Abstract
Pharmacovigilance, the domain concerned to monitoring and assessing the safety and efficacy of pharmaceuticals, has entered a new era with the onset of the digital age.This revolutionary shift is identified by the integration of digital technologies, data sources, and advanced analytical techniques. This article's goal is to investigate the various ways that the digital era has modified pharmacovigilance and to highlight the possible benefits and challenges that this age has brought.We explore the historical context of pharmacovigilance, tracing its evolution and adaptation to the digital landscape. Key themes discussed include the expanding array of data sources include wearable devices, social media, and electronic health records. We also investigate the integration of artificial intelligence (AI), machine learning (ML), and natural language processing (NLP) for signal detection and risk assessment. Additionally, the regulatory framework associated with digital pharmacovigilance is being examined, highlighting the vital role of global regulatory bodies Patient-oriented PV and the Patient involvement in reporting adverse events are also explored. Case studies and examples from the case studies field illustrate practical applications, highlighting the real-world impact of digital pharmacovigilance. The review concludes by discussing the implications for drug safety, public health, and clinical practice and suggests future directions for research and development in this dynamic field.
Keywords
Pharmacovigilance, Digital age, Signal Detection, Artificial intelligence, Big data analytics, Machine learning
Introduction
The term "pharmacovigilance" has its origins in the Greek name pharmakon, which means medicine or drug, as well as the Latin term vigilia, which means to watch. (1) Pharmacovigilance define as the science and activities relating to the detection, assessment, understanding, and prevention of adverse effects and other drug-related safety problems.(2) Pharmacovigilance involves collecting and analyzing spontaneous reports of adverse drug events from various sources, including manufacturers, healthcare professionals, and patients. To improve future pharmacovigilance, electronic medical records (EMR) should be saved on the cloud allowing for big data analysis. The rise of online social networking has also facilitated communication and information sharing worldwide. Social media helps consumers and patients share experiences related to drug efficacy and safety, bridging geographical and social limitations. Platforms like blogs, discussion boards, emails, and chats are possible resources for exploring these experiences. (3) Recognizing the “safety” signals that call for escalation and triage, is a critical difficulty in PV, which is how to interpret these massive and heterogeneous data sets .Considering the rapid growth of artificial intelligence (AI) in the last ten years, driven by major advancements in machine learning (ML) across many scientific and medical domains (4) Artificial intelligence helps decision-making in difficult situations. Cognitive services are designed to evaluate PV users ability to make decisions. Thus, a strategy for overcoming AEs with unreported data using AI.(5) Hospitals requires a system that enable them to regularly, quickly, and widely monitor the occurrence of adverse drug events (ADEs) in order to reduce ADEs. In the context of pharmacovigilance, natural language processing (NLP), a computerized method for text data analysis, has demonstrated positive findings for the objective of ADE identification(6) The globalization of healthcare has made international cooperation and pharmacovigilance methods regulation important for ensuring patient safety worldwide. The reporting regulations and methods of many different countries might cause variations in the way data has been collected and analyzed.Since then, the ICH has created standards for pharmacovigilance procedures, including as risk management, signal identification, and adverse event reporting. In addition to the ICH, other global organizations like the European Medicines Agency (EMA) and the World Health Organization (WHO) have been crucial in establishing global collaboration in pharmacovigilance. (7) WHO has created strategies and tools as well as introduced innovations. Many strategies and approaches have been used to enhance and improve pharmacovigilance innovations .WHO has developed user-friendly mobile applications and web platforms. (8) The EudraVigilance system is designed to handle and evaluate data regarding possible side effects of medications that have been approved or are undergoing clinical trials inside the European Economic Area (EEA). The electronically exchanging of individual case safety reports between European medical agency , national authorised administrations , marketing authorisation holders and sponsors of clinical trials in the EEA. (9)(10) The FDA's MedWatch program allows patients, customers as well as health care providers to report medical product safety. (11)Everybody can report injuries and/or deaths caused on by medical products to the FDA through the MedWatch adverse event and reporting system. For the typical user, submitting a report is simple and simply requires an Internet connection. (12) Striking a balance between encouraging innovation and maintaining the safety and effectiveness of drugs is the purpose of the regulatory System in the digital era. (13) Patient-centered pharmacovigilance is an important innovative part of this digital transformation. Over the course of the last ten years, there has been a notable growth in Participation of patients in medical care to the level that all patient-related activities now consistently use the term "patient centricity. (13)(14) Reporting methods must to be inexpensive and easily accessible, according to the World Health Organization (WHO). Patients or users can send their reports over the phone, by fax, email, online form, or on paper by pre-paid postal mail. Paper forms need to be accessible in magazines published by patient organizations, nearby pharmacies, and medical institutions or offices. (15)(16)(17) This review navigates the multifaceted landscape of pharmacovigilance in the digital age, addressing the challenges and opportunities that emerge in the wake of this technological transformation .We hope to offer an in-depth overview of the digital evolution of pharmacovigilance and its significant consequences for the safety and effectiveness of medicines in the twenty-first century by exploring the interaction of data, technology, and patient involvement
Pharmacovigilance in the Digital Age:
- Historical Context:
Around 170 years in the previous century, pharmacovigilance began, though It wasn't known at that point by that name. It's a disciplined initiative in the medical industry with major both social and financial impacts that looks to assess medication risk-benefit ratios and raise the standard of living and safety of patients. (18) The historical eras are also helpful in an understanding of the causes behind the significant advancements in pharmacology and human health that have been made possible by pharmacovigilance, as well as the difficulties that still lie ahead for this field. (18) (1) Pharmacovigilance's history began 169 years ago, on 29 January 1848, when Hannah Greener, a young girl from northern England, passed away following the administration of a chloroform anesthetic.and Continued till 2020-2022 for EMAS PV Risk assessment committee adopted Eudravigilance Plan .(1) A diagrammatic representation of the sequence of events in the history of pharmacovigilance may be seen in Figure No.1
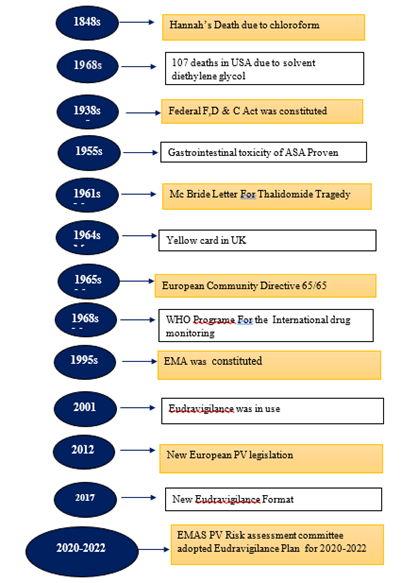
Figure 1:Timeline for the historical evolution of Pharmacovigilance
Impact of the Digital Age:
The primary method used of collecting ADRs was always through paper-based reporting. However, paperless methods are being explored, such as text messaging, calling, websites (e-reporting), and social media, to enhance ADR reporting. (19) Furthermore, it is well known that IT has become a part of clinical safety protocols and has sparked the development of global PV systems for the identification of safety signals.(3)(20) The newly introduced e-systems are built or set up to record the necessary details, including unexpected ADRs (UADRs). (20) The need to immediately gather and maintain such a large amount of data has put current databases under strain, which has accelerated up the development of new data analysis tools. (21) The Food and Drug Administration (FDA), pharmaceutical companies, and healthcare organizations can use big data analytics to help create a more effective and efficient surveillance system. Specifically, they they can analyze trends using computational models On the basis of information taken from already-existing databases. Algorithmic models can be used to auto-code medications, sort adverse event data.(21)(22) Insufficient reporting of adverse incidents to the post-marketing databases is the system's major issue. The medical team may overlook mild symptoms since reporting is not given attention.A large amount of data regarding drug usage in the real world and in real time is available through EHR. Among its drawbacks are the narrative information's unstructured nature and difficulty in analysis. (23) Pharmacovigilance is a single sector that is changing as a result of technological improvements. In the healthcare industry, artificial intelligence (AI) has become an innovative force that presents previously unheard-of chances to completely overhaul medication safety monitoring and surveillance. (24)
Data Source
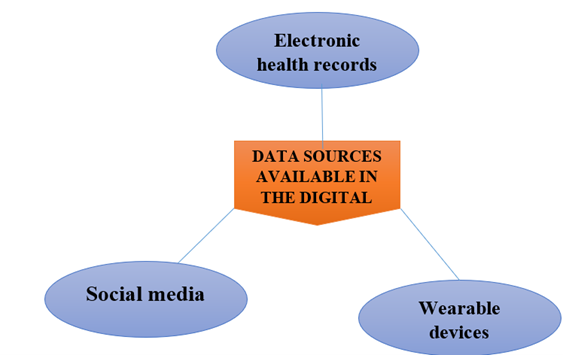
Figure 2 : Data sources available in the digital age
Electronic health records
Data from EHRs are used to provide real-time, on-the-ground monitoring of medication safety. These records contain data acquired from standard clinical care, such as symptoms, prescriptions, health outcomes, test results, physical exam findings, and hospitalizations of patients. (25) As EHR databases hold clinical data from real-world settings in real time, they may offer a more proactive method for pharmacovigilance. (26)(27) EHRs and other healthcare databases are likely the most important sources of RWD. EHR data can also be used to address a variety of safety concerns, specifically long-term safety data, which is frequently not collected during the short duration of Phase- III and Phase IV Clinical trials. (27).EHR databases offer the chance to determine the scope of a medication safety issue since they store records related to a significant amount of patients. A group of patients using the same medication for the same condition can be found and examined using EHR data In order evaluate the effect of a possible link in between a medication and a specific ADE. (25)(26) EHRs are also regarded as a high-quality data source because they are typically generated and maintained by healthcare providers. (25)
Advantages
- The information is accurate since details including dosages for drugs, lab findings, and diagnosis records are preserved.
- The information gathered varies among patients due to differences in genetic background, diet, and ethnicity, providing high-quality data for research and study.
Limitations:
- For the bare minimum amount of patients in a hospital, the sample size is The data might not be adequate for an ADR identification because of the small patient population with short hospitalizations.
- Information can be susceptible to bias because of the disparity that results from mistakes made by people in hospitals.
- Social media:
In comparison with SRS or EHR databases, social media ,which includes social medias, chat rooms, health blogs, and patient community websites offers a more patient-focused form of ADE reporting. (25) Furthermore, every post made on social networking sites like Facebook, Twitter, Snapchat, Instagram, and YouTube collects a significant amount of broad, fast-moving data from millions of users. This data can be evaluated using data mining techniques to find ADEs that were previously unnoticed. (25)(28) When a user shares their experience on a social media for a medication, with other users in the community are likely to discuss similar experiences, which can lead to an increase in reports that could act as "an early warning system" for adverse drug events (ADE). Social media also offers a way to keep an eye on ADEs that don't demand urgent attention or reporting to an SRS. (25) Using social media websites for ADR detection has increased, as has a desire to use social media in pharmacovigilance. These contain all-purpose social networking platforms like Twitter and health and support networks like PatientsLikeMe , DailyStrength and MedHelp. (29)
Advantages:
- Information is diverse and comes from several global demographic groups
- Many patients are discussing their experiences receiving medical treatment. online, which might offer useful information for physicians.
Limitations:
- Sophisticated language processing is necessary to accommodate for grammatical or spelling mistakes in vernacular English
- For example, rather of using the word "fatigue," some choose to refer to their conditions as "flatout."Because people don't always publish every detail online, there is limited information available, which makes evaluating the data associated to ADRs more challenging.
- Wearable devices
Wearable devices , which includes smartphones, smart watches, fitness trackers, and mobile apps, collects and monitors data in real-time, making it possible to identify, classify, and detect adverse events as soon as they occur. (30) Wearable technology and mobile health apps track physiological indicators, physical state, and individual analytics can support the scheduling of medication. (31) Wearable technology can produce dynamic, real-time data that providers can evaluate with software on PCs, tablets, or smartphones. The outcomes happen promptly.
accessible and enable healthcare professionals to quickly make the necessary modifications as opposed to waiting for lab findings. (31)(30)
Advanced Analytical Techniques
Advanced methodologies including artificial intelligence (AI), machine learning techniques, natural language processing (NLP) into pharmacovigilance are as follows. (32)
Artificial intelligence (AI) in pharmacovigilance:
Fortunately AI has been started and used in a number of PV areas, it is not yet developed enough for broad use. (22) Pharmacovigilance-related data can be processed and analyzed by artificial intelligence (AI) algorithms, but they must first be trained with large amounts of high-quality data, which is the main problem that needs to be solved. (33) Pharmacovigilance processes can benefit from the usage of AI and automation in signal identification, monitoring, risk management, AE intake, and report generation. (22) There are two technological obstacles for AI-base PV are - data integration and the data annotation.
Machine learning in PV:
The accessibility of machine learning (ML) is expanding including those who focus their work via analytics into the pharmacovigilance in order to improve a drug's benefit-to-risk ratio. (34) The manual entry, reporting, and analysis of data is a major component of traditional pharmacovigilance methods. Still, this method is challenging, can make mistakes, and frequently missing similarities and signals in huge amounts of data. ML and AI can automate the gathering and assessment of information from numerous sources, such as social media, adverse event reports, and electronic health records. (35)
Natural language processing (NLP) in PV :
NLP for understanding text generated by users for pharmacovigilance. (34) Natural language processing (NLP), a computerized method for textual analysis of data, has demonstrated encouraging outcomes in order to fulfill ADE identification with reference to pharmacovigilance.(36)(25) NLP makes it possible to manage huge amounts of unstructured data like social media posts and hospital records efficiently. These types of data are full of real-world information regarding the effects of drugs.
Transformative Effects in Pharmacovigilance:
The digital era has made a significant impact on pharmacovigilance, changing the profession and bringing with it new difficulties and opportunities. Here, we examine these impacts, highlighting the chances and challenges that have arisen as a result of this change.
