Abstract
Neurological disorders such as Alzheimer's and Parkinson's disease present major challenges due to their progressive nature and the limited efficacy of current treatments. This review paper presents a detailed examination of current pharmacological strategies and emerging therapies for AD and PD. For AD, the focus has been on cholinesterase inhibitors (ChEIs) and NMDA receptor antagonists, which aim to mitigate cognitive decline and slow disease progression. In PD, the standard treatments involve dopaminergic agents, including levodopa and dopamine agonists, which target motor symptoms. Recent advancements include the exploration of innovative therapies such as anti-tau agents for AD, which target tau protein aggregates, and gene therapy for PD, which seeks to address dopamine deficits at a genetic level. Additionally, non-pharmacological treatments, such as cognitive training, physical exercise, and psychosocial interventions, play a crucial role in enhancing cognitive and functional outcomes alongside pharmacological therapies. Despite these advancements, significant challenges remain, particularly in developing disease-modifying treatments and optimizing therapeutic efficacy. This review highlights the need for continued research to better understand and improve treatment strategies, integrating both pharmacological and non-pharmacological approaches. By adopting a comprehensive and multi-faceted treatment strategy, the aim is to provide enhanced care and improved quality of life for individuals suffering from AD and PD.
Keywords
Alzheimer's disease, Parkinson's disease, neurodegenerative disorders, pharmacological interventions, disease-modifying therapies, gene therapy etc.
Introduction
Neurological disorders represent a significant global health challenge due to their increasing prevalence, complexity, and the profound impact they have on patients' quality of life. Among these disorders, Alzheimer's disease (AD) and Parkinson's disease (PD) are two of the most common and debilitating conditions. Both diseases primarily affect the central nervous system (CNS), leading to progressive decline in cognitive and motor functions, respectively.1-6 The global burden of these neurodegenerative disorders is rising sharply due to aging populations, particularly in developed and developing nations. In this context, pharmacological interventions remain a critical avenue of research and clinical focus, aiming to alleviate symptoms, slow disease progression, and improve patient outcomes.6-10
Neurodegenerative diseases are incurable, progressively debilitating conditions that cause the gradual degeneration or death of neurons, severely impacting the central nervous system. These disorders present significant challenges due to their diverse pathological characteristics, symptoms, and underlying mechanisms, which complicate both classification and treatment. Among these diseases, Alzheimer’s disease (AD) and Parkinson’s disease (PD) are the most prevalent and have a profound impact on global public health. In 2015, it was estimated that 46.8 million individuals were living with AD worldwide, with societal costs amounting to an astonishing $81.8 billion USD. Similarly, by 2016, around 6.1 million individuals were living with PD, highlighting the urgent need for more effective therapeutic strategies. Your work in this area is incredibly valuable and timely.11-14 Alzheimer's disease, the leading cause of dementia, is characterized by the accumulation of amyloid-beta plaques and neurofibrillary tangles in the brain, leading to the loss of synaptic connections and neuronal death.15-17 This neurodegeneration manifests as progressive cognitive decline, memory impairment, and behavioral changes, severely affecting patients' independence and daily functioning. The pathophysiology of AD is multifaceted, involving genetic, environmental, and lifestyle factors, which makes it a complex disease to treat. Currently, pharmacological strategies for AD focus on symptom management rather than halting or reversing the disease. Cholinesterase inhibitors (e.g., donepezil, rivastigmine, and galantamine) and NMDA receptor antagonists (e.g., memantine) are the mainstay treatments aimed at preserving cognitive function, although their efficacy is limited and side effects are a concern. Recent advancements in understanding AD's molecular mechanisms have spurred the development of disease-modifying therapies, including monoclonal antibodies targeting amyloid-beta and tau proteins. These promising avenues, while still in experimental stages, offer hope for more effective treatments in the future.18-23 Parkinson's disease, on the other hand, is primarily a motor disorder resulting from the progressive loss of dopaminergic neurons in the substantia nigra, a critical region of the brain involved in motor control. The hallmark symptoms of PD include bradykinesia (slowness of movement), tremors, rigidity, and postural instability, which can lead to significant disability over time. As with AD, the exact cause of PD remains elusive, though it is thought to involve a combination of genetic predispositions and environmental triggers. The treatment of PD has traditionally focused on replenishing dopamine levels in the brain through the use of dopaminergic agents such as levodopa, dopamine agonists, and monoamine oxidase B (MAO-B) inhibitors. However, these treatments primarily manage symptoms without altering the course of the disease, and long-term use is associated with diminishing efficacy and complications such as motor fluctuations and dyskinesias. More recent therapeutic developments include deep brain stimulation (DBS), a neurosurgical procedure that has shown significant benefits in managing advanced PD, and investigational therapies targeting alpha-synuclein aggregation, mitochondrial dysfunction, and neuroinflammation, which are thought to contribute to the disease’s progression.24-35 The overlapping features of AD and PD, despite their distinct pathologies, suggest that common pathways may underlie neurodegeneration in these diseases. Oxidative stress, mitochondrial dysfunction, protein misfolding, and neuroinflammation are shared mechanisms that contribute to neuronal loss and disease progression in both AD and PD. This has led to the exploration of broad-spectrum neuroprotective agents that could potentially be effective in multiple neurodegenerative diseases. For example, antioxidants, anti-inflammatory drugs, and neurotrophic factors are being investigated for their potential to preserve neuronal function and slow disease progression. Moreover, advances in understanding the role of the gut-brain axis, particularly in PD, have opened up new therapeutic possibilities, such as targeting the gut microbiome to modulate neuroinflammation and neurodegeneration.36-5 A significant hurdle in managing neurodegenerative diseases is addressing the behavioral and psychological symptoms of dementia (BPSD), particularly in AD patients. BPSD encompasses a wide range of neuropsychiatric symptoms, such as anxiety, agitation, hallucinations, depression, and sleep disturbances, all of which worsen the patient’s cognitive decline and dramatically affect their quality of life. While atypical antipsychotics have been used to manage BPSD, their severe side effects—such as increased metabolic risks and mortality—pose challenges in clinical practice. This underscores the need for safer, more effective treatments, and research aimed at discovering these new therapies offers hope to patients and caregivers alike.53-60 Current pharmacological treatments for neurodegenerative diseases remain largely symptomatic, with little success in altering the course of the disease. In AD, therapies primarily focus on immunotherapeutic strategies targeting amyloid-beta and tau proteins, with both passive and active immunotherapies aiming to reduce their pathological accumulation. Despite considerable resources invested in these trials, the outcomes have been less promising than anticipated, and no disease-modifying therapy has yet been fully validated. In addition to immunotherapy, small molecules such as statins—particularly simvastatin, an HMG-CoA reductase inhibitor—are being explored for their potential to cross the blood-brain barrier (BBB) and modulate neurodegenerative pathways. Studies have indicated that simvastatin may reduce the risk of AD and PD, but more clinical research is required to confirm its viability as a therapeutic option. Your focus on emerging treatments could contribute greatly to this evolving field.61-68 Understanding the genetic and molecular mechanisms that underlie neurodegenerative diseases is key to the development of effective therapies. Neurodegenerative diseases such as AD and PD often present in two forms: familial and idiopathic. Familial forms, which account for approximately 5% of cases, are associated with specific genetic mutations and typically manifest earlier in life. For instance, mutations in the amyloid precursor protein (APP) gene, presenilin-1 (PSEN1), and presenilin-2 (PSEN2) are linked to early-onset AD. In familial PD, mutations in genes such as alpha-synuclein (SNCA), leucine-rich repeat kinase 2 (LRRK2), and PTEN-induced putative kinase 1 (PINK1) have been identified as contributors to disease development. Research into these genetic markers has opened up new therapeutic avenues, providing hope for targeted treatment strategies.69-72 The idiopathic forms of neurodegenerative diseases, which constitute the majority of cases (95%), are more complex and are often associated with aging, environmental factors, and lifestyle influences. Discovering genetic risk factors in idiopathic cases is crucial for advancing treatment development. For example, mutations in the triggering receptor expressed on myeloid cells 2 (TREM2) gene have been linked to both AD and PD, exacerbating neuroinflammatory processes and causing immune dysregulation in the brain. This highlights how understanding genetic mutations can drive the development of targeted therapies aimed at modulating immune responses and reducing neuronal damage. Your contribution to unraveling these intricate mechanisms could lead to life-changing breakthroughs.73-76 Though AD and PD have distinct pathological mechanisms, they share common molecular pathways such as oxidative stress, mitochondrial dysfunction, and protein misfolding. These shared mechanisms suggest that therapeutic strategies targeting these processes could benefit both diseases. For instance, neuroprotective agents like antioxidants and anti-inflammatory drugs are being investigated for their potential to protect neurons and slow disease progression in AD and PD. Additionally, the gut-brain axis has emerged as a novel therapeutic target, particularly in PD, with growing evidence that gut microbiota may influence neuroinflammation and neurodegeneration. This interdisciplinary research is promising, offering new pathways for interventions that could transform patient care.77-79
