Abstract
This research investigated the synergistic antimicrobial effects of extracts derived from endophytic fungi isolated from Ashwagandha (Withania somnifera) and Turmeric (Curcuma longa). Endophytic fungi were isolated from sterilized plant tissues, and secondary metabolites were extracted using ethyl acetate. Concurrently, methanolic extracts from the host plants were prepared. The antimicrobial efficacy of these extracts, both individually and in combination, was assessed broth microdilution methods. Individual extracts from Ashwagandha and Turmeric exhibited moderate antimicrobial activities, whereas extracts from the endophytic fungi demonstrated slightly lower activity. However, when plant extracts were combined with their respective fungal extracts, a significant enhancement in antimicrobial activity was observed. The combination of Ashwagandha, Turmeric, and their associated endophytic fungal extracts showed the highest antimicrobial activity, confirmed by larger zones of inhibition and lower minimum inhibitory concentrations (MICs) compared to individual extracts. These findings suggest that the symbiotic relationship between plants and their endophytic fungi can be leveraged to develop potent natural antimicrobial agents. The enhanced efficacy observed in the combinations highlights the potential for these natural extracts to serve as effective alternatives to conventional antibiotics. This study opens avenues for further research into the mechanisms underlying this synergy and the development of sustainable antimicrobial therapies utilizing plant-fungal extract combinations.
Keywords
Potato Dextrose Agar, Ashwagandha, Turmeric, Endophytic Fungi, Centrifugation, Synergistic.
Introduction
The escalating threat of antimicrobial resistance (AMR) has spurred a pressing need for novel antimicrobial strategies. In this context, natural products have gained prominence for their diverse pharmacological properties, including antimicrobial activity. Ashwagandha (Withania somnifera) and turmeric (Curcuma longa) are two botanicals with a rich history in traditional medicine and known antimicrobial potential. Additionally, endophytic fungi residing within these plants have been recognized as prolific producers of bioactive secondary metabolites, which may contribute to the host plant's therapeutic effects. Ashwagandha, also known as Indian ginseng or winter cherry, has been a cornerstone of Ayurvedic medicine for centuries, esteemed for its adaptogenic, immunomodulatory, and antimicrobial properties. Turmeric, a member of the ginger family, has similarly enjoyed a longstanding tradition of use in Ayurveda and Traditional Chinese Medicine for its anti-inflammatory, antioxidant, and antimicrobial activities. The antimicrobial efficacy of ashwagandha and turmeric is largely attributed to bioactive constituents such as alkaloids, withanolides, curcuminoids, and essential oils.
In recent years, there has been a growing interest in exploring the synergistic interactions between natural products to enhance their therapeutic potential and mitigate resistance mechanisms. Synergy, characterized by the cooperative action of two or more agents resulting in an effect greater than the sum of their individual effects, offers promise in optimizing antimicrobial therapies.
This study aims to investigate the synergistic effects of extracts isolated from endophytic fungi residing within ashwagandha and turmeric for antimicrobial activity. Endophytic fungi, which colonize plant tissues without causing overt harm, have been found to biosynthesize an array of bioactive compounds with diverse pharmacological activities. By harnessing the collective bioactivity of these endophytic fungi extracts alongside their host plants, this research endeavors to elucidate novel antimicrobial combinations with potential therapeutic applications.
The exploration of synergistic interactions between endophytic fungi extracts from ashwagandha and turmeric represents a significant advancement in leveraging natural products for combating infectious diseases and addressing the challenge of antimicrobial resistance. Understanding the underlying mechanisms of synergy and identifying optimal combinations of bioactive compounds hold promise for the development of innovative antimicrobial therapies. This study aims to contribute to the growing body of knowledge on natural product synergy and its applications in antimicrobial therapy, with implications for drug discovery and public health.[1] Endophytic fungi play various crucial roles and functions within their host plants and in broader ecosystems. Here are some of their key functions and roles:
Functions of Endophytic Fungi
Promotion of Plant Growth:
Endophytic fungi produce hormones like auxins, gibberellins, and cytokinins, which can enhance plant growth and development. They facilitate nutrient uptake by solubilizing phosphates and fixing nitrogen, thus improving plant nutrition.
