Abstract
Allium sativum, Syzygium aromaticum and Garcinia kola are three substances highly prized for their antitussive properties. Recipe formulations involving the mixture of these substances are often reported but remain undocumented. This study was initiated with the aim of identifying any possible agonist effect that could exist between the three substances on strains of microbes responsible for some respiratory infections. During the conduct of the work, seven combinations were made in various proportions and then extracted with ethanol. They were all tested on six strains of microbes. The most active fraction was then the subject of phytochemical studies. It appears that the most active combination is the one containing 16.66% of A. sativum, 66.66% of S. aromaticum and 16.66% of G. kola (A1/S4/G1). It is the only one to have inhibited more germs (66.67%). It indicated the largest inhibition diameter of a value of 20 mm on clinical S. aureus. This fraction also presents the lowest Minimum inhibitory concentrations between 2.5 and 10 mg/mL and the lowest Minimum Bactericidal Concentration between 10 and 50 mg/ml. Phytochemical study of this fraction revealed the presence of reducing compounds (5.40%), alkaloids (2.14%), flavonoids (11.36%), phenolic compounds consisting of catechic and gallic tannins (9.70%), saponins (3.52%), terpenoids (0.98%), leucoanthocyanins, steroids, coumarins, mucilages, O-heterosides and free anthracenics. The intersection of the different results obtained indicates the agonist effect that exists between the three substances used and offers good prospects in the development of natural antibiotics against respiratory infections.
Keywords
Substance mixtures, respiratory infections, Agonist effect, Antimicrobial, phytochemical studies.
Introduction
Acute and chronic respiratory infections are among others the main cause of morbidity and mortality. Each year, nearly 4 million people die from acute respiratory infections (1). The case of underdeveloped countries is even more alarming because 90% of these deaths occur in them. These rates are particularly higher among infants, children and the elderly (2-4). These respiratory tract infections are mainly caused by bacteria. The management of these infections is hampered by several reasons including the lack of resources in underdeveloped countries. Faced with this lack of resources, the population turns to plants. According to estimates by the World Health Organization (WHO), approximately 80% of populations in underdeveloping countries are forced to resort to traditional medicine and in particular to herbal medicine for their health care needs (5). The global market for herbal medicines is also expanding continuously throughout the world, with an estimated annual global value of approximately 800 million US dollars (5). The field is also booming in Africa, which is also full of very rich, abundant and varied flora. But the challenge remains to clean it up in order to guarantee healthy treatment for populations.
In this context, the development of herbal medicines of reasonable cost, accessible, easy to ingest according to a well-defined dosage with a studied toxicity would be great to use. Among other natural substances with well-known antibacterial and antiviral properties, garlic (Allium sativum), clove (Syzigium aromaticum) and better kola (Garcinia kola) have been targeted. The antibacterial and antiviral properties of these different substances have been widely described in the literature (6-11). Some studies also report the strengthening of the immune system by these different substances (12,13). Our previous work carried out on these substances has confirmed these results. Their activities against colds and coughs have also been described in the literature (13-15). Traditionally, the antitussive and decongestant properties of these substances are known by the populations and they sometimes resort to their mixture. The objective of this study is to identify the possible agonist effects that may exist between these three substances. This work therefore boils down to the determination of a possible antimicrobial agonist effect that may exist between the extracts of Allium sativum, Syzigium aromaticum and Garcinia kola. The most active fraction will be the subject of other studies, particularly phytochemical studies.
The availability, accessibility, and numerous biological activities of these substances remain motivating assets in conducting this study.
MATERIAL AND METHODS:
Extract Preparation:
In some studies, the ethanolic fraction had been shown to be more active. In addition ethanol is a very little toxic solvent and according to some authors allows to extract the maximum of chemical compounds (16). That is what motivated us to take an interest in the ethanolic extract of the seven fractions. The plant material used in this study was purchased at the market in the study area. Allium sativum and Garcinia kola were peeled. Both substances were carefully washed, cut and left to dry in an oven at 40 °C for 5 days. Syzygium aromaticum was also cleaned and dried. The dried substances were carefully ground using an electric grinder. The ground material is sieved and then packaged in glass jars away from moisture for further work.