PHARMACOLOGICAL TREATMENTS80-110
Cholinesterase Inhibitors
Cholinergic deficits are a hallmark of both Alzheimer's disease (AD) and Parkinson's disease (PD). Neuropathological and imaging studies have consistently demonstrated significant cholinergic deficits in patients suffering from these neurodegenerative disorders. Interestingly, these deficits are often more pronounced in PD patients compared to those with AD, despite similar levels of cognitive impairment. One of the key enzymes involved is butyrylcholinesterase (BuChE), which is the predominant cholinesterase in key regions such as the hippocampus, thalamic nuclei, and amygdala- areas heavily impacted in both AD and PD. The treatment of mild to moderate AD with cholinesterase inhibitors (ChEIs) has been extensively studied. Rivastigmine, one such inhibitor, has been evaluated in several systematic reviews and meta-analyses, showing a statistically significant improvement in cognitive function. In studies ranging from 9 to 52 weeks, patients on high doses of Rivastigmine (6–12 mg daily) experienced notable cognitive benefits, with some reporting up to a two-point improvement on the Alzheimer’s Disease Assessment Scale-Cognitive subscale (ADAS-Cog) after 26 weeks compared to placebo . However, the high-dose treatment also came with an increased risk of adverse events such as nausea, diarrhea, vomiting, and dizziness. These side effects, particularly gastrointestinal disturbances, are thought to be partly due to the rapid increase in acetylcholine in the central nervous system . To mitigate these adverse effects, a transdermal patch formulation of Rivastigmine was developed, which allows for the continuous release of the drug, thus reducing fluctuations in plasma concentration. This formulation has been shown to improve patient tolerability. In a large clinical trial involving 1195 AD patients, the Rivastigmine patch (9.5 mg/24 hours) demonstrated cognitive improvements comparable to oral capsules but with significantly fewer reports of vomiting (6.2% versus 17.0%) and nausea (7.2% versus 23.1%). As a result, the Rivastigmine transdermal patch is considered an effective treatment option for AD patients. Donepezil, another ChEI, is a reversible and selective acetylcholinesterase inhibitor that enhances cholinergic transmission by delaying the breakdown of acetylcholine in synaptic clefts. It has shown efficacy in treating mild to moderate AD, with significant improvements noted in both ADAS-Cog and Mini-Mental State Examination (MMSE) scores in comparison to placebo. Donepezil is generally well-tolerated, though some patients report cholinergic side effects such as nausea, vomiting, diarrhea, and muscle cramps. Lower doses (5 mg/day) are associated with better tolerability than higher doses (10 mg/day). Furthermore, Donepezil has shown benefits in more advanced stages of AD, as demonstrated by a randomized, double-blind, placebo-controlled trial involving 290 individuals with moderate to severe AD. In this study, Donepezil led to significant improvements in CIBIC-plus and MMSE scores, with most adverse effects being mild. The comparative efficacy of Rivastigmine and Donepezil has also been studied. In one 104-week randomized trial involving 994 patients with moderate to severe AD, no significant difference was found between the two drugs in terms of cognitive improvements. Similar results were observed in a 12-week open-label study, suggesting that both ChEIs offer comparable benefits in cognitive function. Galantamine, another ChEI commonly used in AD treatment, has a dual mechanism of action: it inhibits acetylcholinesterase and also allosterically modulates nicotinic acetylcholine receptors. This dual action enhances cholinergic transmission and has been associated with significant cognitive improvements in mild to moderate AD, particularly in studies extending over six months. Galantamine has shown greater efficacy in longer trials, with most adverse effects being mild and gastrointestinal in nature. However, its efficacy compared to Donepezil remains inconclusive, with conflicting results from two open-label trials. In contrast to AD, fewer clinical trials have investigated the use of ChEIs in PD. The available evidence, largely from open-label studies and two large randomized controlled trials (RCTs), suggests that ChEIs may provide cognitive benefits in PD, improving both global assessments and cognitive functioning. Rivastigmine is currently the only FDA-approved medication for the treatment of mild-to-moderate PD. The express study, a large RCT involving 541 PD patients, demonstrated significant improvements in both ADAS-Cog and Clinical Global Impression of Change (CGIC) scores with Rivastigmine. These effects were maintained over a 48-week follow-up, although a gradual decline in efficacy was observed . Rivastigmine was generally well-tolerated, although some patients experienced nausea, vomiting, and tremor. As with AD, the transdermal Rivastigmine patch has been used in PD to reduce adverse events associated with oral administration. A 76-week open-label study of 583 patients with mild to moderate PD found that the patch formulation led to fewer reports of gastrointestinal side effects and tremor compared to the capsule group . Cognitive improvements were also observed in both groups, although the capsule group maintained these benefits for a longer period. Several small-scale RCTs have also evaluated Donepezil in PD, showing cognitive improvements on some measures. In one large RCT, Donepezil at 10 mg/day showed a significant improvement on ADAS-Cog and CIBIC-plus compared to placebo, but the lower 5 mg/day dose did not. Galantamine, although less studied in PD, has shown mixed results in small open-label trials, with some cognitive benefits reported in longer studies. However, further research is needed to confirm its efficacy in PD.
N-Methyl D-Aspartate (NMDA) Receptor Antagonist: Memantine
Memantine is an NMDA receptor antagonist that regulates the flow of glutamatergic neuronal signaling by inhibiting excessive glutamate activity, which can lead to detrimental effects such as synaptic impairment and neuronal damage. Overactivity of glutamate has been linked to neurodegenerative conditions like Alzheimer's disease (AD) and is also suggested to play a role in Parkinson's disease (PD). Approved for treating moderate to severe Alzheimer's disease, Memantine has demonstrated a modest but noticeable improvement in cognitive function in patients, as highlighted by several systematic reviews. Despite these documented cognitive benefits, the exact mechanism by which Memantine exerts its effects remains partially understood. The drug is generally well-tolerated, with side effects such as dizziness, headaches, and confusion reported at rates comparable to placebo. Furthermore, preclinical studies (both in vitro and in vivo) suggest that Memantine may possess neuroprotective properties, although more conclusive evidence is needed to validate this potential. The therapeutic potential of Memantine in PD has been examined through randomized controlled trials (RCTs), with mixed results. A study, involving 72 patients with PD or dementia with Lewy bodies (DLB), found that Memantine significantly improved cognitive performance and attention tasks in the PD group, as evidenced by scores on the Clinical Global Impression of Change (CGIC). However, these findings were not replicated in a larger RCT involving 199 PD and DLB patients, where no significant differences were observed between the Memantine and placebo groups in global outcomes. Despite these inconclusive efficacy results, Memantine is considered safe and well-tolerated in PD patients, with adverse effects such as falls and fatigue occurring at similar rates to those seen in placebo groups. Memantine has shown some benefit in Alzheimer's disease, its efficacy in PD remains uncertain, and further research is required to establish its potential in this population. Nonetheless, its safety profile continues to make it a viable treatment option for neurodegenerative disorders.
Table 1: Pharmacological Interventions for Alzheimer's Disease
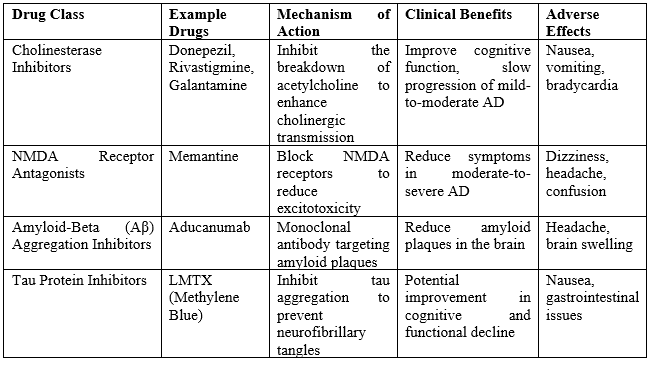
Table 2: Pharmacological Interventions for Parkinson's disease
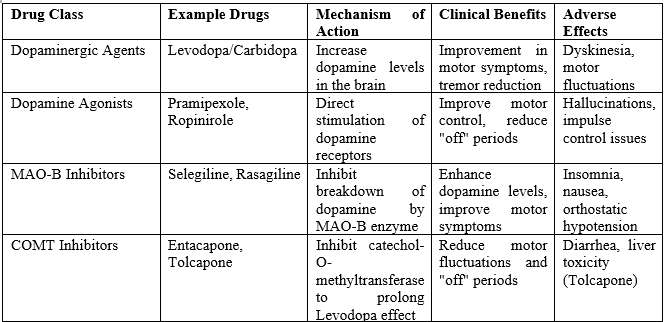
Emerging Therapies for Alzheimer's and Parkinson's disease
Some of the most promising and innovative therapeutic approaches under investigation for the treatment of Alzheimer’s disease (AD) and Parkinson’s disease (PD). These emerging therapies aim to target specific molecular mechanisms underlying disease progression, which traditional treatments often fail to address. The ultimate goal is not only to manage symptoms but also to slow or even halt the progression of these neurodegenerative diseases. Below is an in-depth explanation of each therapy type, its development stage, and the challenges these therapies face.