Real-time Data Access
The digital age has transformed data accessibility in pharmacovigilance. Electronic health records (EHRs) are an essential a source of information for identification of adverse drug reactions (ADRs). (37) Electronic health records (EHRs) data mining has become an appropriate alternative technique for post-marketing drug safety monitoring. (38) Fortunately adverse reactions are frequently not the result of prescription drugs alone, but rather originate from underlying medical conditions in specific patients. Challenges must be taken into account while mining EHR data to find ADRs.(37) The growing accessibility of electronic health records (EHRs) offers chances for inquiry into a variety of adverse reactions to medications and to identify signs that are closer to the point of event. (38)
Patient-Centered Reporting
Patients' viewpoints have recently been included in pharmacovigilance (PV) activities like ADR reporting and signal detection & assessment, risk communication, benefit-risk assessment, pharmaceutical error assessment, and risk management . (16) According to certain research, patients report ADRs more quickly than healthcare professionals do, and they also provide more information about them. Information on their standard of living Additionally, direct patient reporting enhances patients' participation in their own healthcare management and protects their rights. The World Health Organization (WHO) recommended that reporting methods be simple to use and available. (39)(16) Health care professionals (HCPs), patients, and NRAs from wealthy countries have been drawn to mobile phone applications (apps) created for ADR reporting. An Android smartphone app called "ADR PvPI" has been launched for ADR reporting in order to provide administrative control over data with IPC (NCC-PvPI). This will help both patients and healthcare professionals for ADR reporting including features like XML generation, PDF generation, and support for document and picture attachments. Additionally, the app features different reporting windows for patients and HCPs. (40)(41)
Figure 3:Patient-Centered Reporting
Big data analytics in pharmacovigilance:
Big data is a term applied to describe huge amounts of dynamic, diversified, distributed structured or unstructured data.(25) In PV, big data is utilized to examine vast amounts of electronic data regarding ADEs and reactions.(40) Big data analytics applications were developed growing throughout America, Europe, and most recently Asia .(42)Big data has been collected from administrative data, health and drug monitoring registries, and electronic health records. In a healthcare center, pharmacists and physicians are typically the ones who provide this data. The primary disadvantage of this data, still, is that it needs to be verified for accuracy because there might be some underlying issues, such duplicates.(42)(43)
The following steps are included in the systematic execution of the big data approach to Pv :
- Explain the key format that big data should exist in and describe the appropriate sources of the data.
- Analyze whether areas of data are applicable and usable.
- Using a gap analysis, describe the existing state of ability, anticipated needs, and challenges.
- Create a wish list and big data framework.
- The steps mentioned above will improve the most accurate method of analyzing big data by minimizing manual errors and analyzing time. (43)(25)
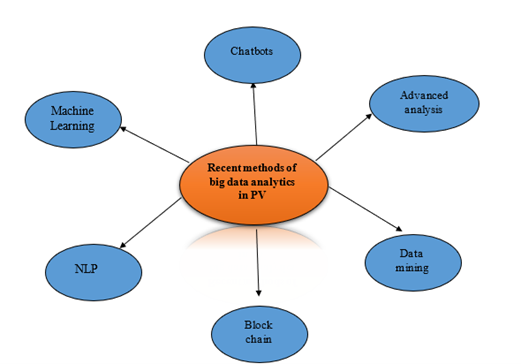
Figure 4: Recent methods of big data analytics in PV
Global collaboration in PV:
Global collaboration in PV can help to enhance the safety of medication in a number of ways. First, it can help to identify and analyse risks more quickly. This is due to data can be collected from a larger number of patients, which can help to identify rare or prolonged adverse effects. (44)Safety concerns must be carefully and clearly addressed because data is collected from various sources (pre-marketing trials, post-approval safety studies, spontaneous reports, etc.) and from different places (FDA-FAERS, Vigibase, Eudravigilance, etc.) in accordance with various standards. In order to minimize bias and enable analysis and interpretation from a global perspective, consistency in the gathering of safety data is essential. (45)By working together, countries can create efficient risk management strategies, exchange knowledge, and combine data. (46)
Advantages of Global Collaboration in PV:
- Enhanced risk identification as well as assessment
- Improved patients safety
- Enhanced risk management.(46)
Challenges to Global Collaboration in PV :
- Data harmonization
- Fostering collaboration and trust
- Limitations on resources. (44)
Challenges in the Digital Age:

Figure 5: Challenges in the Digital Age
Data Privacy and Security:
Data privacy is a crucial concern. whenever data is posted accessible as well as research using this data. It is the users' obligation to ensure that the information linked to their identity is not neglected. (3) A primary issue involves protecting patient privacy and making ensuring data is secure, particularly in the digital age when cyberattacks are frequent. In the digital age, healthcare organizations face immense cybersecurity issues due to the increasing reliance on technology and the storage of large amounts of confidential patient data. (47) Patient medical records, or electronic health records, are becoming more and more digital (EHR). Since electronic data is readily maintained and altered by software, electronic health records (EHRs) are more effective than paper records for improved healthcare and medical research These priceless data are maintained in various health information systems (HIS) in hospitals, research centers and diagnostic laboratories. (48)Privacy issues arise whenever sensitive data, including personally identifiable information, is gathered and kept in any format. Sharing data between medical professionals while safeguarding private information presents significant challenges in the field of health data privacy. Information privacy can be implemented in a variety of ways, such as data masking, authentication, and encryption, all of which aim to guarantee that information is available to authorized individuals only. (48)(47)
Information Overload :
Information overload is applied in many fields such as medicine, social sciences, marketing, computer science, education and psychology.Big data i.e is information Overload the term used to describe large and extensive quantities of computerized medical data that has been collected from administrative data, health and drug monitoring registries, and electronic health records.A different kind of big data employed in Pv is electronic health records. Databases of hospital discharges, results of outpatient diagnostic tests, laboratory test results, and emergency department, disease registries, mortality, and assessment records for children and the elderly . PV is additionally promoted through social media. (42)(48). This material is still in the development stage and makes use of text mining Many patients publish their personal medication therapy experiences on social media networks like Facebook and Twitter, which might be a valuable resource for early signal detection.All of this electronic data that is computerized is analyzed and evaluated for the purpose of detecting different ADRs. (48)(3)
Regulatory Frameworks :
Regulatory Frameworks in PV can help to enhance the safety of drugs in a many ways. Firstly , it can help to identify and assess risks more quickly. because data can be integrating from a larger number of patients, which can help to detect identify rare or delayed adverse events.A major hurdle in the field of pharmacovigilance is the absence of uniformity whenever it comes to the reporting of adverse events. Data collection and analysis may be different between countries due to differences in reporting regulations and practices. This may make it more difficult for regulators to recognize safety signals and pattern and to take the necessary measures. (48) To deal with this issue the ICH has created standards for pharmacovigilance procedures, including as risk management, signal identification, and adverse event reporting.Along with the ICH, other global institutions like the European Medicines Agency (EMA) and the World Health Organization (WHO) have been important in promoting international collaboration in pharmacovigilance. To begin with To promote the responsible use of medications everywhere, the WHO launched the Programme for International Drug Monitoring (PIDM) in 1968. This program gathers and examines data on adverse drug reactions. In contrast, the European Medicines Agency (EMA) is in charge of conducting scientific assessments of drugs approved for use in the EU. To ensure that medications are safe, the EMA has set up a network of cpharmacovigilance centers across the European Union. (48)(49)
Algorithm Interpretation:
Since AI and machine learning offer powerful resources for data analysis, it can be challenging to understand the results and verify decision-making is complex . Data completeness and accuracy are essential for reliable AI and ML findings. trash in, trash out: incorrect or incomplete data will lead to malfunctioning of results. (35)(50) .Regulation governing the use of AI in pharmacovigilance must be followed, and decisions made using AI must be transparent. Using ML and AI in Pharmacovigilance requires large investments in data management, training, and technology. (22)(50) A key challenge to the use of AI is the lack of structured and verified data to train the algorithms that detect possible medicine-related issues. (5) The deficiency of superior datasets inadequate resources for humans, insufficient AI technology, and a lack of government support are technological difficulties to AI-based pharmacovigilance in areas with restricted resources. (33).Experts in lots of areas of AI are also required for AI-based PV., such as engineers that work on creating machine learning and natural language processing this is different challange for countries having low income .In AI-based Pharmacovigilance , data annotation is essential since the training models and algorithms depend on annotated data to predict unannotated data. The expensive cost of annotation and a lack of high-quality data for model training restrict research on AI-based PV.