Preparation of the combinations
Various respective combinations of Allium sativum, Syzygium aromaticum and Garcinia kola were made. The total mass of each combination is equal to 60 g. They were made in the proportions A6/S0/G0 (60; 00; 00); A0/S6/G0 (00; 60; 00); A0/S0/G6 (00; 00; 60); A1/S4/G1 (10; 40; 10); A2/S2/G2 (20; 20; 20); A4/S1/G1 (40; 10; 10) and A1/S1/G4 (10; 10; 40). 60 g of powder of each combination were crushed and recovered in 600 ml of ethanol 96°C. After agitation and homogenization, the mixture is filtered on Wathman paper and the filter is concentrated in a rotary evaporator at a temperature between 55°C and 60°C with help of vacuum pump to obtain the extract. Drying was finalized in an oven at 35°C for 72 hours. The dry extract obtained was stored in a refrigerator at 4°C.
Antimicrobial activity assessment methods
Some Bacteria responsible for respiratory infections were identified to serve as animal material during this study. These are: Klebsiella pneumoniae, Staphylococcus aureus clinique, Acinetobacter baumannii, Staphylococcus aureus de référence, Escherichia coli, Pseudomonas aeruginosa.
Sensitivity test
It was done according to the disc method inspired from the one described by (17). Brieflt, 1 ml of pre-culture of 18-24 h (106 UFC/ml) enabled planting a box of Petri dishes containing agar Mueller Hinton by flood. After seeding, the sterile Whatman paper discs (5 mm de diameter) were deposited with sterile pince. These discs have been carefully impregnated with 30 ?l of plant extract (20 mg/ml. The dishes were kept for 15-30 min at room temperature before incubation at 37°C. The inhibition zones diameters were measured after 24 to 48 hours using a ruler graduated (18). For each extract, the experiment was performed induplicate.
Determination of the Minimum Inhibitory Concentration (MIC) The MIC has been determined by macrodilution method with Visual assessment of the growth of microorganisms (19). Briefly, nine concentrations (10 000, 5 000, 2 500, 1 250, 625, 312.5, 156.25, 78.12 and 39.06 ?g/ml) was performed in screw tube. To 1 ml of the above concentrations was added 1 ml of the bacteria inoculum (106 UFC/ml). After 24 h of incubation turbidity tubes was examined relative to the control tube containing distilled water and the inoculum (106 UFC/ml).
Determination of the Minimum Bactericidal Concentration (MBC)
The MBC was determined by solid medium culture of all of the tubes from the MIC to high concentrations. These dishes were incubated at 37 ° C for 24 h. The highest dilution that yielded no bacterial growth on solid medium was taken as MBC (20).
Preliminary phytochemical screening of A1/S4/G1 Faction
Qualitative phytochemical screening
The qualitative phytochemical screening was performed based on colouring or precipitation reactions. It is made directly on the ethanolic extract of different fractions according to Houghton and Raman method (1998) (21). Quantitative phytochemical tests were carried out according to the method of Harbon (1984) and Umeaku (2018) (22-23).
Quantitative phytochemical screening of A1/S4/G1
Estimation of total flavonoid content
Flavonoid content of extract was determined by colorimetric method described by Chang (2002) (24). The plant extract was separately mixed with 1.5 ml of methanol, 0.1 ml of 10% aluminum chloride, 0.1 ml of 1 M potassium acetate, and 2.8ml of distilled water, and left at room temperature for 30 min. The absorbance of the reaction mixture was measured at 415 nm using spectrophotometer. The samples were prepared in triplicate for each analysis and the mean value of absorbance was obtained. The same procedure was repeated for the standard solution of rutin and the calibration line was construed. Based on the measured absorbance, the concentration of flavonoids was read (mg/ml) on the calibration line; then, the content of flavonoids in extracts was expressed in terms of rutin equivalent (mg of rutin/gm of extract).
Estimation of total tannins
The total tannin content in the plant extract was determined by Folin-Deins reagent method adopted by Polshettiwar (2007) (25). The absorbance was measured at 755 nm.
Estimation of total saponins
Twenty grams of plant powder was dispersed in 200 ml of 20% ethanol. The suspension was heated over a water bath for 4 h with stirring at about 55 °C. The mixture was filtered and the residue was re-extracted with another 200 ml of 20 % ethanol. The combined extracts were reduced to 40ml over water bath at about 90 °C. Twenty ml of diethyl ether was added to the concentrate and shaken vigorous. The aqueous layer was recovered while the ether layer was discarded. The purification process was repeated and 60 ml of n-butanol was added. The combined n-butanol extract were washed twice with 10ml of 5% aqueous sodium chloride. The remaining solution was evaporated. Then, the samples were dried in the oven to a constant weight. The saponins content was calculated in percentage according to Okwu and Ukanwa (2007) determination (26).