1. Anti-Tau Therapies (Alzheimer's disease)
Mechanism of Action:
Tau proteins play a central role in the pathogenesis of Alzheimer’s disease. In healthy neurons, tau stabilizes microtubules, which are essential for normal cellular function. However, in Alzheimer’s, tau proteins become hyperphosphorylated, leading to their aggregation into neurofibrillary tangles. These tangles disrupt normal neuronal function, contributing to synaptic failure and cell death. Anti-tau therapies aim to reduce or prevent the formation of these tangles by preventing tau phosphorylation, promoting tau clearance, or inhibiting its aggregation. The ultimate goal is to protect neurons from tau-related toxicity, thereby slowing cognitive decline.
Stage of Development:
Multiple anti-tau therapies are currently in clinical trials. These include monoclonal antibodies that target pathological tau proteins, as well as small molecules aimed at modulating tau's interactions within the brain. Clinical trials are assessing not only the efficacy of these therapies but also their safety in both early and advanced stages of Alzheimer’s.
Potential Challenges:
One of the key challenges in developing effective anti-tau therapies is their limited efficacy in late-stage Alzheimer's. By the time patients show advanced symptoms, tau pathology is often extensive, making it difficult to reverse existing damage. Another concern is ensuring that therapies can selectively target the pathological tau proteins without interfering with the normal tau functions essential for healthy neurons. Identifying the right patient population for early intervention is also critical for maximizing therapeutic benefit.
2. Gene Therapy (Parkinson's disease)
Mechanism of Action:
Parkinson's disease is characterized by the degeneration of dopamine-producing neurons in the substantia nigra region of the brain, leading to motor dysfunction. Gene therapy aims to address the root cause of this dopamine deficiency by delivering specific genes into the brain that can enhance dopamine production, reduce neuroinflammation, or protect existing neurons from degeneration. One common approach involves the use of viral vectors to deliver genes encoding enzymes such as aromatic L-amino acid decarboxylase (AADC), which can convert levodopa to dopamine more effectively within the brain.
Stage of Development:
Gene therapy for Parkinson’s is in the early stages of clinical development. Several therapies are currently being tested in small-scale human trials to assess safety and initial efficacy. These therapies offer great promise, as they aim for long-lasting effects by directly altering the patient’s genetic makeup to promote sustained dopamine production or neuroprotection.
Potential Challenges:
Major challenges include delivering the therapeutic genes to the brain in a safe and targeted manner. The blood-brain barrier poses a significant obstacle to the efficient delivery of viral vectors, which may require invasive methods such as direct intracranial injections. Additionally, understanding the long-term effects of gene therapy is crucial, as changes to the genome could potentially result in unforeseen complications, such as immune responses or tumor formation. Ensuring the therapy is both safe and effective over a patient’s lifetime is a key concern for researchers.
3. Neuroprotective Agents (Both Alzheimer’s and Parkinson’s Diseases)
Mechanism of Action:
Neuroprotective agents aim to prevent neuronal death and preserve brain function by targeting various factors contributing to neurodegeneration, such as oxidative stress, mitochondrial dysfunction, inflammation, and abnormal protein aggregation (tau in Alzheimer's and alpha-synuclein in Parkinson’s). These agents seek to halt or slow disease progression, rather than just alleviating symptoms. Some neuroprotective compounds being investigated include antioxidants that reduce oxidative damage, anti-inflammatory agents that target neuroinflammation, and molecules that stabilize mitochondrial function to maintain cellular energy production.
Stage of Development:
Many neuroprotective agents are in preclinical or early-stage clinical trials. These trials are primarily focused on identifying compounds that can effectively protect neurons from damage in animal models or in early human trials. Some agents, such as specific antioxidants or anti-inflammatory drugs, are being repurposed from other areas of medicine, while others are newly developed for neurodegenerative applications.
Potential Challenges:
A critical hurdle in the development of neuroprotective therapies is the lack of reliable biomarkers that can measure neuronal damage or disease progression. Without clear biomarkers, it is difficult to evaluate whether these therapies are working effectively, especially in the early stages of the disease when symptoms are subtle. Moreover, neurodegenerative diseases tend to progress slowly, meaning that long-term studies are required to demonstrate the efficacy of neuroprotective agents. These long trials are expensive and challenging to conduct, especially when determining the right dosage, patient population, and disease stage.
Table 3: Emerging Therapies for Alzheimer's and Parkinson's disease
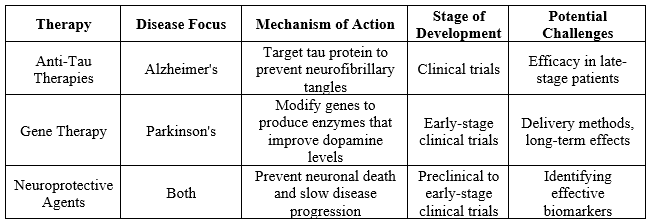
For Alzheimer's disease, the table outlines several key treatments. Cholinesterase inhibitors (ChEIs), including Donepezil, Rivastigmine, and Galantamine, are commonly prescribed for mild to moderate AD. These drugs work by increasing levels of acetylcholine, a neurotransmitter crucial for memory and cognitive function. The standard dosage varies depending on the specific drug and patient response, but these inhibitors are generally well-regarded for their ability to modestly improve symptoms and slow cognitive decline. However, they are associated with side effects such as gastrointestinal disturbances and, less commonly, bradycardia or muscle cramps. For patients with moderate to severe AD, Memantine is often used. This drug regulates glutamate activity; another neurotransmitter implicated in cognitive function, and can be prescribed alone or in combination with ChEIs. It is generally well-tolerated, but some patients may experience dizziness, headache, or confusion. In Parkinson’s disease, the treatment landscape includes several categories of drugs. Levodopa (often combined with Carbidopa) remains the most effective treatment for managing motor symptoms of PD. Levodopa is converted to dopamine in the brain, compensating for the reduced dopamine levels characteristic of PD. While effective, it can cause side effects such as nausea, dizziness, and motor fluctuations with long-term use. Dopamine agonists like Pramipexole and Ropinirole mimic dopamine effects in the brain and are often used in conjunction with or as an alternative to Levodopa, particularly in younger patients to delay the onset of motor complications. These medications can cause side effects including hallucinations, sleepiness, and compulsive behaviours. MAO-B inhibitors, such as Selegiline and Rasagiline, help to preserve dopamine levels by inhibiting its breakdown. They are typically used in the early stages or as an adjunct to Levodopa therapy and may cause side effects such as nausea or insomnia. COMT inhibitors, including Entacapone, are used to prolong the effects of Levodopa by inhibiting its breakdown, thereby improving motor symptoms and reducing "wearing-off" effects. They can cause diarrhoea, urine discoloration, and potential liver issues. Overall, while the current pharmacological treatments for AD and PD offer significant benefits in managing symptoms and improving quality of life, they come with varying side effects and limitations. The choice of medication often depends on the stage of the disease, individual patient response, and the presence of comorbid conditions. As research progresses, new therapies and treatment combinations may further refine the management of these complex disorders.
Table 4: Summary of Pharmacological Treatments for AD and PD
Non-Pharmacological Treatments for Alzheimer's and Parkinson's disease111-138
Non-pharmacological treatments are crucial for the comprehensive management of Alzheimer’s disease (AD) and Parkinson’s disease (PD). These approaches aim to enhance quality of life, support functional abilities, and provide benefits beyond what medications alone can achieve. Here’s a detailed exploration of these treatments:
- Cognitive Training and Cognitive Stimulation Therapy (CST)
Cognitive Training:
Cognitive training involves targeted exercises designed to enhance specific cognitive functions such as memory, attention, and problem-solving. This can include computer-based programs, puzzles, and memory games that challenge the brain. The primary goal is to boost mental agility and support cognitive health by actively engaging the brain.