Bias and Misinformation:
Misinformation is defined as misleading or inaccurate data that unintentionally spreads on social media site. (51) AI algorithms are subject to biases in data and design, some patient groups may be treated unfairly or unequally as a result. It is essential to handle with these partialities and ensure justice in AI-driven pharmacovigilance detection in order to avoid unfairness and unanticipated outcomes in the healthcare industry. (24) Health misinformation on a social media refers to a genuine remark concerning health that is not supported by scientific proof and professional judgment(52) Social media platforms like Facebook with their quick growth and rapid nature, give a broad range of topics related to health. It implies that these websites may use to more quickly detect possible new adverse drug reactions (ADRs). (53)(54) According to a recent survey, between 3 and 4 percent of respondents who use the internet had openly shared their concerns on social media regarding adverse reactions to drugs. Authorities are becoming more and more interested in analyzing this kind of information from social media posts and supportive group websites as a possible new source for information on pharmacovigilance about ADRs. (54) In conclusion, the digital age has brought about significant transformative effects in pharmacovigilance, Facilitating real-time data access, patient engagement, and advanced analytical capabilities. However, it has also introduced challenges related to data privacy, data security, information overload, regulatory frameworks, algorithm interpretation, and the management of bias and misinformation. Addressing these challenges while utilizing the capabilities of the digital age is crucial to ensuring drug safety in the 21st century.
Future Challenges in pharmacovigilance:
The amount and speed at which data is generated by social media sites could present exciting chances for advancements in pharmacovigilance. (28)(29)So, numerous issues need to be fixed before social media can fully achieve its promise.While some of these are technological in nature, Others need to be carefully examined. from ethical and legal views in order to effectively understand and gain the advantages that social media provides.An existing technological obstacle relates to the complexity of algorithms in understanding non-technical information.differences between markings throughout the annotation process, which makes it extremely difficult to truly understand ADRs in social media. (29)(30)A few problems need to be solved is an attempt to enhance pharmacovigilance's incorporation of direct patient reporting.The first is patient reports and their quality. There is no usefulness in patient reports if they are unclear and lack sufficient necessary information that would allow authorities or MAHs to take appropriate action. (14)Making forms more user friendly for patients , and as electronic forms are typically simpler to use, electronic reporting can also be quite helpful..It can use various techniques (such as images) to represent a the side effects experienced by the patient. (13)The use of artificial intelligence (AI) in PV is still quite new and developing. the main challenges in adopting to AI is raises privacy concerns because data may be utilized for other purposes without their consent. (5)There are so many future challenges to global collaboration in PV like need to harmonize data standards and procedures, this is because in order to ensure that data can be shared precisely between different countries.Another challenge is the need to building cooperation and confidence between different stakeholders, including patient entities the pharmaceutical industry, and regulatory bodies.By building cooperation counties together can can collect data, share experience, and develop effective risk management plans. (43)(44)
Future opportunities in pharmacovigilance:
Encourage direct patient reporting:
Direct patient communication in pharmacovigilance is remaining unidentified practice as much as possible of patients, essentially because they are unaware of it or don't think they will complete it correctly In fact, the absence of data / One reason why patients don't report well has been associated to their education.Still, some of these challenges might be resolved with simple solutions. Some of the examples are physicians and hospital personnel should let patients aware that they can report adverse effects on their own.Additionally, patient organizations have a significant role to play in accurately spreading information regarding the value of pharmacovigilance's direct reporting.Teaching patients to report adverse effects directly is a goal that can be achieved by partnership across various stakeholders rather than necessarily being an expensive or challenging task. By encouraging patients and customers to report ADRs by means of electronic reminders sent to them via email or phone calls.Verifying the receipt of reports through correspondence, emails, or phone conversations.Ensuring that reporting methods are widely accepted and conveniently accessible. (13)(16)
Artificial intelligence in pharmacovigilance:
The past years have experienced a significant increase in the availability of healthcare data, and this tendency will likely continue in the future due to increasing marketing of digital tools which collect patient-derived data: Vast volumes of digital data present an opportunity for using artificial intelligence (AI) techniques for improving assessment of drug safety. Information extraction, extracts useful data from easily accessible, mainly unstructured sources using text mining and natural language processing (NLP) tool which has been becoming more important in the pharmacovigilance field. In regard to pharmacovigilance, text mining and natural language processing techniques can be highly helpful in compiling data from a variety of textual sources on adverse drug reactions (ADRs). (32)(33)
Big data analytics:
Big data analytics has been important in finding novel ADEs/ADRs connected to drugs for drug safety monitoring. despite so many obstacles still to overcome, big data clearly has an opportunity to significantly assist PV efforts.Though still in the early phases of development, modified techniques, instruments, and data sources utilized in drug safety surveillance are helpful in advancing the application of big data in PV. The most crucial measure of big data's value will be how effectively it allows for drug safety concerns. (43)
Regulatory Framework:
The regulatory framework for post-marketing drug surveillance of adverse events (AEs) usually depends on self-reporting to surveillance systems through the regulatory authority of the country or region.Below, we discuss through just a few of these systems. (55)
Adverse Event Reporting Databases at the Food and Drug Administration (FDA):
FAERS and VAERS- These are FDA Adverse Event Reporting System (FAERS) and the Vaccine Adverse Event Reporting System (VAERS)Data submitted to the FDA on adverse events (AEs) for pharmaceuticals, such as drugs and therapeutic biological products or vaccines, may be accessed in these databases..(11)(56)Reports were offered by manufacturers and customers., or healthcare professionals and they can be obligatory or optional.Reports from patients as well as healthcare professionals can be made voluntarily through the MedWatch program for medications and non-vaccine biological products, and VAERS for vaccines. FAERS is exclusively sponsored by the FDA, while VAERS is co-sponsored by the Centers for Disease Control and Prevention.