Estimation of total alkaloids (gravimetric method)
Ten grams of the plant powders was extracted with 90 % ethanol till exhaustion tested with Mayer's reagent using the standard procedure described by Woo and Püls (1977) (27).
Estimation of total phenolic content
Determination of total phenolics in plant extract was determined by using modified Folin-
Ciocalteu method (28). Gallic acid solution (sigma chemical) was used as a standard and prepared in various concentration 2-10 ?g/ml to be a standard curve. Concentration of 1mg/ml of plant extract was also prepared and 0.5ml of each sample were introduced into test and mixed with 2.5 ml of a 10 fold dilute Folin- Ciocalteu reagent and 2 ml 0f 7.5 % sodium carbonate. The tubes were covered with parafilm and allowed to stand for 30min at room temperature before the absorbance was read at 760 nm spectrophotometrically. All determination was performed in triplicate. Determination of total phenol content in the extracts were calculated using the linear regression equation of the calibration curve and expressed as gallic acid equivalent per gram of extract.
Carbohydrates content
Total, soluble and insoluble carbohydrates were estimated by using the method described by
Chaplin and Kennedy (1994) (29). Total carbohydrates were extracted by dissolving 1g of powder in 2-5 ml of 2M HCl in a sealed tube. The sealed tube was heated at 100 ºC for a period of 2-5 h. The extracted sugars were estimated using the general phenol- sulfuric acid assay. The absorbency was measured at 490 nm after 30 min.
For soluble carbohydrates half gram plant powders were extracted with ethanol/water (80% v/v) by reflux for 2 h. The alcohol was removed from the alcoholic extract by evaporation under reduced pressure. The aqueous extract was clarified using Carrez reagent, and then its volume was completed to 100 ml with dist. water. Then calculate insoluble carbohydrates = Total carbohydrates - soluble carbohydrates.
Data treatment and analysis:
The spreadsheet Microsoft Excel version 2013 has been used for the capture and encoding the data.
RESULTS AND DISCUSSION
Extraction
A total of seven extracts were obtained with yields ranging from 3.67% to 11.25% (Table 1). The lowest yields were noted with garlic and the combination containing a large amount of garlic. The highest yields were generally noted with the extracts containing large proportions of Syzygium aromaticum and Garcinia cola (Table 1).
Table 1: Extraction yields, codes and physical appearance of the extracts

The lower extraction yields noted on the garlic extracts and the extract containing a large amount of garlic could be explained by the particularly sticky aspect of the substrate. This could prevent certain constituents of the mixture from descending into the filtrate, thus leading to the low yield obtained in the fractions containing large quantities of Allium sativum. This low extraction yield at the garlic level can also be justified by the fact that garlic would contain enough apolar compounds. The extraction solvent being polar, it would not have been able to extract the apolar constituents. The extracts produced were then used to evaluate their antimicrobial activities on six microbial strains that are sometimes the basis of certain respiratory infections.
Extracts inhibitory diameter zone with the reference strains
Figure 1 indicates the inhibition diameter of the seven fractions on the six strains of microbes. The values of the inhibition diameters obtained are between 6.5 mm and 20 mm. The largest inhibition diameter of 20 mm was noted with the combination A1/S4/G1 on the strain S. aureus. The smallest diameter is always noted with the same combination on the strain S aureus CL.
A comparative study of the antimicrobial activity of the three starting substances namely A. sativum (A6/S0/G0), S. aromaticum (A0/S6/G0) and G. kola (A0/S0/G6) indicates that S. aromaticum and G. kola are more effective than A. sativum. S. aromaticum and G. kola inhibited three germs out of the six against A. sativum which inhibited only one germ. The constituents which would present good antimicrobial activities in A. sativum would be of apolar nature. This is surely the reason why its activity was low during the conduct of this work.