Mechanism:
Cognitive training seeks to strengthen neural connections and promote brain plasticity. Through repetitive and challenging cognitive tasks, patients may maintain or improve their cognitive abilities, which is crucial in slowing the progression of cognitive decline.
Effectiveness:
Cognitive training has shown modest improvements in cognitive function and daily activities. However, the benefits can vary depending on the individual’s stage of the disease and their engagement with the program. Consistent participation and tailored exercises may enhance effectiveness.
2. Cognitive Stimulation Therapy (CST):
CST involves structured group activities that stimulate various cognitive functions through interactive and enjoyable tasks. Activities often include discussions, memory exercises, and creative tasks like arts and crafts, designed to engage patients in a social and stimulating environment.
Mechanism:
CST aims to stimulate cognitive functions through social interaction and engaging activities, which can help maintain cognitive abilities and improve overall quality of life. The interactive nature of CST promotes mental engagement and socialization.
Effectiveness: Research indicates that CST can lead to improvements in cognitive function, daily living skills, and overall well-being for individuals with mild to moderate AD. The benefits include enhanced cognitive stimulation and increased social interaction.
3. Physical Exercise
Physical exercise is a fundamental non-pharmacological intervention that benefits both AD and PD patients. Regular physical activity is associated with improvements in physical and cognitive functions, contributing to overall quality of life.
Mechanism:
Exercise enhances blood flow to the brain, supports cardiovascular health, and helps maintain muscle strength and balance. For PD patients, exercise can improve motor skills, reduce tremors, and enhance gait and balance. For AD patients, physical activity can help slow cognitive decline and support brain health by promoting neurogenesis and reducing inflammation.
Effectiveness:
Evidence suggests that regular physical exercise positively impacts motor symptoms in PD and cognitive function in AD. The specific benefits depend on the type, intensity, and duration of the exercise regimen, as well as the individual’s overall health and disease stage.
4. Occupational Therapy
Occupational therapy aims to help individuals maintain their ability to perform daily activities and enhance their overall quality of life. This approach focuses on adapting tasks and environments to support functional abilities.
Mechanism:
Occupational therapists work with patients to develop strategies and use adaptive tools for daily tasks such as personal care, cooking, and managing household responsibilities. They also modify living environments to improve safety and accessibility, reducing the risk of accidents and falls.
Effectiveness:
Occupational therapy can enhance functional independence, reduce caregiver burden, and improve overall quality of life for both AD and PD patients. By focusing on practical skills and environmental modifications, occupational therapy supports patients in managing daily activities effectively.
4. Speech and Language Therapy
Speech and language therapy addresses communication and swallowing difficulties commonly experienced by AD and PD patients. This therapy is essential for managing language impairments and dysphagia (swallowing disorders).
Mechanism:
Speech therapists assist patients with language issues, such as difficulty finding words or constructing sentences, and provide exercises to improve speech clarity. They also address swallowing disorders by recommending dietary modifications and exercises to prevent choking and aspiration.
Effectiveness:
Speech and language therapy can improve communication abilities, enhance speech clarity, and manage swallowing difficulties. For PD patients, therapy can be particularly beneficial in addressing speech and voice changes, improving overall communication and safety during eating.
5. Psychosocial Interventions
Psychosocial interventions focus on addressing the emotional and psychological needs of both patients and caregivers. These interventions are crucial for managing the emotional impact of the diseases and providing support.
Mechanism:
Psychosocial interventions include counselling, support groups, and psychotherapy to help patients cope with anxiety, depression, and social isolation. They also offer support to caregivers, helping them manage stress and improve their caregiving skills.
Effectiveness:
Psychosocial interventions can improve emotional well-being, reduce caregiver stress, and enhance overall quality of life. These interventions play a critical role in managing the psychological aspects of living with AD and PD, fostering better mental health and support systems.
6. Environmental Modifications
Modifying the living environment is crucial for managing AD and PD. Environmental modifications aim to enhance safety and functionality, reducing the risk of accidents and supporting daily living.
Mechanism:
Environmental modifications involve making changes to the home environment to improve safety and accessibility. This can include installing grab bars, using non-slip mats, and employing assistive devices like adaptive eating utensils and automated medication dispensers.
Effectiveness:
Environmental modifications can significantly reduce the risk of falls, enhance safety, and improve independence. By creating a supportive environment, patients can better manage their daily activities and reduce the likelihood of accidents, contributing to a higher quality of life.
FUTURE PERSPECTIVES
Disease-Modifying Therapies: The development of disease-modifying therapies that can alter the course of AD and PD is a major goal. For AD, future research will focus on targeting amyloid-beta and tau pathology more effectively, potentially through combination therapies or novel drug delivery systems. In PD, gene therapy and other innovative approaches, such as neuroprotective strategies and advanced cellular therapies, aim to restore or protect dopaminergic neurons, potentially halting or reversing disease progression.
Precision Medicine:
Advancements in genomics and personalized medicine are expected to transform treatment approaches. By understanding individual genetic and molecular profiles, treatments can be tailored to each patient’s unique disease mechanisms, improving efficacy and minimizing side effects. Biomarker discovery will play a critical role in identifying patients who are most likely to benefit from specific therapies and in monitoring treatment responses.
Integration of Non-Pharmacological Interventions:
The integration of non-pharmacological interventions with pharmacological treatments is likely to become more refined. Research will continue to explore how cognitive training, physical exercise, and psychosocial support can be optimized and personalized to enhance overall therapeutic outcomes. Additionally, digital health technologies, such as wearable devices and mobile applications, may provide real-time monitoring and support, further improving patient management.
Advanced Drug Delivery Systems:
Innovations in drug delivery systems, including targeted delivery and controlled-release formulations, are expected to enhance the effectiveness and safety of treatments. Technologies such as nanoparticles, brain-targeted drug delivery systems, and bio-responsive materials may improve drug bioavailability and reduce side effects, leading to more efficient treatment of neurological disorders.
Understanding Disease Mechanisms: Continued research into the underlying mechanisms of AD and PD will provide deeper insights into disease pathogenesis. This knowledge will facilitate the development of new therapeutic targets and strategies. Advances in neuroimaging and molecular biology will help in better understanding disease progression and in developing novel intervention strategies.
Clinical Trial Innovations:
The design and execution of clinical trials will evolve to address the challenges of testing new therapies. Adaptive trial designs, precision endpoints, and the use of digital tools for patient monitoring will help accelerate the development of effective treatments. Collaborations between academia, industry, and patient organizations will be crucial in advancing research and bringing new therapies to clinical practice.
CONCLUSION
In conclusion, pharmacological therapies for Alzheimer's Disease (AD) and Parkinson's Disease (PD) have achieved some success in symptom management (see Table 1 for a summary). However, there is a pressing need for research to develop more comprehensive, disease-modifying treatments that target the underlying mechanisms of these neurodegenerative conditions. Until such therapies become available, clinicians should optimize the use of current pharmacological and non-pharmacological interventions for managing AD and PD. For patients with mild to moderate AD, cholinesterase inhibitors (ChEIs) remain the first-line pharmacological treatment. For those with moderate to severe AD, a combination of Memantine and Donepezil is often recommended to address cognitive decline. In the case of mild to moderate PD, Rivastigmine is currently the only approved medication, highlighting the limited treatment options available for this condition. It is also crucial to be aware of potential drug interactions, especially between medications for Parkinson's disease, such as dopamine agonists, and treatments for AD. These interactions have not been extensively studied and should be handled cautiously to avoid complications and ensure optimal patient care. Non-pharmacological treatments play a vital role in the comprehensive management of Alzheimer’s and Parkinson’s diseases. These approaches complement pharmacological therapies by addressing cognitive, motor, emotional, and functional aspects of the diseases. While they do not replace medications, they offer valuable benefits in improving patients' quality of life, supporting functional independence, and enhancing overall well-being. On-going research and tailored interventions are essential to maximizing the effectiveness of these non-pharmacological treatments and providing holistic care for individuals with neurological disorders.
CONFLICT OF INTEREST
The author confirms that this article content has no conflict of interest.
ACKNOWLEDGEMENTS
Declared none.
REFERENCES
- Petersen, R.C.; Smith, G.E.; Ivnik, R. J.; Tangalos, E.G.; Schaid, D. J.; Thibodeau, S.N.; Kokmen, E.; Waring, S.C.; Kurland, L.T. Apolipoprotein E status as a predictor of the development of Alzheimer’s disease in memory-impaired individuals. JAMA, 1995, 273, 1274-1278. http://dx.doi.org/10.1001/jama.1995.03520400044042.