Adverse Event Reporting Databases at the European Medicines Agency (EMA):
EMA Eudravigilance database- The European Drugs Agency (EMA) is the European data processing network and management system for the purpose of reporting and assessing suspected adverse events (AEs) throughout a new drug's development and after its authorization for marketing in the European Economic Area (EEA)
It has an entirely automated message processing and safety system that uses XML-based messaging, along with a big database for pharmacovigilance which includes tracking and query options. (55)(57)
The EudraVigilance system deals with the:
- EudraVigilance Clinical Trial Module (EVCTM): For the reporting of Suspected Unexpected Serious Adverse Reactions (SUSARs)
- EudraVigilance Post-Authorization Module (EVPM): It improves the secure and efficient execution of medications by allowing the electronic interchange of ICSRs between the European Medicines Agency (EMA), national competent authorities, marketing authorization holders, clinical trial sponsors in the European Economic Area (EEA), and early identification and assessment of potential safety signals. (9)(10)
EU Vaccine Adverse Event Surveillance and Communication (VAESCO):
The goal of the European Union's (EU) Vaccine Adverse Event Surveillance and Communication (VAESCO) project is to create a collaborative network comprised of educational institutions, public health organizations, and regulatory agencies throughout Europe that is capable of gathering data on adverse events (AEs) that occur after vaccination. (55)
WHO safety database:
VigiBase - The World Health Organization (WHO) maintains reports offered by participating countries registered in its international drug monitoring program, which are kept in the global an ICSR database called as VigiBase. It is the global largest single repository for medication safety data its sort, with more than 30 million reports of possible drug side effects. (9)(58) The VigiBase system has connectivity with medical and medication databases like WHODrug, MedDRA, WHO ICD, and WHO-ART in addition to its data management and quality assurance advantages.
Safety signal detection and management:
The term ‘signal’ is frequently used with biomedical products during the Phase-4, while It is applicable during premarketing phase in clinical trials. The definition of a signal is given by the Council for International Organizations of Medical Sciences (CIOMS) Working Group VIII is as follows(59) . ‘. . . information that arises from one or multiple sources (including observations and experiments), which suggests a new potentially causal association, or a new aspect of a known association, between an intervention and an event or set of related events, either adverse or beneficial, that is judged to be of sufficient likelihood to justify verificatory action’. Signal management refers to actions taken to determine whether a medicine's reported hazards have shifted and require action to review its safety profile, or because there are newly discovered risks connected to the drug.The signal management procedure consists of the following steps are as follows in Fig No .6.(60)(59)
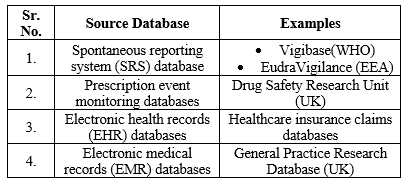
Table 1:Data Sources and Statistical Data Mining Methods used in Safety Signal Detection
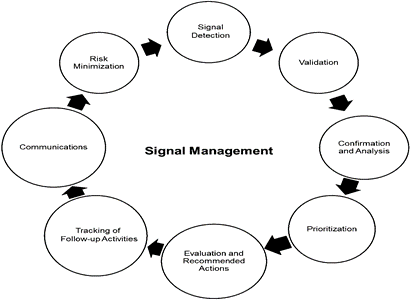
Figure 6:Signal management (61)
Automation in signal management in pharmacovigilance:
In addition to signal management being only available in the phase-4, the capacity of earlier databases remain still being developed. But current regulations resulted in the procedure of signal management as a necessary step for any drug's a preapproval validation. (61)In the post-marketing phase, signal management becomes increasingly necessary due to the vulnerable populations' diverse medical histories, including children, the elderly, and pregnant women in addition administer conmed medications that need to be well monitored. It could have an impact on the efficacy and safety standards, including the benefit-risk ratio of any medicinal product.The 2008 heparin disaster raised awareness of the importance of signal control and monitoring across numerous regulatory bodies of medications during every phase of a medical product's development. (62)
Need of automation in signal management:
- The development of medical research has made it easier for adverse events to occur, which could affect public safety and health. Because of this, there is now a demand for greater confidence
- Pharmacovigilance reporting system in order to safeguard public health and develop the pharmacovigilance sector. (61)
- Sometimes, reported data are very hazy, and even information related to clinical trials may not always be available in public
- An automated database specifically intended for pharmacovigilance needs to be created in compliance with the global ICH E2B (R3) guideline. ICH E2B (R3) recommendation includes different areas that every pharmacovigilance database has to have. (61)(62)
Safety Communication and Risk Management:
In pharmacovigilance, a branch of risk management is in responsible for signal detection and drug risk-benefit profile monitoring. (2)In order to reduce drug risks, the goal of RMP is for drug threats to be monitored on a regular basis or in reaction to developments in pharmacovigilance and post-marketing surveillance. (63)
Pharmacovigilance Plan:
- The contents of the Pharmacovigilance Plan should be Considering the ICH E2E Guideline as a reference, the following.
- MAHs should regularly evaluate the drug's safety specification in compliance with the ICH E2E Guideline. The Safety Specification should be reviewed immediately if any new safety concerns result from post-marketing pharmacovigilance efforts, etc.(64)(63)
- The following criteria should be used to evaluate the Pharmacovigilance Plan's content, with the ICH E2E Guideline serving as a guide.
Routine Pharmacovigilance Practices:
MAHs ought to offer a brief description of their system of implementation and regular pharmacovigilance practices.(64)
Additional Pharmacovigilance Activities:
Evaluating the Safety Specification, the need for, reasons for, approaches used, etc., new pharmacovigilance activities should be reviewed and reported using the implementation system. (64)(63)
Risk Minimization Plan
The term "risk minimization plan" refers to the aggregate individual's risk-minimization activities implemented to reduce the drug's risk and retain the proper benefit-risk balance, information that was gathered when the safety clearance was given and other data gathered by post-marketing PV initiatives, as well as analysis of the data.
There are two groups into which the risk minimization actions fall: i.e., routine activities and extra activities carried out , if necessary. (64) Routine Risk Minimization Practices:
Routine risk minimization practices consist of Creating packages inserting with information about authorized items, such as "Dosage and Administration," "Indications," and "Precautions
Additional Risk Minimization Activities:
Additional risk minimisation measures comprise, for e.g , educating medical personnel, patients receiving medication treatment, and creating requirements for using the medication, which are explained here, in addition to the typical distribution of package insert information. The MAHs must develop an extra risk minimizing plan and take into account whether these risk minimization measures, or by combinations of them, are required based on the features, etc., of specific pharmaceuticals. (63)(60)
PV for centrally authorised products reporting of adverse reactions and other information about safety
Reporting of adverse reactions and other safety-related information :
Post authorisation phase :
Adverse Drug Reactions ADRs/ Individual Case safety Reports (ICSRs)
Electronic reporting:
- Mandatory e-reporting of the ICSRs
- Define exceptional conditions (mechanical, programmatic, electronic, or communication breakdown) that prevent electronic reporting.