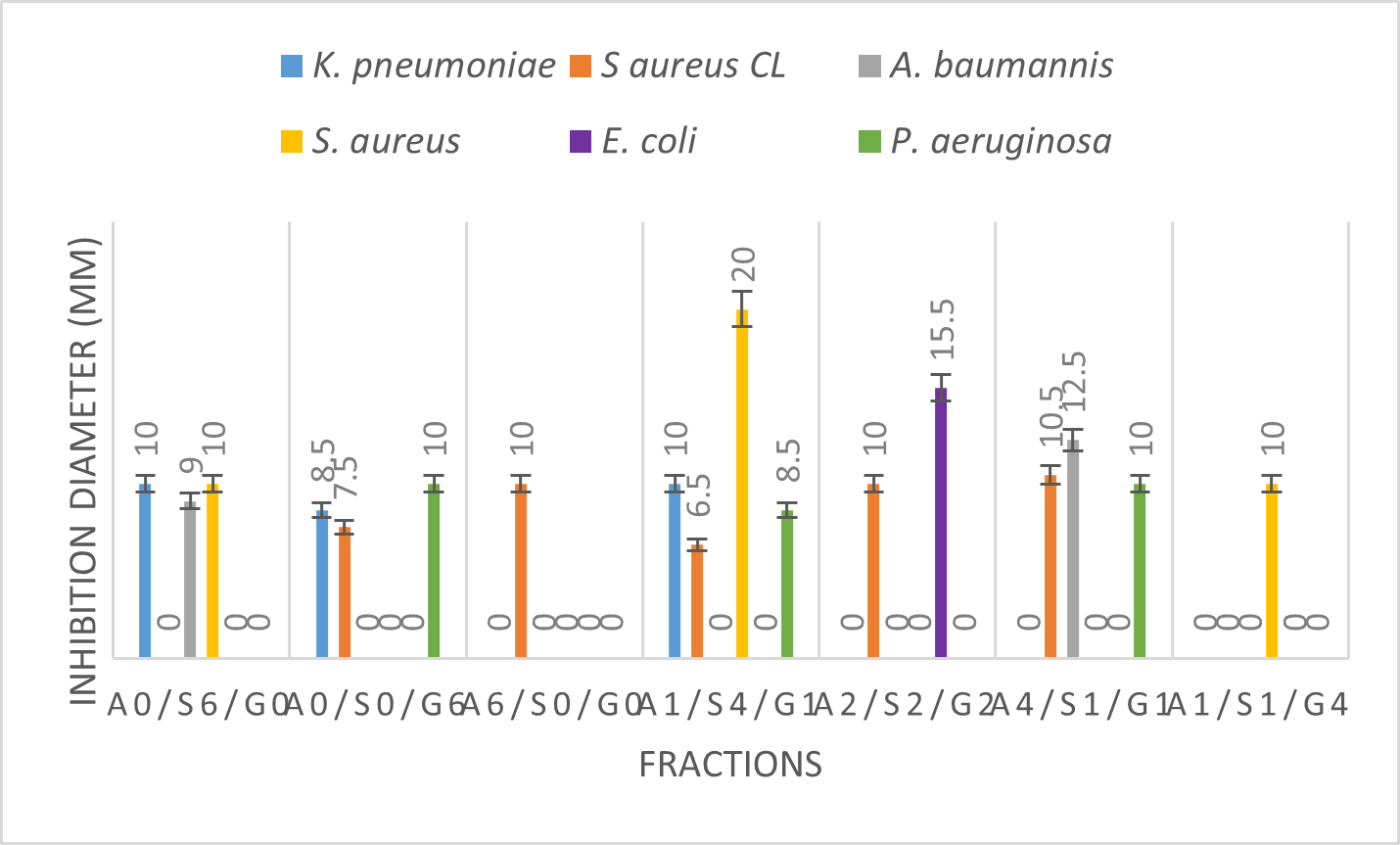
CL =Clinic
Figure 1: Diameters of inhibition of different fractions
Among combinations, the combination A1/S4/G1 inhibited four strains out of six with 66.67%. It is therefore the one that inhibits more germs. It was also more effective than the starting substrates taken individually (A6/S0/G0; A0/S6/G0; A0/S0/G6). In order to identify the fraction that inhibits more germs and presents the highest diameters of inhibition, the average diameters of inhibition taking into account all the strains used were evaluated and led to Figure 2.