Stress Tolerance:
These fungi help plants withstand abiotic stresses such as drought, salinity, and extreme temperatures by inducing stress tolerance mechanisms and producing stress-related metabolites. They enhance the plant’s antioxidant defense system, reducing oxidative damage under stress conditions.
Disease Resistance:
Endophytic fungi produce antimicrobial compounds that protect plants from pathogenic microbes. This can reduce the incidence of diseases caused by bacteria, fungi, and viruses.
They can induce systemic resistance in plants, enhancing the plant's overall defense mechanisms.
Insect and Herbivore Deterrence:
By producing secondary metabolites like alkaloids, terpenoids, and phenolics, endophytic fungi deter herbivores and insect pests, reducing damage to the plant.
Bioremediation:
Some endophytic fungi can degrade pollutants and assist in the detoxification of contaminated soils, helping plants grow in polluted environments.
Roles of Endophytic Fungi
Symbiotic Relationships:
Endophytic fungi form mutualistic relationships with plants, where both partners benefit. The fungi gain a habitat and nutrients from the plant, while the plant receives growth-promoting and protective benefits.
Ecological Interactions:
Endophytic fungi contribute to plant diversity and ecosystem stability. They can influence plant community dynamics and succession by affecting plant health and competition.
Biodiversity and Conservation:
These fungi contribute to the biodiversity of microbial communities within ecosystems. Their presence can be critical for the survival and health of plant species, especially in harsh environments.
Biotechnological Applications:
Endophytic fungi are sources of novel bioactive compounds with potential applications in medicine, agriculture, and industry. They are studied for developing new antibiotics, anticancer agents, and biofertilizers.
Agricultural Benefits:
By promoting plant health and yield, endophytic fungi can reduce the need for chemical fertilizers and pesticides, contributing to sustainable agricultural practices.
Ashwagandha (Withania somnifera):
Biological Source: Withania somnifera, commonly known as ashwagandha, belongs to the Solanaceae family.
Description: It is a small shrub with yellow flowers and red fruit, native to the dry regions of India, the Middle East, and parts of Africa.
Parts Used: The roots and leaves of the ashwagandha plant are primarily used for medicinal purposes
Chemical Constituents: Ashwagandha contains several bioactive compounds, including alkaloids (such as withanine), steroidal lactones (withanolides), and saponins.
Uses: Ashwagandha has been traditionally used in Ayurvedic medicine for its adaptogenic, immunomodulatory, anti-inflammatory, and anxiolytic properties. It is also believed to possess antioxidant and anti-cancer properties.[2]
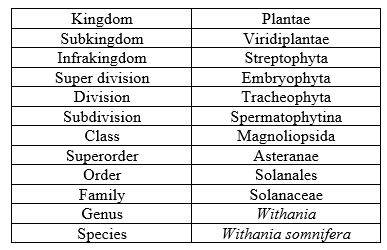
Table 1: Taxonomy of Ashwagandha
Turmeric (Curcuma longa):
Biological Source: Curcuma longa, known as turmeric, is a member of the Zingiberaceae family.
Description: It is a perennial herbaceous plant with underground rhizomes (root-like stems) and tall stems bearing long leaves and yellowish-white flowers. Turmeric is native to South Asia and is cultivated extensively in tropical regions.
Parts Used: The rhizomes of the turmeric plant are harvested, dried, and ground into a bright yellow powder, which is the form commonly used in cooking and traditional medicine.
Chemical Constituents: Turmeric is rich in curcuminoids, with curcumin being the most abundant and pharmacologically active compound. It also contains volatile oils, including turmerone and zingiberene.
