Pioletti DP. Improving hydrogels? toughness by increasing the dissipative properties of their network. Journal of the mechanical behavior of biomedical materials. 2015 Jan 1;41:161-7.
- Li J, Illeperuma WR, Suo Z, Vlassak JJ. Hybrid hydrogels with extremely high stiffness and toughness. ACS Macro Letters. 2014 Jun 17;3(6):520-3.
- Strachota B, Oleksyuk K, Strachota A, Šlouf M. Porous hybrid poly (N-isopropylacrylamide) hydrogels with very fast volume response to temperature and pH. European Polymer Journal. 2019 Nov 1;120:109213.
- Zhao Y, Shi C, Yang X, Shen B, Sun Y, Chen Y, Xu X, Sun H, Yu K, Yang B, Lin Q. pH-and temperature-sensitive hydrogel nanoparticles with dual photoluminescence for bioprobes. ACS nano. 2016 Jun 28;10(6):5856-63.
- Imran AB, Seki T, Takeoka Y. Recent advances in hydrogels in terms of fast stimuli responsiveness and superior mechanical performance. Polymer journal. 2010 Nov;42(11):839-51.
- Fu J. Strong and tough hydrogels crosslinked by multi?functional polymer colloids. Journal of Polymer Science Part B: Polymer Physics. 2018 Oct 1;56(19):1336-50.
- Kong HJ, Wong E, Mooney DJ. Independent control of rigidity and toughness of polymeric hydrogels. Macromolecules. 2003 Jun 17;36(12):4582-8.
- Yang S, Wang F, Han H, Santos HA, Zhang Y, Zhang H, Wei J, Cai Z. Fabricated technology of biomedical micro-nano hydrogel. Biomedical Technology. 2023 Jun 1;2:31-48.
- Lo?wenberg C, Balk M, Wischke C, Behl M, Lendlein A. Shape-memory hydrogels: evolution of structural principles to enable shape switching of hydrophilic polymer networks. Accounts of chemical research. 2017 Apr 18;50(4):723-32.
- Lv Y, Chen X, Zhang P, Zhou S, Li J, Bai R, Wang L. Hydrophilic shape memory polymer hydrogels with virous pore structures and shape changing performance. Polymers for Advanced Technologies. 2022 Oct;33(10):3532-9.
- Zhu R, Wu G, Liu X, Shi D, Cao B, Gu R, Xiao J, Liao H. PNIPAM hydrogel induces skeletal muscle inflammation response. RSC Advances. 2015;5(36):28023-9.
- Darnell MC, Sun JY, Mehta M, Johnson C, Arany PR, Suo Z, Mooney DJ. Performance and biocompatibility of extremely tough alginate/polyacrylamide hydrogels. Biomaterials. 2013 Nov 1;34(33):8042-8.
- Lu B, Han X, Zou D, Luo X, Liu L, Wang J, Maitz MF, Yang P, Huang N, Zhao A. Catechol-chitosan/polyacrylamide hydrogel wound dressing for regulating local inflammation. Materials Today Bio. 2022 Dec 1;16:100392.
- Lin P, Ma S, Wang X, Zhou F. Molecularly engineered dual?crosslinked hydrogel with ultrahigh mechanical strength, toughness, and good self?recovery. Advanced Materials. 2015 Mar;27(12):2054-9.
- Hua J, Ng PF, Fei B. High?strength hydrogels: Microstructure design, characterization and applications. Journal of Polymer Science Part B: Polymer Physics. 2018 Oct 1;56(19):1325-35.
- Assad H, Fatma I, Thakur A, Sharma S, Ganjoo R, Singh N, Kumar A. An overview of hydrogels and their potential applications in drug delivery. InAIP Conference Proceedings 2023 Sep 8 (Vol. 2800, No. 1). AIP Publishing.
- Awasthi S. A review on hydrogels and ferrogels for biomedical applications. Jom. 2021 Aug;73(8):2440-51.
- Bercea M. Bioinspired hydrogels as platforms for life-science applications: Challenges and opportunities. Polymers. 2022 Jun 11;14(12):2365.
- Kaliaraj GS, Shanmugam DK, Dasan A, Mosas KK. Hydrogels—A Promising Materials for 3D Printing Technology. Gels. 2023 Mar 22;9(3):260.
- Jose AJ, Mohan K, Vavachan A. Alginate Hydrogel and Aerogel. Alginates: Applications in the Biomedical and Food Industries. 2019 Feb 13:59.
- Radoor S, Karayil J, Jayakumar A, Kandel DR, Kim JT, Siengchin S, Lee J. Recent advances in cellulose-and alginate-based hydrogels for water and wastewater treatment: A review. Carbohydrate Polymers. 2024 Jan 1;323:121339.
- Akter M, Bhattacharjee M, Dhar AK, Rahman FB, Haque S, Rashid TU, Kabir SF. Cellulose-based hydrogels for wastewater treatment: A concise review. Gels. 2021 Mar 18;7(1):30.
- Ghiorghita CA, Dinu MV, Lazar MM, Dragan ES. Polysaccharide-based composite hydrogels as sustainable materials for removal of pollutants from wastewater. Molecules. 2022 Dec 5;27(23):8574.
- Benhalima T, Chicha W, Ferfera-Harrar H. Sponge-like biodegradable polypyrrole-modified biopolymers for selective adsorption of basic red 46 and crystal violet dyes from single and binary component systems. International Journal of Biological Macromolecules. 2023 Dec 31;253:127532.
- Shaghaleh H, Hamoud YA, Sun Q. Functionalized nanocellulose nanocomposite hydrogels for soil and water pollution prevention, remediation, and monitoring: A critical review on fabrication, application properties, and potential mechanisms. Journal of Environmental Chemical Engineering. 2024 Jan 6:111892.
- Sun Y, Li D, Yu Y, Zheng Y. Insights into the role of natural polysaccharide-based hydrogel wound dressings in biomedical applications. Gels. 2022 Oct 12;8(10):646.
- Lv A, Lv X, Xu X, Shao ZB. Tailored ultra-tough, antimicrobial and recyclable hydrogels based on chitosan and ionic liquid modified montmorillonite with different chain lengths for efficient adsorption of organic dyes in wastewater. International Journal of Biological Macromolecules. 2023 Dec 14:128752.
- Su T, Wu L, Pan X, Zhang C, Shi M, Gao R, Qi X, Dong W. Pullulan-derived nanocomposite hydrogels for wastewater remediation: Synthesis and characterization. Journal of colloid and interface science. 2019 Apr 15;542:253-62
- Crini G, Torri G, Lichtfouse E, Kyzas GZ, Wilson LD, Morin-Crini N. Dye removal by biosorption using cross-linked chitosan-based hydrogels. Environmental Chemistry Letters. 2019 Dec;17(4):1645-66.


 Kajal Kumari* 1
Kajal Kumari* 1
 K. Rajeswar Dutt 2
K. Rajeswar Dutt 2
 Arnab Roy 3
Arnab Roy 3
 Abrarul Haque 4
Abrarul Haque 4
 Nur Hasan 5
Nur Hasan 5
 Maheshwar Prasad Deep 6
Maheshwar Prasad Deep 6
 Manav Kumar 7
Manav Kumar 7
 Sahil Singh 8
Sahil Singh 8
 Sakshi Kumari 9
Sakshi Kumari 9
 Sonu Sharma 10
Sonu Sharma 10
 Ayush Kumar 11
Ayush Kumar 11
 Sajid Ansari 12
Sajid Ansari 12
 Subham Kumar Lohani 13
Subham Kumar Lohani 13








 10.5281/zenodo.13995287
10.5281/zenodo.13995287