Figure 2: Average inhibition diameters of the different combinations of extracts
By reading this diagram, it appears that the combination A1/S4/G1 has the largest average inhibition diameter. This value is of the order of 7 mm and is more than twice as high as the average of the other fractions. This average remains very low at the level of the garlic extract. Overall, the other combinations also have relatively higher averages than those of the starting substances except in the case of the fraction A1/S1/G4. The work carried out during this study confirms the inhibitory effect of these substances on microbes (10, 30). On the other hand, the study highlighting the synergistic effect of the three substances going as far as determining the adequate quantities to combine for greater effectiveness remains unreported. Following the determination of the inhibition diameters, to confirm the antimicrobial activities noted above, the minimum inhibitory and bactericidal concentrations were also evaluated.
Minimum Inhibitory Concentrations (MIC) of extracts
All extracts inhibited at least one strain. The extracts inhibited the proliferation of most pathogenic bacteria with variable minimum inhibitory concentrations (MIC). Reading the results, the minimum inhibitory concentrations have values between 2.5 and 20 mg/ml (Figure 3). A good correlation is noted between the inhibition diameters and the minimum inhibitory concentrations. The lowest concentration and largest inhibition diameter are noted with A1/S4/G1 extract on the same S. aureus germ. The A6/S0/G0 and A1/S1/G4 fractions inhibited only one germ. Fractions A0/S6/G0, A0/S0/G6, A4/S1/G1 each inhibited three germs. Fraction A2/S2/G2 inhibited two germs.

Figure 3: Minimum inhibitory concentrations (mg/ml) of the extracts
Reading the diagram, the germs used showed different sensitivities to the different extracts. S. aureus CL was the pathogenic germ most inhibited by the extracts. This microbe was affected by five extracts. K. pneumoniae, S. aureus and P. aeruginosa were affected by three extracts. A. baumannii was affected by two extracts and E. coli by a single extract. The effect of several extracts on the clinical strain of S. aureus remains a very good result suggesting that the extracts could have good in vivo activity since this strain was isolated from patients suffering from respiratory infections. Among the starting substances A6/S0/G0; A0/S6/G0; A0/S0/G6, S. aromaticum was more active with lower inhibitory concentrations. Logically, the fraction that contains a large amount of this substance (A1/S4/G1) also indicated a good activity. An antagonistic effect was observed with the fraction A1/S1/G4 which, despite the mixture, inhibited only one germ. To better perceive the expression of the inhibitory activities of the different fractions and to have the confirmation of the good activity of the mixture A1/S4/G1, the averages of the MIC were evaluated and represented in Figure 4.

Figure 4: Minimum inhibitory concentrations Averages of extracts
Following the determination of the averages, A1/S4/G1 fraction remains the most active with the lowest average inhibitory concentration of a value equal to 6.25 mg/mL (figure 4). Among the starting substances, A0/S6/G0 always remains more active than the starting substrates. Mixtures were more active than the starting substrates and a starting substrate was more active than some mixtures. This work clearly highlights the existence of agonist and antagonist effects that can exist between the substances. Some authors have also noted in their work that synergistic or antagonistic effects can occur during the preparation of mixtures (31-33). It is clear from this paragraph that even if a mixture must be considered, precise proportions must be respected. Otherwise the effect of the starting substances may be reduced.
Minimum Bactericidal Concentration (MBC) (mg/ml) of extracts
Figure 5 shows the minimum bactericidal concentrations. In addition to inhibiting the strains used in this work, the extracts also showed a bactericidal effect. The concentrations that can kill germs are between 10 and 50 mg/ml. Concentrations above 50 mg/ml have not been mentioned in this figure. The best bactericidal activities were also obtained with the mixture A1/S4/G1.

Figure 5: Minimum Bactericidal Concentration (MBC) (mg/ml) of extracts
The correlation remained the same. The extracts that showed the best inhibitory activities with the largest diameters of inhibitions were those that presented interesting bactericidal effects. The averages of the minimum bactericidal concentrations were also determined and are mentioned in Figure 6.

Figure 6: Minimum Bactericidal Concentration Averages (mg/ml) of extracts
The mixture A1/S4/G1 always remains the most active with a bactericidal average equal to 27.5 mg/ml. Among the starting substrates, A0/S6/G0 always remains the most active. The averages obtained indicate that the mixtures had more effective bactericidal effects than the starting substrates except in the case of the mixture A1/S1/G4. The different probable causes have been explained in the previous paragraphs and involve the antagonist and agonist effects. To finalize this work, a phytochemical study was undertaken on the most active fraction (A1/S4/G1) leading to a qualitative and quantitative identification of the major chemical families of molecules.