Uses: Turmeric has a long history of use in traditional medicine systems, including Ayurveda and Traditional Chinese Medicine. It is renowned for its anti-inflammatory, antioxidant, antimicrobial, and anticancer properties. Turmeric is used to treat a variety of conditions, including arthritis, digestive disorders, skin diseases, and infections.[3]
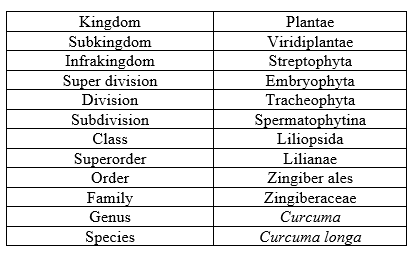
Table 2: Taxonomy of Turmeric
Contribution of the study:
The exploration of endophytic fungi has a rich history spanning several decades, marked by significant advancements in our understanding of their roles and applications across various fields. Initially regarded as mere inhabitants of plant tissues, endophytic fungi gained attention in the mid-20th century as researchers began to recognize their potential contributions to plant health and ecology. In the early years, studies primarily focused on identifying endophytic fungi and understanding their basic biology. Researchers isolated and characterized fungal strains from diverse plant species, laying the foundation for subsequent investigations into their ecological roles. Notable milestones include the discovery of novel fungal species and the development of methods for isolating endophytes from plant tissues. During the latter half of the 20th century, research on endophytic fungi expanded significantly, driven by growing interest in plant-microbe interactions and their implications for agriculture and ecology. These findings underscored the potential of endophytic fungi as natural allies in promoting plant health and productivity. In the 21st century, advances in molecular biology and genomic technologies revolutionized the study of endophytic fungi, enabling researchers to unravel their complex interactions with host plants at the molecular level
Furthermore, biotechnological applications of endophytic fungi have emerged as a major area of research interest. The discovery of bioactive compounds produced by endophytic fungi has sparked investigations into their pharmaceutical, agricultural, and industrial potential. These fungi have yielded a plethora of secondary metabolites with diverse biological activities, including antimicrobial, anticancer, and immunomodulatory properties, opening new avenues for drug discovery and bioprospecting. Looking ahead, research on endophytic fungi continues to advance rapidly, driven by ongoing efforts to elucidate their ecological significance, harness their biotechnological potential, and address pressing challenges in agriculture, medicine, and environmental sustainability.
Methodology
Isolation Process:
Collection and Preparation of Samples:
The isolation of endophytic fungi begins with the careful collection and preparation of plant samples. Healthy plant tissues from Ashwagandha (*Withania somnifera*) and Turmeric (*Curcuma longa*) are selected from their natural habitats. These samples are collected with caution, ensuring they are free from visible signs of disease, damage, or contamination
Surface Sterilization:
Upon arrival at the laboratory, the plant samples undergo surface sterilization to eliminate external contaminants. This process involves washing the samples with sterile distilled water to remove any soil or debris. Subsequently, the samples are treated with a series of disinfectants such as ethanol to sterilize the surface. This step is crucial to ensure that only endophytic fungi are isolated without any interference from external microorganisms.
Isolation on Selective Media:
Following surface sterilization, small amount of extracted powder was plated onto selective growth media. Potato Dextrose Agar (PDA) supplemented with antibiotics are commonly used to inhibit the growth of contaminants while promoting the growth of endophytic fungi. The plated tissues are then incubated under controlled conditions to allow the growth of fungal colonies.[14]
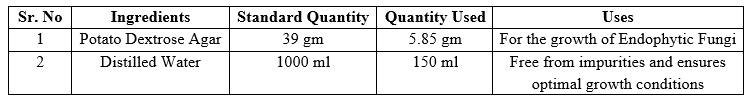
Table 3: Preparation of Media
Identification Process:
Morphological Characterization:
The isolated fungal colonies are initially characterized based on their morphological features such as colony morphology, color, texture, and reproductive structures (e.g., spores, conidia). Microscopic examination using techniques like light microscopy and staining methods (e.g., lactophenol cotton blue) provides further insights into the morphological characteristics of the fungi.