- Fallback protocols are in place to ensure expedited reporting compliance.
- Periodic Safety Update Reports (PSURs) from MAH to the Competent Authorities
- Legal basis: Volume 9A of EudraLex- Pharmacovigilance. (64)
Monitoring of the safety profile:
Signal Identification :
- Signal detection involves investigating AEs data patterns that lead to a novel, possible causal relationship between an event and a drug or a set of related events. (64)(60)
- A signal of potentially unanticipated harms or variations in severity, Characteristics or occurrences of anticipated adverse effects can be determined by:
- The Marketing Authorisation Holder (MAH)
- The Rapporteur
- The Member States
- The Agency in agreement with the Rapporteur.
Signal Evaluation:
Since signals of possible unexpected adverse reactions or differ in the severity, features, or frequency of expected adverse reactions may appear from a variety of data sources (see above), it is necessary to compile the pertinent data for an efficient evaluation on a timeline commensurate with the significance and the signal's expected impact. No matter who detected the signal, the Member State where the signal's origination or the Reporter should conduct a signal review. (60)
Evaluation of Periodic Safety Update Reports:
The Agency and each Member State must receive Periodic Safety Update Reports (PSURs) from the Marketing Authorization Holder (MAH). It is the duty of the Agency to check that the Marketing Authorisation Holder fulfils the timeframes.
Case studies:
The study was carried out using the WHO pharmacovigilance database, VigiBase®, It keeps track of all Individual Case Safety Reports (ICSRs) from greater than 130 nations worldwide.CSRs undergoes thorough quality checks and deduplication before being added to VigiBase®. Every ICSR registered from January 1, 2000, and December 31, 2019 have been retrieved and classified as "fatal outcomes" in the System Organ Class High Level Group Term (HLGT). They did not include ICSRs from patients whose sex was unknown, nor from patients who were younger than 18 or older than 64, as our first review of VigiBase® did not reveal any fatal outcome in patients under the age of 18 and older than 64 with the medication combination (hydroxychloroquine + metformin) They conducted a analysis of disproportionality (case/non-case analysis) adhering to the latest protocols to identify a Pharmacovigilance signal in huge databases for pharmacovigilance.Cases were defined as the incident of "fatal outcomes" with hydroxychloroquine (or chloroquine) (P01BA according to ATC drug classification), as well as non-cases were all other cases Isochloroquine (or chloroquine)-only ICSRs. The interaction within hydroxychloroquine (or chloroquine) + metformin (A10BA) and There was a comparison of results using hydroxychloroquine (or chloroquine) alone.

Table 2:Total No of fatal non-suicidal reports, along with the ratios associated along metformin and hydroxychloroquine, that were enrolled in the WHO PV database VigiBase® between January 1, 2000, and December 31, 2019
RESULT:
Out Of the 10,771 Individual Case Safety Reports (ICSR) including hydroxychloroquine, 52 were recorded as ‘fatal outcomes In comparison along with hydroxychloroquine alone, hydroxychloroquine+metformin.This study identifed a signal for the combination of hydroxychloroquine+metformin that appears to be more at risk to result in death (particularly by completed suicides) than one of the two drugs when given alone. (65)
FUTURE DIRECTIONS:
A few challenges must be overcome in order to boost the use of direct patient reporting in pharmacovigilance. (13) The first involves linked to patient reports and their level of accuracy. There is no value in patient reports if they are unclear and lack sufficient information that is relevant for authorities or MAHs to take appropriate action. (14) Enhancing the ease of use of forms is clearly a practical are; and as electronic forms are typically simpler to utilize, electronic reporting can also be very useful. (13)(16) The key to AI technology is data. As a result, establishing an extensive PV database is important. When establishing its PV database, every country is in a different scenario. There are two technical barriers for AI-based PV in resource-limited settings are data integration and data annotation. (32) Furthermore, a further challenge is the lack of funding. This covers maintaining current systems, enhancing the information infrastructure, employing HCPs and AI engineers, and providing education. (33) The government plays an essential role for the decision-making procedure in AI-based PV. Since most LMICs' PV systems remain in early stages and aren't supported by strong legal or regulatory frameworks, more stringent rules are required to involve local companies and HCPs The future of pharmacovigilance is expected to involve social media monitoring in routine practice. For this purpose, a thorough assessment of social media's use as a tool for pharmacovigilance is required, along with to new infrastructure, software tools, and data processing methods adapted to the quantity, velocity, structure, and veracity of social media data. (28) As this topic continues to be investigated, web and social listening powered with artificial intelligence (AI) appears likes an option that might boost accuracy levels,improve reliability of human-directed tracking reduce the need for manual data labelling which makes coordinated and effective systems for creating useful information on the efficacy and safety of drugs possible. (28)(34) Big data may identify patient information connections between multiple data sets. The following are some possibilities that will expand beyond the present limitations of big data analytics in PV: Enhancement of data source inconsistency, verification of data source use, creation of signal detection criteria, and use of an integrative approach to signal detection, enhancing data mining instruments and software, and applying data mining to further product safety and legal concerns . (43)
CONCLUSION:
As a result, Pharmacovigilance involves collecting and analyzing spontaneous reports of adverse drug events from various sources, including manufacturers, healthcare professionals, and patients. To improve future pharmacovigilance, electronic medical records (EMR) should be recorded on the cloud, allowing for big data analysis.Considering the rapid growth of artificial intelligence (AI) in the last ten years, driven by major advancements in machine learning (ML) across many scientific and medical domains . The globalization of healthcare has made international cooperation and pharmacovigilance methods regulation important for ensuring patient safety worldwide. Patient-centered pharmacovigilance is an important innovative part of this digital transformation. The primary method used for collecting ADRs was always through paper-based reporting. However, paperless methods are being explored, such as text messaging, calling, websites (e-reporting), and social media, to enhance ADR reporting. Data from EHRs are used to provide real-time, on-the-ground monitoring of medication safety. social media ,which includes social networks, chat rooms, health blogs, and patient community websites offers a more patient-centered form of ADR reporting. The application of big data analytics has been growing throughout America, Europe, and most recently Asia . Big data has been collected from administrative data, health and drug monitoring registries, and electronic health records.There are some Challenges in the Digital Age like Data Privacy and Security, Information Overload, Regulatory Frameworks, Algorithm Interpretation, Bias and Misinformation. Future opportunities for PV in direct patient reporting, in Artificial intelligence, in Big data analytics. The regulatory framework for Post-marketing drug surveillance of AEs has usually depended on spontaneous reporting to the surveillance systems that are through country’s or a region’s regulatory agency. Signal management refers to actions taken to determine whether a medicine's reported hazards have shifted and require action to review its safety profile, or because there are newly discovered risks connected to the drug. The signal management process includes following steps are as follows : signal detection, validation, confirmation, analysis, prioritization, evaluation, and recommended actions, tracking of follow-up activities, communication, and risk minimization.In pharmacovigilance, a branch of risk management is in responsible for signal detection and drug risk-benefit profile monitoring. The term "risk minimization plan" refers to the aggregate individual risk-minimization activities implemented to minimize the drug's risk and retain the proper benefit-risk balance. A few challenges must be overcome in order to boost the use of direct patient reporting in pharmacovigilance.