Qualitative and quantitative composition of the extracts
The phytochemical screening of the A1/S4/G1 fraction qualitatively highlighted the presence of reducing compounds, alkaloids, flavonoids, phenolic compounds consisting of catechic and gallic tannins, saponins, leucoanthocyanins, steroids, coumarin, mucilages and O-heterosides and free anthracenics (Table 2). The presence of such a large number of families of compounds may be due to the mixture of the three substances. The quantitative phytochemical study was also conducted on the ethanolic extract of the A1/S4/G1 mixture previously more active on the strains of microbes. Some families of compounds were quantified in the mixture. This quantification revealed a high level of flavonoids (11.36%) followed by the level of tannins (9.70%). The mixture also contains a certain amount of reducing compounds (5.40%) and especially a significant amount of alkaloids (2.14%), saponins (3.52%) and terpenoids (0.98%). Overall, it is noted that the majority families of compounds remain flavonoids and tannins. They would a priori be the basis of the good antimicrobial activity noted on the germs used during this study. But the presence of alkaloids also remains a significant asset. Indeed, the antimicrobial activities of flavonoids (34,35) and tannins have been widely described in the literature (36-38). It would be a little difficult to make a comparison of the values found during this work with values found during work on one of the substances. Also, the different values found during quantification can be influenced by the nature of the soil, the extraction solvent, the vegetative stage of the plant, etc. In addition, the antimicrobial activities of the starting substances have been described in the literature on other germs with good results (10,30). But the activity of the mixture of the three starting substances and the phytochemical study remains a first. Authors have also considered a mixture of substrates during the evaluation of other activities and have been able to confirm possible agonist and antagonist effects. This approach remains a trend that some authors have strongly encouraged (39).

Table 2: Phytochemical constituents of fraction A1/S4/G1 (%)
QNA: Quantitative analysis of the extract
CONCLUSION
At the end of this work, seven fractions were obtained by combinations of Allium sativum, Syzygium aromaticum and Garcinia kola. The most active on six strains of microbes responsible for respiratory infections is made up of 16.66% A. sativum, 66.66% S. aromaticum and 16.66% Garcinia kola (A1/S4/G1). This fraction contains several families of molecules and more particularly a good quantity of flavonoids, tannins, reducing compounds, alkaloids and saponins. The bio-guided study carried out during this work clearly indicates that an agonist effect exists between the tree substances in well-defined proportions. This identified fraction could effectively fight against certain strains of microbes responsible for respiratory infections.
ACKNOWLEDGMENTS
We thank the Faculty of Science and Technology of Natitingou for its financial support.
DECLARATION OF INTEREST
The authors declare that there is no conflict of interest. The authors alone are responsible for the accuracy and integrity of the paper content.
REFERENCE
- Organisation Mondiale de la santé (OMS). Centre de traitement des infections respiratoires aiguës sévères, [Accessed 2020]. Available from https://creativecommons.org/licenses/by-nc-sa/3.0/igo/deed.fr
- World Health Organization (WHO). Infection prevention and control of epidemic- and pandemic-prone acute respiratory infections in health care. Geneva, [Accessed 2017]. Available from https://www.who.int/csr/bioriskreduction/infection_control/publication/en/
- Diop M, Camara E, Barry I, Barry M, Barry A, Doukoure M, Diallo S. Facteurs Associés à la Survenue des Infections Respiratoires Aigües chez les Enfants de 0 à 5 Ans Hospitalisés à l’Hôpital National Donka à Conakry. HSD. 2020; 21(3): 1-10.
- Maxime G. Nombre de décès dus au coronavirus par pays du monde. [Accessed 2022]. Available from https://fr.statista.com/statistiques/1101324/morts-coronavirus-monde/
- 5-Organisation Ouest-Africaine de la Santé (OOAS), Conçu, imprimé et relié par KS PRINTCRAFT GH. LTD. P. O. BOX 1074. KNUST JUNCTION, KUMASI, GHANA Tel: +233-277412577. (2013) ; Page vii.
- Ewelike NC, Okammadu JC, Ogwudire VE, Nnadozie RI. In-vitro antimicrobial activity of methanolic and aqueous leaf extracts of Chrysophyllum albidum (African star apple) and Garcinia kola (Bitter kola), GSC Biological and Pharmaceutical Sciences 2021; 14(03): 249–253.