Microscopic Characterization:
Microscopic characterization of endophytic fungi involves several detailed observations. The process begins with mounting and staining fungal samples to visualize their structures clearly. Lactophenol cotton blue is a commonly used stain, while calcofluor white can be used for fluorescence microscopy due to its ability to bind to chitin. Under the microscope, the hyphal structures are examined for characteristics such as septation, branching patterns, width, and the presence of special structures like chlamydospores and sclerotia. The reproductive structures, including conidiophores, spores, and conidia, are scrutinized for their structure, size, shape, and arrangement. Accurate identification often requires measuring the dimensions of these structures. Hyphae, conidia, spores, asci, and other reproductive structures are measured for their precise size. High-resolution photomicroscopy is employed to capture detailed images of these structures, allowing for comparison with known species and aiding in accurate identification.[15]
Fermentation Process for Endophytic Fungi
Fermentation Setup:
Inoculum Preparation: The fermentation process begins with the preparation of the fungal inoculum. Endophytic fungi isolated from plant samples are grown on agar plates containing suitable growth media. After incubation, fungal spores or mycelial fragments are harvested from the plates and used to inoculate the fermentation medium.
Fermentation Medium: A nutrient-rich fermentation medium is prepared to support the growth and metabolite production of endophytic fungi. The composition of the medium varies
depending on the nutritional requirements of the specific fungal strain. Common components of the medium include carbon sources (e.g., glucose, sucrose), nitrogen sources (e.g., peptone, yeast extract), minerals, and vitamins.
Fermenter Setup: The inoculated fermentation medium is transferred to a flask containing glucose broth for fermentation, temperature control, pH monitoring, and aeration systems. These parameters are carefully controlled to optimize fungal growth and metabolite production throughout the fermentation process.
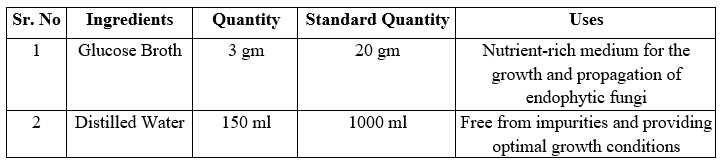
Table 4: Preparation of Fermentation Medium
Fermentation Process:
Optimization of Culture Conditions: The fermentation conditions, including temperature, pH, agitation rate, and aeration, are optimized to enhance fungal growth and the production of bioactive metabolites. These parameters are adjusted based on the specific requirements of the fungal strain and the desired metabolite production.
Fermentation Duration: The duration of the fermentation process varies depending on the growth kinetics of the fungal strain and the production kinetics of the target metabolites. Fermentation may last anywhere from a few days to several weeks, with periodic sampling to assess progress.
Extraction Process
Harvest the Fermentation Broth
Transfer the fermented broth from the fermenter vessel to a suitable container such as a centrifuge tube or flask. If necessary, separate the biomass from the fermentation broth by centrifugation or filtration to obtain a clear supernatant.
Preparation of Extraction Solvent
Choose an appropriate organic solvent or solvent mixture for extracting the metabolites from the fermentation broth. Common solvents include ethyl acetate, methanol, ethanol, or a mixture of these solvents. Prepare the extraction solvent by adding it to the harvested fermentation broth in a suitable ratio. Typically, a solvent-to-sample ratio of 1:1 or higher is used.
Extraction
Shake or vortex the mixture of fermentation broth and extraction solvent vigorously to ensure thorough mixing. Allow the mixture to stand for a period of time to facilitate partitioning of the metabolites into the organic solvent phase.
Partitioning
After allowing sufficient time for extraction, separate the organic solvent phase containing the extracted metabolites from the aqueous phase. Perform liquid-liquid extraction by carefully decanting or pipetting off the organic solvent layer while leaving behind the aqueous layer.