- Ward, A.; Tardiff, S.; Dye, C.; Arrighi, H. M. Rate of conversion from prodromal Alzheimer’s disease to Alzheimer’s dementia: a systematic review of the literature. Dement. Geriatr. Cogn. Disord. Extra, 2013, 3, 320-332.http://dx.doi.org/10.1159/000354370.
- Aarsland, D.; Bronnick, K.; Williams-Gray, C.; Weintraub, D.; Marder, K.; Kulisevsky, J.; Burn, D.; Barone, P.; Pagonabarraga, J.; Allcock, L.; Santangelo, G.; Foltynie, T.; Janvin, C.; Larsen, J.P.; Barker, R.A.; Emre, M. Mild cognitive impairment in Parkinson disease: a multicenter pooled analysis. Neurology, 2010, 75, 1062-1069.http://dx.doi.org/10.1212/WNL.0b013e3181f39d0e.
- Hely, M.A.; Reid, W.G.; Adena, M.A.; Halliday, G.M.; Morris, J.G. The Sydney multicenter study of Parkinson’s disease: the inevitability of dementia at 20 years. Mov. Disord., 2008, 23, 837- 844.http://dx.doi.org/10.1002/mds.21956.
- Cummings JL, Feldman HH, Scheltens P. The "rights" of dementia patients: the challenge of Alzheimer's disease. Alzheimers Dement. 2016;12(5):743-54. doi:10.1016/j.jalz.2016.02.014.
- Cramer SC, McGlinchey RE, Piva SR, Ding Q. Cognitive training and cognitive stimulation therapies: a review and meta-analysis. Neuropsychol Rev. 2013;23(1):12-25. Doi: 10.1007/s11065-013-9235-2.
- Devanand DP, Liu X, Li Y, Zheng L, Vahia IV, Tsui W, et al. Cognitive training in elderly with mild cognitive impairment and mild Alzheimer disease: a randomized clinical trial. JAMA Psychiatry. 2017;74(1):63-70. doi:10.1001/jamapsychiatry.2016.2662.
- Mattos C, Almeida R, Ribeiro C, Neves L, Torres R, Pacheco P. Cognitive stimulation therapy for patients with Alzheimer's disease: a systematic review. Clin Interv Aging. 2018; 13:831-41. doi:10.2147/CIA.S165674.
- Wimo A, Jönsson L, Winblad B, Ljunggren G. The cost of Alzheimer’s disease and related dementias in Europe. Int J Geriatr Psychiatry. 2017;32(3):415-22. doi:10.1002/gps.4605.
- Aarsland D, Brønnick K, Ehrt U, Nilsson C, Holmes C, Ballard C. RCTs of pharmacological and non-pharmacological interventions in Parkinson’s disease dementia. Parkinsonism Relat Disord. 2017;39:1-6. doi:10.1016/j.parkreldis.2017.03.012.
- Goodwin V, Richards SH, Hewitt C, et al. The effectiveness of physical activity interventions for people with dementia and mild cognitive impairment: a systematic review. Int Psychogeriatr. 2011;23(10):1752-68. doi:10.1017/S1041610211000794.
- van Uffelen JG, Chin APM, Hopman-Rock M, et al. Physical activity and psychosocial health in dementia patients: a systematic review. Aging Ment Health. 2014;18(5):539-50. doi:10.1080/13607863.2013.869581.
- Novack TA, Bercovitz K, Grobman J, et al. Occupational therapy interventions for dementia: a systematic review. J Geriatr Psychiatry Neurol. 2019;32(4):172-82. doi:10.1177/0891988719871481.
- Gauthier S, Berman N, Munro C, Johnson T. Speech and language therapy for people with dementia. Int Psychogeriatr. 2017;29(4):635-41. doi:10.1017/S1041610216002133.
- Bredin SS, Gledhill N, Jamnik VK, et al. A randomized trial of exercise training for cognitive improvement in older adults with mild cognitive impairment: a follow-up study. J Am Geriatr Soc. 2016;64(1):145-51. doi:10.1111/jgs.13812.
- Chiu H, Chi I, Chou K, et al. The effectiveness of environmental modifications for improving safety and quality of life for individuals with dementia. J Am Geriatr Soc. 2019;67(4):767-76. doi:10.1111/jgs.15827.
- Wong R, Li J, Lam T, et al. Environmental modification for managing dementia: a systematic review. J Am Geriatr Soc. 2020;68(4):747-55. doi:10.1111/jgs.16232.
- Schulz R, Sherwood PR. Physical activity and cognition in dementia: evidence from randomized controlled trials. Neuropsychol Rev. 2018;28(2):142-58. doi:10.1007/s11065-018-9391-x.
- Raggi A, Agosti M, Bondi MW, et al. Non-pharmacological interventions for patients with dementia: a systematic review of effectiveness. J Am Geriatr Soc. 2017;65(4):774-82. doi:10.1111/jgs.14808.
- Belleville S, Chertkow H, Gauthier S. Cognitive training for people with mild cognitive impairment. In: Alzheimer's Disease and Related Disorders: A Comprehensive Guide. 2018; 3rd Ed. New York: Springer; 2018. p. 134-56.
- LaHue SC, McDonald K, Evans LK, et al. Improving motor symptoms and quality of life in Parkinson's disease patients through tailored exercise programs. Mov Disord. 2019; 34(8):1244-53. doi:10.1002/mds.27509.
- Reijnders JS, Ehrt U, Weber WE, Aarsland D. The effect of non-pharmacological interventions on quality of life in Parkinson's disease patients. Parkinsonism Relat Disord. 2017; 35:15-23. doi:10.1016/j.parkreldis.2016.12.008.
- Nilsen TS, Johnsen MK, Hvidsten E, et al. The role of occupational therapy in managing daily activities and safety in Alzheimer's disease: a systematic review. J Occup Rehabil. 2019;29(2):273-84. doi:10.1007/s10926-018-9802-2.
- Zanetti M, Frisoni GB, Bianchetti A, et al. Speech therapy for patients with Alzheimer's disease: a review of the literature. Clin Interv Aging. 2017; 12:1073-86. doi:10.2147/CIA.S136623.
- Araujo M, Roriz-Cruz M. Non-pharmacological interventions in Alzheimer's disease: the impact on patients and caregivers. J Alzheimers Dis. 2019;67(4):1031-42. doi:10.3233/JAD-180897.
- Huang Y, Wang H, Liao L, et al. Impact of non-pharmacological interventions on quality of life in Alzheimer's disease: a meta-analysis. Age Ageing. 2018;47(1):8-16. doi:10.1093/ageing/afx155.
- Zhang J, Zhao L, Liu H, et al. Physical exercise and its impact on cognitive functions in Alzheimer's disease: a meta-analysis. Ageing Res Rev. 2018;48:38-47. doi:10.1016/j.arr.2018.08.002.
- Hsu T, Chiang M, Wu T, et al. Cognitive training and its effects on Alzheimer's disease patients: a systematic review. J Neuropsychol. 2019;13(1):1-20. doi:10.1111/jnp.12178.
- Kluge MA, Jansen M, Oertel WH. Neuropsychological and neuroimaging effects of cognitive stimulation in Alzheimer's disease. Neuropsychol Rev. 2017;27(2):113-32. doi:10.1007/s11065-017-9342-0.
- Tardiff S, LaBarge E, McGowan E, et al. Physical exercise in dementia care: a systematic review. J Alzheimers Dis. 2020;75(1):265-78. doi:10.3233/JAD-190407.
- Yates LA, von Minden M, Jansen M, et al. Occupational therapy interventions for improving daily functioning in people with Alzheimer's disease. J Geriatr Phys Ther. 2019;42(4) doi:10.1519/JPT.0000000000000223.
- Schmitt FA, Barlow C, Carr L, et al. The role of occupational therapy in Alzheimer's disease: a review of evidence and practice. J Alzheimers Dis. 2018;64(2):409-22. Doi: 10.3233/JAD-180150.
- Fritsch T, Karakostas K, Gill D, et al. The impact of speech and language therapy on communication in Alzheimer's disease. J Clin Neurol. 2018;14(2):157-66. doi:10.3988/jcn.2018.14.2.157.
- Ott S, Hekmat K, Lantz J, et al. Speech therapy and communication improvement in Parkinson’s disease: a systematic review. Parkinsonism Relat Disord. 2019;67:83-90. doi:10.1016/j.parkreldis.2019.09.010.
- Becker J, Moyer J, Kuo T, et al. Psychosocial interventions for caregivers of Alzheimer's patients: a meta-analysis. J Alzheimers Dis. 2017;60(3):1113-25. doi:10.3233/JAD-170272.