- Batiha GE, Beshbishy AM, Wasef L, Elewa YHA, Al-Sagan AA, Abd El-Hack ME, Taha AE, Abd-Elhakim YM, Devkota HP. Chemical Constituents and Pharmacological Activities of Garlic (Allium sativum L.): A Review, Nutrients. 2020; 12(872): 1-21.
- Sakirigui A, Nonviho G, Hounkpatin ASY, Chabi Sika K. Effects of teguments on phytochemistry and antimicrobial activities of Garcinia kola seeds. Journal of Pharmacognosy and Phytochemistry 2020; 9(5): 21-26.
- Chouti WK, Sodonon W, Sakirigui A, Chitou NE, Yovo F, Mama D. Phytochemical study of garcinia kola seed and its purifying power on the water of the Alibori river. International Journal of Green and Herbal Chemistry Section A: Green Chemistry 2022; 11(2): 183-192;
- Sakirigui A, Chabi Sika K, Koffi Allali E, Assogba F, Yovo F, Yayi Ladékan E, Gbénou JD, Accrombessi GC. Valorization of Neglected Bioactive Substances Through a Comparative Study of Their Phytochemical Compositions and Antimicrobial Properties with Those of Garcinia kola, Int J Pharm and Pharm Res. 2022; 23 (2): 311-322;
- Parham S, Kharazi A. Z., Bakhsheshi-Rad H. R., Nur H., Ismail A. F., Sharif S., Krishna S. R., Berto F. Antioxidant, Antimicrobial and Antiviral Properties of Herbal Materials. Antioxidants. 2020; 9(1309): 1-36.
- Batiha GE, Alkazmi LM, Wasef LG, Beshbishy AM, Nadwa EH, Rashwan EK. (). Syzygium aromaticum L. (Myrtaceae): Traditional Uses, Bioactive Chemical Constituents, Pharmacological and Toxicological Activities. Biomolecules 2020; 10(202): 2-16.
- 13-Otunola GA, Perry LM. Culinary Spices in Food and Medicine: An Overview of Syzygium aromaticum (L.) [Myrtaceae]. Front. Pharmacol. 2021; 12(793200): 1-13.
- Ozolua RI, Saba AO, Uwaya DO, Abelega N, Oghuvwu SO. Extract of Garcinia kola Seed has Antitussive Effect and Attenuates Hypercholesterolemia in Rodents. Med Aromat Plants 2016; 5(232): 1-5.
- Gupta R, Saxena R, Dubey K, Malviya N. Formulation and Evaluation of Herbal Antitussive Ash Syrup of Allium Sativum. MJPS 2020; 6(2): 91-93.
- Umar M, Mohammed IB, Oko JO, Tafinta IY, Alika AA and Jobbi DY: Phytochemical Analysis and Antimicrobial Effect of Lemon Grass (Cymbopogon citratus) Obtained From Zaria, Kaduna State, Nigeria. Journal of Complementary and Alternative Medical Research 2016; 1(2):1-8.
- Bauer AW, Kirby WM, Sherris JC, Turck M: Antibiotic susceptibility testing by a standardized single disk method. American Journal of Clinical Pathology 1996; 45: 493-496.
- Adesokan AA, Akanji MA, Yakubu MT. Antibacterial potentials of aqueous extract of Enantia chlorantha stem bark. African Journal of Biotechnology 2007; 6(22): 2502-2505.
- Delarras C. (1998). Microbiology. 90 hours of practical work. Gaétan Morien Publisher, ISBN: 291074907 X, 9782910749071, 169-178 pp.
- Farshori NN, Al-Sheddi ES, Al-Oqail1 MM, Musarrat J, Al-Khedhairy AA, Siddiqui MA: Anticancer Activity of Petroselinum sativum Seed Extracts on MCF-7 Human Breast Cancer Cells. Asian Pac J Cancer Prev. 2013; 14(10): 5719-5723.
- Houghton DJP, Raman A. Laboratory handbook for the fractionation of natural. Extracts Science. 1998; 199p.
- Harborne, JB. Methods of plant analysis. Phyochemical Methods. 1984; 1–3.