Concentration and Evaporation
Concentrate the organic solvent containing the extracted metabolites using techniques such as rotary evaporation or nitrogen blow-down. Remove the solvent under reduced pressure or gentle heating to obtain a concentrated extract.[16]
Anti-Microbial Activity
Anti-microbial activity of selected endophytic isolates was carried out using the modified well diffusion method. Three wells were created in each plate previously incubated with the 100 ?l of tested fungi at concentration of 1, 2, 3 µl using 100 µl of semisolid crude organic extract. The isolate was dissolved in 5 ml of ethyl acetate to give a final concentration of 20 mg/ml. Then 100 µl of the late test solution was placed in one of the wells; the other two wells were served as positive and negative controls with 100 ?l of Ampicillin (0.05 mg/ml), respectively plates were incubated at 37°C for 24 hours after which they were examined for inhibition zones. Experiments were performed in triplicate to ensure reliability.[17]
Result and Discussion
Isolation Process:

Table 5: Inoculation of Sample
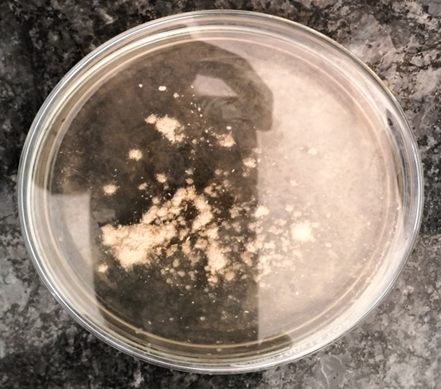
Fig 1: Ashwagandha Plate

Fig 2: Turmeric Plate
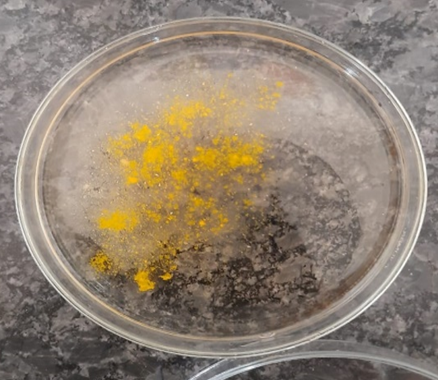
Fig 3: Synergistic Plate
Identification Process:
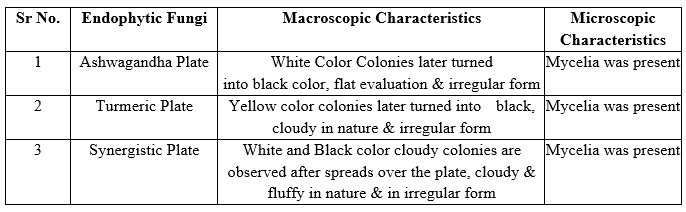