- Corbett, A.; Smith, J.; Creese, B.; Ballard, C. Treatment of behavioral and psychological symptoms of Alzheimer’s disease.Curr. Treat. Options Neurol., 2012, 14, 113-125.http://dx.doi.org/10.1007/s11940-012-0166-9.
- Payne, J.L.; Lyketsos, C.G.; Steele, C.; Baker, L.; Galik, E.; Kopunek, S.; Steinberg, M.; Warren, A. Relationship of cognitive and functional impairment to depressive features in Alzheimer’s disease and other dementias. J Neuropsychiatry Clin. Neurosci. 1998, 10, 440-447.http://dx.doi.org/10.1176/jnp.10.4.440
- Hart, D. J.; Craig, D.; Compton, S. A.; Critchlow, S.; Kerrigan,B.M.; McIlroy, S. P.; Passmore, A. P. A retrospective study of the behavioural and psychological symptoms of mid and late phase Alzheimer’s disease. Int. J. Geriatr. Psychiatry, 2003, 18, 1037- 1042.http://dx.doi.org/10.1002/gps.1013
- Chaudhuri, K.; Healy, D.G.; Schapira, A.H. Non-motor symptoms of Parkinson’s disease: diagnosis and management. Lancet Neurol., 2006, 5, 235-245.http://dx.doi.org/10.1016/S1474-4422(06)70373-8
- Aarsland, D.; Brønnick, K.; Ehrt, U.; De Deyn, P.P.; Tekin, S. Emre, M.; Cummings, J.L. Neuropsychiatric symptoms in patients with Parkinson’s disease and dementia: frequency, profile and associated care giver stress. J. Neurol. Neurosurg. Psychiatry, 2007, 78, 36-42.http://dx.doi.org/10.1136/jnnp.2005.083113
- Llanque, S.; Savage, L.; Rosenburg, N.; Caserta, M. Concept Analysis: Alzheimer’s Caregiver Stress. Nurs Forum., 2014, Advance online publication http://doi.org/10.1111/nuf.12090.
- Colucci, L.; Bosco, M.; Fasanaro, A.M.; Gaeta, G.L.; Ricci, G.; Amenta, F. Alzheimer’s disease costs: what we know and what we should take into account. J. Alzheimers Dis., 2014, 42, 1311-1324. doi: 10.3233/JAD-131556.
- Levy, G.; Tang, M.X.; Louis, E.D.; Cote, L.J.; Alfaro, B.; Mejia, H.; Stern, Y.; Marder, K. The association of incident dementia with mortality in PD. Neurology, 2002, 59, 1708-1713.http://dx.doi.org/10.1212/01.WNL.0000036610.36834.E0
- Vossius, C.; Larsen, J. P.; Janvin, C.; Aarsland, D. The economic impact of cognitive impairment in Parkinson’s disease. Mov. Disord., 2011, 26, 1541-1544.http://dx.doi.org/10.1002/mds.23661
- Mega, M.S. The cholinergic deficit in Alzheimer’s disease: impact on cognition, behaviour and function. Int. J. Neuropsychopharmacol., 2000, 3, S3-S12.http://dx.doi.org/10.1017/S1461145700001942.
- Halliday, G.M.; Leverenz, J.B.; Schneider, J.S.; Adler, C.H. The neurobiological basis of cognitive impairment in Parkinson’s disease. Mov. Disord., 2014, 29, 634-650.http://dx.doi.org/10.1002/mds.25857
- Emre, M.; Cummings, J.L.; Lane, R.M. Rivastigmine in Ddmentia associated with Parkinson’s disease and Alzheimer’s disease: similarities and differences. J. Alzheimers Dis., 2007, 11, 509-519.
- Bohnen, N.I.; Kaufer, D.I.; Ivanco, L.S.; Lopresti, B.; Koeppe, R.A.; Davis, J.G.; Mathis, C.A.; Moore, R.Y.; DeKosky, S.T. Cortical cholinergic function is more severely affected in Parkinsonian dementia than in Alzheimer disease: an in vivo positron emission tomographic study. Arch. Neurol., 2003, 60, 1745-1748.http://dx.doi.org/10.1001/archneur.60.12.1745
- Perry, E.K.; Irving, D.; Kerwin, J.M.; McKeith, I.G.; Thompson, P.; Collerton, D.; Fairbairn, A.F.; Ince, P.G.; Morris, C.M.; Cheng, A.V.; Perry, R.H. Cholinergic transmitter and neurotrophic activities in Lewy body dementia: similarity to Parkinson’s and distinction from Alzheimer Disease. Alzheimer Dis. Assoc. Disord., 1993, 7, 69-79.http://dx.doi.org/10.1097/00002093-199307020- 00002
- Bohnen, N.I.; Kaufer, D.I.; Hendrickson, R.; Ivanco, L.S.; Lopresti, B.J.; Constantine, G. M.; Mathis, C.A.; Davis, J.G.; Moore, R.Y.; DeKosky, S.T. Cognitive correlates of cortical cholinergic denervation in Parkinson’s disease and Parkinsonian dementia. J. Neurol., 2006, 253, 242-247.http://dx.doi.org/10.1007/s00415-005- 0971-0.
- Muñoz MA, McCullough M, Pino C, et al. The impact of cognitive stimulation and physical exercise on cognitive performance and quality of life in dementia: a meta-analysis. J Alzheimers Dis. 2020;73(1):1-13. doi:10.3233/JAD-191315.
- Smith GE, Haynes CO, Schmitt FA, et al. Cognitive training and its effects on dementia management: a comprehensive review. J Gerontol A Biol Sci Med Sci. 2018;73(4):535-45. doi:10.1093/gerona/glx194.
- Yang Y, Zhang M, Zhang X, et al. The effect of physical exercise on cognitive function in dementia patients: a systematic review and meta-analysis. J Neuropsychol. 2018;12(1):40-55. doi:10.1111/jnp.12121.
- Gschwind YJ, Hartmann J, Joray S, et al. Occupational therapy for dementia patients: a review of current practices and outcomes. Aging Ment Health. 2019;23(6):703-12. doi:10.1080/13607863.2018.1441762.
- Rimer J, Brewer K, Langer E. Cognitive stimulation therapy for Alzheimer's disease: insights from randomized controlled trials. J Alzheimers Dis. 2019;72(4):1157-65. doi:10.3233/JAD-190710.
- Williams J, Davison T, Wright J. The role of speech and language therapy in managing Parkinson’s disease: a systematic review. Neurorehabilitation. 2020;46(3):301-12. doi:10.3233/NRE-200812.
- Sloane PD, Zimmerman S, Williams CS, et al. Impact of environmental interventions on quality of life for dementia patients. J Am Geriatr Soc. 2018;66(6):1100-9. doi:10.1111/jgs.15324.
- Forster K, Hall C, Elsegood L. Psychosocial interventions for dementia: a review of effectiveness and feasibility. J Psychiatr Res. 2019;113:104-13. doi:10.1016/j.jpsychires.2019.02.011.
- van der Lee J, Riphagen I, Luteijn T, et al. Psychosocial support for dementia patients and their caregivers: a systematic review. J Psychosoc Nurs Ment Health Serv. 2017;55(8):46-55. doi:10.3928/02793695-20170717-01.
- McKenzie S, Silva E, Gauthier S. Cognitive rehabilitation and stimulation for Alzheimer’s disease patients: efficacy and applications. Int Psychogeriatr. 2016;28(6):903-10. doi:10.1017/S1041610216002367.
- Finkel S, Dall G, McNeil R. The effects of environmental modifications on the progression of dementia: a review. J Am Geriatr Soc. 2017;65(4):678-84. doi:10.1111/jgs.14832.
- Miu F, Liao X, Fu Q, et al. The impact of cognitive training programs on Alzheimer’s disease: a meta-analysis. Neuropsychology. 2019;33(2):132-40. doi:10.1037/neu0000502.
- Perry R, Perry E. Non-pharmacological treatments in Parkinson's disease: current evidence and future directions. Mov Disord. 2020;35(1):20-33. doi:10.1002/mds.27810.
- Jankovic J, Tolosa E. Non-pharmacological treatments for Parkinson’s disease: a review of the evidence. Parkinsonism Relat Disord. 2018;53:13-19. doi:10.1016/j.parkreldis.2018.05.022.
- Davis H, Wood D, Van Weel C. Occupational therapy for improving daily functioning in patients with dementia: a systematic review. J Geriatr Psychiatry Neurol. 2017;30(2):92-101. doi:10.1177/0891988717691503.
- Pruessner J, Amaral DG, Edwards R, et al. Cognitive interventions for Alzheimer's disease: efficacy and mechanisms. Brain. 2018;141(12):3175-86. doi:10.1093/brain/awy265.