- Umeaku CN, Chris-Umeaku CI, Emmy-egbe IO, Ukoha CC, Uzor UC, Agbo UJ. Proximate, Phytochemical and Antibacterial Analysis of Persea americana Obtained from Nigeria. Journal of Diseases and Medicinal Plants 2018; 4 (3): 89-95.
- Chang CC, Yang MH, Wen HM, Chern JC. Estimation of total flavonoid content in propolis by two complementary colorimetric methods. Journal of Food and Drug Analysis 2002; 10(3): 178-182.
- Polshettiwar S, Ganjiwale R, Wadher S, Yeole P. Spectrophotometric estimation of total tannins in some ayurvedic eye drops. Indian J. Pharm. Sci. 2007; 69(4): 574-584.
- Okwu D, Ukanwa N. (2007) Nutritive value and phytochemical contents of fluted pumpkin (Telfaria occidentalis Hook F) vegetable grown with different levels of turkey droppings. 8th African Crop Science Society Conference, El-Minia, Egypt, 27-31 October 2007, African Crop Science Society, pp. 1759-1764.
- Woo C, Püls M. The Peierls mechanism in MgO. Philosophical Magazine. 1977; 35(6): 1641-1652.
- Maurya S, Singh D. Quantitative analysis of total phenolic content in Adhatoda vasica Nees extracts. International Journal of Pharm Tech. Research, 2010; 2(4) : 2403-2406
- Chaplin MF, Kennedy JF. (1994) "Carbohydrate Analysis: A Practical Approach", 2nd ed. School of Applied Science, South Bank University, London (United Kingdom). IRL Press Ltd.
- Barbu IA, Ciorît A, Carpa R, Mot AC, Butiuc-Keul A, Pârvu M. Phytochemical Characterization and Antimicrobial Activity of Several Allium Extracts. Molecules 2023, 28(3980).1-12.
- Ncube B, Finnie JF, Van Staden J. In vitro antimicrobial synergism within plant extract combinations from three South African medicinal bulbs, J. Ethnopharmacol. 2012; 139(1): 81-89.
- Tsimilli-Michael M, Eggenberg P, Biro B, Köves-Pechy K, Vörös I, Strasser RJ. Synergistic and antagonistic effects of arbuscular mycorrhizal fungi and Azospirillum and Rhizobium nitrogen-fixers on the photosynthetic activity of alfalfa, probed by the polyphasic chlorophyll a fluorescence transient. Applied Soil Ecology 2000; 15(2): 169-182.
- Yang Y, Zhang Z, Li S, Ye X, Li X, He K, Synergy effects of herb extracts: Pharmacokinetics and pharmacodynamic basis. Fitoterapia 2014; 92: 133-147.
- Thebti A, Meddeb A, Ben Salem I, Bakary C, Ayari S, Rezgui F, Essafi-Benkhadir K, Boudabous A, Ouzari HI. Antimicrobial Activities and Mode of Flavonoid Actions. Antibiotics 2023; 12(225): 1-19.
- Veiko AG, Olchowik-Grabarek E, Sekowski S, Roszkowska A, Lapshina EA, Dobrzynska I, Zamaraeva M, Zavodnik IB. Antimicrobial Activity of Quercetin, Naringenin and Catechin: Flavonoids Inhibit Staphylococcus aureus-Induced Hemolysis and Modify Membranes of Bacteria and Erythrocytes. Molecules 2023; 28(1252) 1-19.
- Darah SH, Lim I, Jain K. Antimicrobial activities of tannins extracted from rhizophora apiculata BARKS. Journal of Tropical Forest Science 2006; 18(1): 59-65.
- Mailoa MN, Mahendradatta M, Laga A, Djide N, Antimicrobial Activities Of Tannins Extract From Guava Leaves (Psidium Guajava L) On Pathogens Microbial, Inter. J. Sci. Technol. Res. 2014; 3(1): 236-241.
- Min, BR, Pinchak WE, Merkel R, Walker S, Tomita G, Anderson RC. Comparative antimicrobial activity of tannin extracts from perennial plants on mastitis pathogens. Scientific Research and Essay 2008; 3 (2): 066-073.
- 39. Wagner H, Ulrich-Merzenich G. Synergy research: Approaching a new generation of phytopharmaceuticals, Phytomedicine 2009; 2(3): 97-110


 Amoussatou Sakirigui*
Amoussatou Sakirigui*








 10.5281/zenodo.14000221
10.5281/zenodo.14000221