REFERENCES:
- Kumar V, Kumar Bharti S, Shukla D, Suryavanshi A, Kant Jangde K, Kesavan K, et al. Pharmacovigilance in Digital Era: Real-World Data and Safety Monitoring. Chhattisgarh J Sci Technol [Internet]. 2022;19(2):2022. Available from: https://new.ggu.ac.in
- Pharmacovigilance ROF. World Journal of Pharmaceutical Research. 2022;11(2):1223–41.
- Singh J, Kumar R, Sharma P, Singha M. Big Data Analytics and Pharmacovigilance—An Ethical and Legal Consideration. AMEI’s Curr Trends Diagnosis Treat. 2018;2(1):58–65.
- Kompa B, Hakim JB, Palepu A, Kompa KG, Smith M, Bain PA, et al. Artificial Intelligence Based on Machine Learning in Pharmacovigilance: A Scoping Review. Drug Saf [Internet]. 2022;45(5):477–91. Available from: https://doi.org/10.1007/s40264-022-01176-1
- Pranali Wani , Arti Shelke , Mandira Marwadi , Vrushali Somase , Priyanka Borade , Kajal Pansare GS. Role of Artificial Intelligence in Pharmacovigilance: a Concise Review. J Pharm Negat Results. 2022;13(7):6149–56.
- Murphy RM, Klopotowska JE, de Keizer NF, Jager KJ, Leopold JH, Dongelmans DA, et al. Adverse drug event detection using natural language processing: A scoping review of supervised learning methods. PLoS One [Internet]. 2023;18(1 January):1–26. Available from: http://dx.doi.org/10.1371/journal.pone.0279842
- Sigler T. International Collaboration , Challenges and Globalization of Pharmacovigilance. 2023;11(1000423):10–1.
- Pal S, Fukushima A. Pharmacovigilance in WHO: new challenges and opportunities. 2023;(May).
- Banovac M, Candore G, Slattery J, Houÿez F, Haerry D, Genov G, et al. Patient Reporting in the EU: Analysis of EudraVigilance Data. Drug Saf. 2017;40(7):629–45.
- Santoro A, Genov G, Spooner A, Raine J, Arlett P. Promoting and Protecting Public Health: How the European Union Pharmacovigilance System Works. Drug Saf. 2017;40(10):855–69.
- Aschenbrenner DS. A New Treatment for Chronic Hepatitis C. Am J Nurs. 2016;116(5):23.
- Fyfe T. Turning Research Into Practice (TRIP). J Med Libr Assoc. 2007;95(2):215–6.
- Soiza RL, Donaldson AIC, Myint PK. Vaccine against arteriosclerosis: an update. Ther Adv Vaccines. 2018;9(6):259–61.
- Sienkiewicz K, Burzy?ska M, Rydlewska-Liszkowska I, Sienkiewicz J, Gaszy?ska E. The importance of direct patient reporting of adverse drug reactions in the safety monitoring process. Int J Environ Res Public Health. 2022;19(1):1–16.
- Anderson C, Krska J, Murphy E, Avery A. The importance of direct patient reporting of suspected adverse drug reactions: A patient perspective. Br J Clin Pharmacol. 2011;72(5):806–22.
- Saleh A, Fourrier-réglat A, Diogène E. Patient-centered pharmacovigilance?: A review. 2018;17(January):179–88.
- Smith MY, Benattia I. The Patient’s Voice in Pharmacovigilance: Pragmatic Approaches to Building a Patient-Centric Drug Safety Organization. Drug Saf. 2016;39(9):779–85.
- Fornasier G, Francescon S, Leone R, Baldo P. An historical overview over Pharmacovigilance. Int J Clin Pharm [Internet]. 2018;(0123456789):1–4. Available from: https://doi.org/10.1007/s11096-018-0657-1
- Fukushima A, Iessa N, Balakrishnan MR, Pal SN. Smartphone-based mobile applications for adverse drug reactions reporting: global status and country experience. BMC Med Inform Decis Mak [Internet]. 2022;22(1):1–20. Available from: https://doi.org/10.1186/s12911-022-01832-7
- Lu Z. Information technology in pharmacovigilance: Benefits, challenges, and future directions from industry perspectives. Drug Healthc Patient Saf. 2009;1(1):35–45.
- Han M. The Application of Big Data in Pharmacovigilance: A Systematic Review. Proc 2021 Int Conf Public Art Hum Dev ( ICPAHD 2021). 2022;638(Icpahd 2021):533–7.
- Uddin MA, Nagar S, Nandurbar D. ARTIFICIAL INTELLIGENCE-BASED PHARMACOVIGILANCE- A REVIEW. 2023;11(3):131–8.
- Berlin JA, Glasser SC, Ellenberg SS. Adverse event detection in drug development: Recommendations and obligations beyond phase 3. Am J Public Health. 2008;98(8):1366–71.
- Praveen J, Kumar CM K, Channappa AH. Transforming Pharmacovigilance Using Gen AI: Innovations in Aggregate Reporting, Signal Detection, and Safety Surveillance. J Multidiscip Res. 2023;(September):9–16.
- Lee Ventola C. Big data and pharmacovigilance: Data mining for adverse drug events and interactions. P T. 2018;43(6):340–51.
- Eichler HG, Bloechl-Daum B, Broich K, Kyrle PA, Oderkirk J, Rasi G, et al. Data Rich, Information Poor: Can We Use Electronic Health Records to Create a Learning Healthcare System for Pharmaceuticals? Clin Pharmacol Ther. 2019;105(4):912–22.
- Dang A. Real-World Evidence: A Primer. Pharmaceut Med [Internet]. 2023;37(1):25–36. Available from: https://doi.org/10.1007/s40290-022-00456-6
- Pappa D, Stergioulas LK. Harnessing social media data for pharmacovigilance: a review of current state of the art, challenges and future directions. Int J Data Sci Anal [Internet]. 2019;8(2):113–35. Available from: https://doi.org/10.1007/s41060-019-00175-3
- Sloane R, Osanlou O, Lewis D, Bollegala D, Maskell S, Pirmohamed M. Social media and pharmacovigilance: A review of the opportunities and challenges. Br J Clin Pharmacol. 2015;80(4):910–20.
- Cabela R, Whitebrook J. Harnessing safety data from wearable devices Pharmacovigilance professionals propose how to capture , evaluate , and report information across a medicine ’ s lifecycle. 2018;(May).
- Edrees H, Song W, Syrowatka A, Simona A, Amato MG, Bates DW. Intelligent Telehealth in Pharmacovigilance: A Future Perspective. Drug Saf [Internet]. 2022;45(5):449–58. Available from: https://doi.org/10.1007/s40264-022-01172-5
- Lee CY, Chen Y ping P. Machine learning on adverse drug reactions for pharmacovigilance. Drug Discov Today [Internet]. 2019;24(7):1332–43. Available from: https://doi.org/10.1016/j.drudis.2019.03.003
- Liang L, Hu J, Sun G, Hong N, Wu G, He Y, et al. Artificial Intelligence-Based Pharmacovigilance in the Setting of Limited Resources. Drug Saf. 2022;45(5):511–9.