Table 6: Morphological and Microscopic Identification
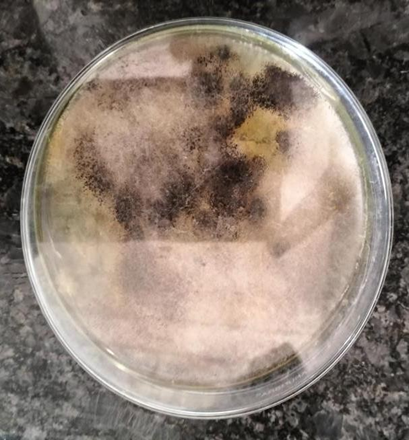
Fig 4: Ashwagandha Endophytic Fungi
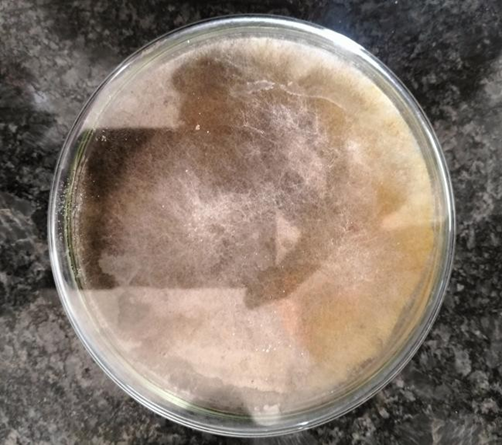
Fig 5: Turmeric Endophytic Fungi
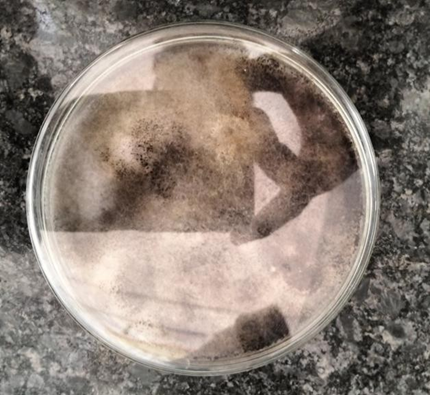
Fig 6: Synergistic Endophytic Fungi
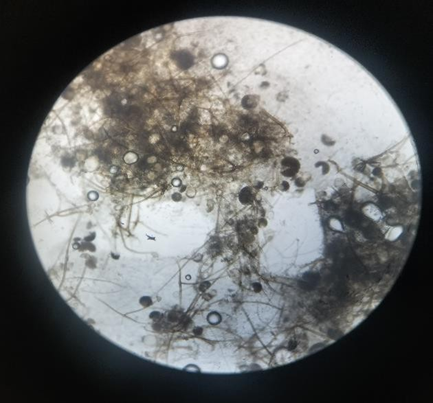
Fig 7: Ashwagandha Identification
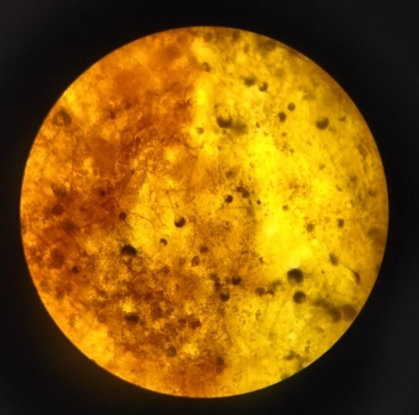
Fig 8: Turmeric Identification
Fig 9: Synergistic Identification
Fermentation Process:

Table 7: Fermentation of Endophytic Fungi

Fig 10: Ashwagandha Fermentation

Fig 11: Turmeric Fermentation

Fig 12: Synergistic Fermentation
Extraction Process:

Table 8: Extraction of Endophytic Fungi

Fig 13: Ashwagandha Extraction

Fig 14: Turmeric Extraction

Fig 15: Synergistic Extraction
Anti-Microbial Activity Process:
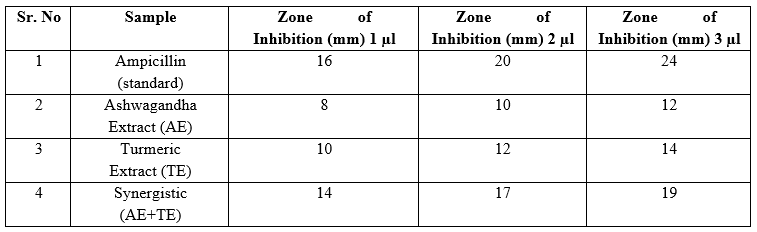
Table 9: Antimicrobial Activity of Extract Isolated from Endophytic Fungi
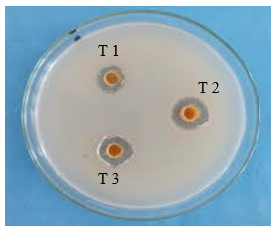
Fig 16: Ampicillin Activity
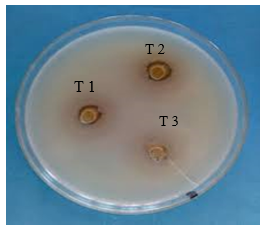
Fig 17: Ashwagandha Activity
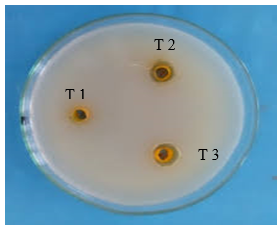
Fig 18: Turmeric Activity
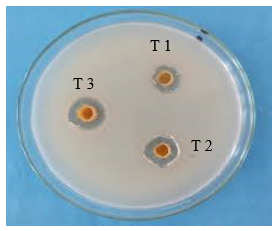
Fig 19: Synergistic Activity
Ampicillin: Demonstrates high zones of inhibition, indicating strong antimicrobial activity at all dilutions.