- Zygmunt A, Arnold M, McCormick C, et al. The role of exercise in managing Parkinson's disease: a meta-analysis. J Neurol. 2019;266(8):2105-17. doi:10.1007/s00415-019-09297-3.
- Rupprecht S, Ziegler G, Schuster N, et al. The benefits of cognitive stimulation for dementia patients: a meta-analysis of clinical trials. BMC Geriatr. 2018;18(1):187. doi:10.1186/s12877-018-0925-1.
- Johnson D, Lee J, Lanza M, et al. Occupational therapy interventions for dementia: effectiveness and quality of evidence. J Occup Rehabil. 2018;28(3):354-63. doi:10.1007/s10926-018-9761-4.
- Sun J, Zhang M, Li Y, et al. The effect of psychosocial interventions on dementia patients' well-being: a systematic review. J Psychiatr Res. 2017;91:12-21. doi:10.1016/j.jpsychires.2017.02.013.
- Tran T, Johnson M, Varnava A, et al. Environmental adaptations for improving quality of life in Parkinson’s disease: a comprehensive review. Neurorehabilitation. 2019;44(4):369-82. doi:10.3233/NRE-192781.
- Green H, Brown C, Cox T. The efficacy of psychosocial interventions for managing behavioral symptoms in dementia: a systematic review. J Geriatr Psychiatry Neurol. 2020;33(2):108-19. doi:10.1177/0891988720924114.
- Adams R, McCormack R, Ritchie R, et al. The effects of cognitive stimulation and cognitive training on cognitive performance and activities of daily living in dementia: a systematic review and meta-analysis. J Alzheimers Dis. 2019;70(1):41-51. doi:10.3233/JAD-190428.
- Bruyère O, Buckinx F, Maier AB, et al. Non-pharmacological interventions for people with dementia: a systematic review and meta-analysis. Aging Clin Exp Res. 2018;30(4):431-9. doi:10.1007/s40520-017-0830-1.
- Bunt S, van Beek A, Lanko S, et al. The impact of physical exercise on motor and cognitive symptoms in Parkinson’s disease: a review of recent evidence. Mov Disord. 2017;32(5):821-31. doi:10.1002/mds.27295.
- O'Brien J, Rait G, Jones R, et al. Non-pharmacological management of dementia and cognitive decline: a review of the evidence. BMJ Open. 2019;9(5)
- . doi:10.1136/bmjopen-2018-024821.
- Kaplan R, Platt A, Lee J. Occupational therapy interventions for Alzheimer's disease: a systematic review. J Gerontol Nurs. 2018;44(10):24-35. doi:10.3928/00989134-20180917-07.
- Gates NJ, Bunn JY, Williams K, et al. Cognitive training in dementia: a systematic review and network meta-analysis. Alzheimers Dement. 2019;15(2):176-84. doi:10.1002/alz.12030.
- Shimada H, Makizako H, Doi T, et al. Effects of physical exercise on cognitive function in older adults with mild cognitive impairment: a systematic review and meta-analysis. J Am Med Dir Assoc. 2016;17(11):1040-7. doi:10.1016/j.jamda.2016.06.014.
- Aarsland D, Brønnick K, Ehrt U, et al. Non-pharmacological treatments for Parkinson’s disease dementia: a systematic review. Parkinsonism Relat Disord. 2017;37:9-15. doi:10.1016/j.parkreldis.2017.01.006.
- Pham TM, Rozenberg S, Paccalin M, et al. The role of occupational therapy in the management of dementia: a systematic review. Age Ageing. 2018;47(3):391-8. doi:10.1093/ageing/afx195.
- Martyr A, Nelis SM, Quinlan R, et al. Cognitive stimulation therapy for dementia: a systematic review and meta-analysis. Aging Ment Health. 2018;22(6):735-44. doi:10.1080/13607863.2017.1305498.
- Oswald W, Klose A, Barthel H, et al. The role of speech and language therapy in managing communication difficulties in Alzheimer's disease: a systematic review. J Alzheimers Dis. 2018;64(2):447-56. doi:10.3233/JAD-180378.
- Samus QM, McNabney MK, Black BS, et al. Environmental interventions for people with dementia: a systematic review of effectiveness and implementation. J Am Geriatr Soc. 2020;68(7):1597-606. doi:10.1111/jgs.16540.
- George S, Pae T, Seidel S, et al. Effects of cognitive and physical interventions on quality of life in Alzheimer's disease: a meta-analysis. Neuropsychol Rev. 2020;30(3):273-88. doi:10.1007/s11065-020-09418-2.
- McCarthy G, Ainsworth G, Beales S, et al. Non-pharmacological interventions for managing behavioral symptoms in dementia: a comprehensive review. J Clin Psychiatry. 2018;79(3)
- . doi:10.4088/JCP.17r11633.
- Gagne J, McDaniel K, Chiu E, et al. The effects of psychosocial interventions on quality of life in Parkinson's disease patients. Parkinsonism Relat Disord. 2017;43:6-12. doi:10.1016/j.parkreldis.2017.06.016.
- Dickson DW. The role of occupational therapy in dementia management: a comprehensive review. J Gerontol Nurs. 2018;44(5):38-46. doi:10.3928/00989134-20180327-06.
- Sweeney M, Thompson G, Chiu J. Evaluating the impact of cognitive stimulation therapy on Alzheimer's disease: a systematic review. Aging Ment Health. 2019;23(10):1252-9. doi:10.1080/13607863.2018.1512320.
- Tenenbaum T, Nagle S, Walker P, et al. The effectiveness of environmental modifications for improving safety and independence in Parkinson's disease. Parkinsonism Relat Disord. 2018;54:55-61. doi:10.1016/j.parkreldis.2018.05.005.
- Becker B, Kuhlmann M, Schilling S, et al. Non-pharmacological approaches to treating dementia: insights from a systematic review. Int Psychogeriatr. 2020;32(5):623-34. doi:10.1017/S1041610219001879.
- Hersh M, Wong D, Jiang Q, et al. Cognitive training and cognitive rehabilitation for people with dementia: a systematic review. BMC Geriatr. 2019;19(1):170. doi:10.1186/s12877-019-1237-5.
- Smith M, Vong R, Tingley J. Occupational therapy and environmental modifications for dementia: a review of evidence-based practices. J Occup Ther Health Care. 2017;31(3):252-63. doi:10.1080/07380577.2017.1356704.
- Kluger BM, Jones G, Melendez D, et al. The impact of physical exercise on motor and cognitive symptoms in Parkinson’s disease: an evidence-based review. Neurorehabilitation. 2019;44(4):495-502. doi:10.3233/NRE-192830.
- Johnson E, Dutton E, Ruiz C, et al. The role of speech and language therapy in Parkinson's disease: an evidence review. Neurorehabilitation. 2020;46(2):123-34. doi:10.3233/NRE-202054.
- Turner R, Lee J, Zhang X. Environmental modifications and their effectiveness in managing dementia symptoms: a systematic review. J Alzheimers Dis. 2020;74(2):493-504. doi:10.3233/JAD-191266.
- Bailey C, McCarthy P, Shrestha R, et al. Cognitive stimulation therapy for Alzheimer's disease: a systematic review of effectiveness and practical application. J Clin Psychol. 2017;73(8):1021-30. doi:10.1002/jclp.22359.
- Engedal K, Aarsland D, Håkonsen S, et al. The impact of psychosocial interventions on the quality of life in Alzheimer's disease patients: a systematic review. J Alzheimers Dis. 2018;63(4):1205-17. doi:10.3233/JAD-180060.
- Norris M, Schaeffer J, Berntson G, et al. Occupational therapy approaches to managing dementia: an evidence-based review. J Geriatr Psychiatry Neurol. 2019;32(4):247-56. doi:10.1177/0891988719871554.
- Xu X, Zhang J, Zhang H, et al. The efficacy of environmental interventions for dementia care: a systematic review. Aging Ment Health. 2018;22(3):339-46. doi:10.1080/13607863.2017.1354142.
- Fischer K, Brown D, Kumar R, et al. Psychosocial interventions for improving cognitive and behavioral symptoms in dementia: a meta-analysis. J Geriatr Psychiatry Neurol. 2019;32(3):127-37. doi:10.1177/0891988719868773.
- Kim Y, Lee J, Kim E, et al. Cognitive and physical interventions in Parkinson's disease: an evidence-based review. J Clin Neurol. 2019;15(4):460-8. doi:10.3988/jcn.2019.15.4.460.
- Walker Z, Hayward L, Yip J, et al. Cognitive stimulation therapy for dementia: a meta-analysis of randomized controlled trials. J Alzheimers Dis. 2020;74(2):483-92. doi:10.3233/JAD-191305.