- Pilipiec P, Liwicki M, Bota A. Using Machine Learning for Pharmacovigilance: A Systematic Review. Pharmaceutics. 2022;14(2):1–25.
- Torous S. Pharmaceutical Regulatory Affairs?: Open Access The Role of Artificial Intelligence and Machine Learning in Pharmacovigilance. 2023;12.
- Chapman AB, Peterson KS, Alba PR, DuVall SL, Patterson O V. Detecting Adverse Drug Events with Rapidly Trained Classification Models. Drug Saf [Internet]. 2019;42(1):147–56. Available from: https://doi.org/10.1007/s40264-018-0763-y
- Ng IYH, Shen X, Sim H, Sarri RC, Stoffregen E, Shook JJ. ?????NIH Public Access. J Neurochem. 2015;4(1):1–15.
- Maguire A, Douglas I, Smeeth L, Thompson M. Determinants of cholesterol and triglycerides recording in patients treated with lipid lowering therapy in UK primary care. Pharmacoepidemiol Drug Saf. 2007;16(September):228–228.
- Paola K, Claudio G. The value of direct patient reporting in pharmacovigilance. Ther Adv Drug Saf. 2020;11:1–7.
- Labh R, Gupta S, Gupta R. Advances in pharmacovigilance in India: Role of mobile application. Indian J Med Spec. 2020;11(3):124.
- Pierce CE, de Vries ST, Bodin-Parssinen S, Härmark L, Tregunno P, Lewis DJ, et al. Recommendations on the Use of Mobile Applications for the Collection and Communication of Pharmaceutical Product Safety Information: Lessons from IMI WEB-RADR. Drug Saf [Internet]. 2019;42(4):477–89. Available from: https://doi.org/10.1007/s40264-019-00813-6
- Maritsch F, Cil I, McKinnon C, Potash J, Baumgartner N, Philippon V, et al. Data privacy protection in scientific publications: process implementation at a pharmaceutical company. BMC Med Ethics [Internet]. 2022;23(1):1–10. Available from: https://doi.org/10.1186/s12910-022-00804-w
- RANI AITHA S, MARPAKA S, T C, E B, RANI KASUKURTHI S. Big Data Analytics in Pharmacovigilance - a Global Trend. Asian J Pharm Clin Res. 2021;(May):19–24.
- Salimi M. Global Collaboration in Pharmacovigilance?: A New Paradigm for Safety. 2023;11(1000430):1000430.
- De Ponti F. Global Perspectives in Pharmacovigilance. J Pharmacovigil. 2013;01(03):3–4.
- Valverde JL. The globalization of medicines as a challenge for governments. Pharm Policy Law. 2016;18(1–4):19–29.
- Filkins BL, Kim JY, Roberts B, Armstrong W, Miller MA, Hultner ML, et al. Privacy and security in the era of digital health: What should translational researchers know and do about it? Am J Transl Res. 2016;8(3):1560–80.
- Shahidul Islam Khan, Abu Sayed Md, Latiful Hoque. Digital Health Data: A Comprehensive Review of Privacy and Security Risks and Some Recommendations. Comput Sci J Mold. 2016;24(2(71)):273–92.
- Hussain R. Big data, medicines safety and pharmacovigilance. J Pharm Policy Pract [Internet]. 2021;14(1):1–3. Available from: https://doi.org/10.1186/s40545-021-00329-4
- Salas M, Petracek J, Yalamanchili P, Aimer O, Kasthuril D, Dhingra S, et al. The Use of Artificial Intelligence in Pharmacovigilance: A Systematic Review of the Literature. Pharmaceut Med [Internet]. 2022;36(5):295–306. Available from: https://doi.org/10.1007/s40290-022-00441-z
- Muhammed T S, Mathew SK. The disaster of misinformation: a review of research in social media. Int J Data Sci Anal [Internet]. 2022;13(4):271–85. Available from: https://doi.org/10.1007/s41060-022-00311-6
- Li YJ, Cheung CMK, Shen XL, Lee MKO. Health misinformation on social media: A literature review. Proc 23rd Pacific Asia Conf Inf Syst Secur ICT Platf 4th Ind Revolution, PACIS 2019. 2019;
- Westergren T, Narum S, Klemp M. Biases in reporting of adverse effects in clinical trials, and potential impact on safety assessments in systematic reviews and therapy guidelines. Basic Clin Pharmacol Toxicol. 2022;131(6):465–73.
- Li Y, Jimeno Yepes A, Xiao C. Combining Social Media and FDA Adverse Event Reporting System to Detect Adverse Drug Reactions. Drug Saf [Internet]. 2020;43(9):893–903. Available from: https://doi.org/10.1007/s40264-020-00943-2
- A., Shafiq CWNSSJRB, Quispe-Tintaya W. ?????? HHS Public Access. Physiol Behav. 2017;176(3):139–48.
- Ahmad SR. Adverse drug event monitoring at the food and drug administration. J Gen Intern Med. 2003;18(1):57–60.
- Postigo R, Brosch S, Slattery J, van Haren A, Dogné JM, Kurz X, et al. EudraVigilance Medicines Safety Database: Publicly Accessible Data for Research and Public Health Protection. Drug Saf. 2018;41(7):665–75.
- Lindquist M. VigiBase, the WHO Global ICSR Database System: Basic facts. Drug Inf J. 2008;42(5):409–19.
- Patel T. Pharmacovigilance and methods of Signal Detection. Indian J Pharm Pract. 2013;6(3):68–72.
- Malikova MA. Practical applications of regulatory requirements for signal detection and communications in pharmacovigilance. Ther Adv Drug Saf. 2020;11:1–15.
- Wadhwa D, Kumar K, Batra S, Sharma S. Automation in signal management in pharmacovigilance - An insight. Brief Bioinform. 2021;22(4):1–9.
- Arnaud M, Bégaud B, Thiessard F, Jarrion Q, Bezin J, Pariente A, et al. An Automated System Combining Safety Signal Detection and Prioritization from Healthcare Databases: A Pilot Study. Drug Saf. 2018;41(4):377–87.
- Pharmaceuticals and Medical Devices Agency. Risk Management Plan Guidance. Pmda. 2012;(0916001):1–17.
- Calvo B, Zuig L. Risk Management Plan and Pharmacovigilance System. Biopharmaceuticals: Biosimilars. Risk Manag Trends. 2011;
Montastruc JL, Toutain PL. A New Drug–Drug Interaction Between Hydroxychloroquine and Metformin? A Signal Detection Study. Drug Saf [Internet]. 2020;43(7):657–60.


 Shruti Aher*
Shruti Aher*
 Manoj Mahajan
Manoj Mahajan








 10.5281/zenodo.10980625
10.5281/zenodo.10980625