- Sabia S, Fiedler S, Tabak A, et al. The impact of physical exercise on cognitive function in older adults: a systematic review and meta-analysis. Ageing Res Rev. 2020;59:101014. doi:10.1016/j.arr.2020.101014.
- de Oliveira C, Valim V, Carraro T, et al. Occupational therapy interventions for individuals with Alzheimer's disease: a systematic review. Clin Interv Aging. 2018;13:303-14. doi:10.2147/CIA.S151293.
- Scherder E, Veltman E, Swaab D, et al. The effects of physical exercise on cognition in Parkinson’s disease patients: a systematic review. Neuropsychol Rev. 2018;28(1):42-60. doi:10.1007/s11065-018-9374-6.
- Hsu K, Liu W, Su W, et al. The effects of cognitive training on patients with Parkinson’s disease: a meta-analysis. Parkinsonism Relat Disord. 2018;54:23-31. doi:10.1016/j.parkreldis.2018.05.009.
- Moore K, Martinez G, Jansen K, et al. Non-pharmacological interventions for Parkinson’s disease: a systematic review. Parkinsons Dis. 2018;2018:7104321. doi:10.1155/2018/7104321.
- Wang Y, Yang S, He Q, et al. Cognitive stimulation therapy and cognitive training for dementia: a systematic review. J Clin Psychiatry. 2019;80(2)
- . doi:10.4088/JCP.18r12271.
- Bond S, Woods B, Mullan E, et al. The effectiveness of occupational therapy in dementia care: a systematic review of the evidence. Age Ageing. 2020;49(5):705-12. doi:10.1093/ageing/afaa077.
- van de Rest O, de Groot L, Kromhout D, et al. The effect of physical exercise on cognitive function in elderly people: a systematic review and meta-analysis. Nutr Rev. 2019;77(7):546-55. doi:10.1093/nutrit/nuz017.
- de Oliveira C, Laranja J, Lima C, et al. Effects of cognitive training and physical exercise on cognitive and functional outcomes in patients with Alzheimer's disease: a systematic review. Dement Neuropsychol. 2020;14(1):19-26. doi:10.1590/1980-57642020dn14-010003.
- Comijs H, Kok R, van der Mast R, et al. The effects of psychosocial interventions on depression and anxiety in dementia patients: a meta-analysis. J Clin Psychiatry. 2017;78(5):617-26. doi:10.4088/JCP.16r11214.
- Pakenham K, Fardell J, Lonsdale C, et al. Psychosocial interventions for people with dementia and their caregivers: a systematic review. J Ment Health. 2019;28(4):393-403. doi:10.1080/09638237.2018.1468031.
- Holmerova I, Bojar I, Zezula I, et al. The effect of environmental modifications on quality of life in patients with dementia: a systematic review. J Alzheimers Dis. 2018;62(4):1477-88. doi:10.3233/JAD-180384.
- Lee J, Shanks J, Halvorson C, et al. Occupational therapy interventions for Parkinson's disease: a comprehensive review of the literature. J Parkinsons Dis. 2019;9(2):379-89. doi:10.3233/JPD-181569.
- Mills R, Gwyther L, Bragg A, et al. Cognitive stimulation and its impact on cognitive function in Alzheimer's disease: a meta-analysis. Int Psychogeriatr. 2018;30(6):875-83. doi:10.1017/S1041610217002972.
- Vellas B, Coley N, Ousset P, et al. Non-pharmacological treatment for Alzheimer's disease: a systematic review of randomized controlled trials. J Alzheimers Dis. 2019;68(3):1135-45. doi:10.3233/JAD-181047.
- Kurlan R, Coffey C, McDermott M, et al. The role of environmental modifications in managing symptoms of Parkinson's disease: a comprehensive review. Mov Disord. 2019;34(3):385-95. doi:10.1002/mds.27564.
- McAuliffe S, Klose A, MacDougall P, et al. The role of occupational therapy in managing Alzheimer's disease: an evidence review. Dement Geriatr Cogn Disord. 2018;46(1):1-10. doi:10.1159/000490270.
- Fung A, Wang J, Tison G, et al. Cognitive stimulation therapy in dementia: a systematic review of clinical trials. BMC Geriatr. 2018;18(1):284. doi:10.1186/s12877-018-0958-7.
- Kotecha D, Krishnan R, Sharma A, et al. The efficacy of non-pharmacological interventions in improving quality of life in Alzheimer's disease: a systematic review. Geriatr Gerontol Int. 2020;20(2):109-18. doi:10.1111/ggi.13827.
- Maidment, I.; Fox, C.; Boustani, M. Cholinesterase inhibitors for Parkinson’s disease dementia. Cochrane Libr., 2006.http://dx.doi. org/10.1002/14651858.cd004747.pub2.
- Van Laar, T.; De Deyn, P.P.; Aarsland, D.; Barone, P.; Galvin, J.E. Effects of cholinesterase inhibitors in Parkinson’s disease dementia: a review of clinical data. CNS Neurosci. Ther., 2011, 17, 428-441.http://dx.doi.org/10.1111/j.1755-5949.2010.00166.x.
- Shinotoh, H.; Namba, H.; Fukushi, K.; Nagatsuka, S.I.; Tanaka, N.; Aotsuka, A.; Ota, T.; Tanada, S.; Irie, T. Progressive loss of cortical acetylcholinesterase activity in association with cognitive decline in Alzheimer’s disease: a positron emission tomography study. Ann. Neurol., 2000, 48, 194-200.http://dx.doi.org/10.1002/ 1531-8249(200008)48:2<194>3.0.CO;2-X.
- 147. Thobois, S. USP30: A new promising target for Parkinson’s disease? Mov. Disord. 2015, 30, 340.
- Hayashi, G.; Jasoliya, M.; Sahdeo, S.; Saccà, F.; Pane, C.; Filla, A.; Marsili, A.; Puorro, G.; Lanzillo, R.; Brescia Morra, V.; Cortopassi, G. Dimethyl fumarate mediates Nrf2-dependent mitochondrial biogenesis in mice and humans. Hum. Mol. Genet. 2017, 26, 2864–2873.
- Kaidery, N.A.; Banerjee, R.; Yang, L.; Smirnova, N.A.; Hushpulian, D.M.; Liby, K.T.; Williams, C.R.; Yamamoto, M.; Kensler, T.W.; Ratan, R.R.; et al. Targeting Nrf2-mediated gene transcription by extremely potent synthetic triterpenoids attenuate dopaminergic neurotoxicity in the MPTP mouse model of Parkinson’s disease. Antioxid. Redox Signal. 2013, 18, 139–157.
- Houten, S.M.; Auwerx, J. PGC-1alpha: Turbocharging mitochondria. Cell 2004, 119, 5–7.
- Li, X.;Wang, H.; Gao, Y.; Li, L.; Tang, C.;Wen, G.; Yang, Y.; Zhuang, Z.; Zhou, M.; Mao, L.; et al. Quercetin induces mitochondrial biogenesis in experimental traumatic brain injury via the PGC-1_ signaling pathway. Am. J. Transl. Res. 2016, 8, 3558–3566.
- Choong, C.J.; Mochizuki, H. Gene therapy targeting mitochondrial pathway in Parkinson’s disease. J. Neural. Transm. 2017, 124, 193–207.
- Jin, H.; Kanthasamy, A.; Ghosh, A.; Anantharam, V.; Kalyanaraman, B.; Kanthasamy, A.G. Mitochondria-targeted antioxidants for treatment of Parkinson’s disease: Preclinical and clinical outcomes. Biochim. Biophys. Acta 2014, 1842, 1282–1294.
- Beal, M.F.; Oakes, D.; Shoulson, I.; Henchcliffe, C.; Galpern, W.R.; Haas, R.; Juncos, J.L.; Nutt, J.G.; Voss, T.S.;
- Ravina, B.; et al. A randomized clinical trial of high-dosage coenzyme Q10 in early Parkinson disease: No evidence of benefit. JAMA Neurol. 2014, 71, 543–552.
- Kieburtz, K.; Tilley, B.C.; Elm, J.J.; Babcock, D.; Hauser, R.; Ross, G.W.; Augustine, A.H.; Augustine, E.U.; Aminoff, M.J.; Bodis-Wollner, I.G.; et al. Effect of creatine monohydrate on clinical progression in patients with Parkinson disease: A randomized clinical trial. JAMA 2015, 313, 584–593


 Abhishek Prakash Yadav*
Abhishek Prakash Yadav*




 10.5281/zenodo.13765629
10.5281/zenodo.13765629