Ashwagandha Extract (AE): Shows moderate antimicrobial activity, slightly lower than Turmeric extract.
Turmeric Extract (TE): Exhibits good antimicrobial activity, outperforming Ashwagandha extract.
Synergistic AE + TE: The combination shows a significant increase in antimicrobial activity compared to the individual extracts, indicating a synergistic effect.
The study investigated the synergistic antimicrobial effects of extracts from endophytic fungi isolated from Ashwagandha (Withania somnifera) and Turmeric (Curcuma longa). Endophytic fungi were isolated from plant tissues, and their secondary metabolites were extracted using ethyl acetate. Methanolic extracts of Ashwagandha and Turmeric were also prepared. The antimicrobial activity of these extracts, both individually and in combination, was tested using standard methods. Results showed that individual extracts of Ashwagandha and Turmeric had moderate antimicrobial activity, while extracts from the endophytic fungi exhibited slightly lower activity. However, combinations of plant extracts with their respective fungal extracts demonstrated significantly enhanced antimicrobial effects. The combination of Ashwagandha, Turmeric, and their respective fungal extracts yielded the highest antimicrobial activity. These findings suggest that combining extracts from endophytic fungi with their host plants can significantly boost antimicrobial properties, potentially leading to the development of potent natural antimicrobial agents.
CONCLUSION:
The research conclusively demonstrated that the synergistic combination of extracts from endophytic fungi isolated from Ashwagandha (Withania somnifera) and Turmeric (Curcuma longa) significantly enhances antimicrobial activity. The individual extracts from both plants and their associated fungi exhibited moderate antimicrobial properties, but when combined, the extracts displayed markedly improved efficacy. The highest antimicrobial activity was observed in the combination of Ashwagandha, Turmeric, and their respective fungal extracts, underscoring the potential of these synergistic combinations to serve as potent natural antimicrobial agents. These findings highlight the importance of exploring symbiotic relationships in plants and their endophytes for novel bioactive compounds. The results suggest that harnessing the synergistic effects between plant extracts and endophytic fungal metabolites could lead to the development of more effective and natural alternatives to conventional antibiotics. This research paves the way for further investigations into the mechanisms behind this synergy and the potential for scaling up these combinations for clinical or agricultural applications. Overall, the study provides a promising outlook on the use of plant-fungal extract combinations in combating microbial infections, offering a sustainable and potent approach to antimicrobial therapy.
REFERENCE
- Dar, N. J., Hamid, A., & Ahmad, M. (2015). Pharmacologic overview of Withaniasomnifera, the Indian Ginseng.Cellular and Molecular Life Sciences, 72(23), 4445-4460.
- Gupta, S. K., Dua, A., Vohra, B. P., &Saxena, G. (2013). Withaniasomnifera (Ashwagandha): A review. Pharmacognosy Reviews, 7(14), 188–198.
- Prasad, S., & Aggarwal, B. B. (2011). Turmeric, the golden spice: From traditional medicine to modern medicine. In Herbal Medicine: Biomolecular and Clinical Aspects (2nd ed.). CRC Press/Taylor &Francis.
- Kumar, S., et al. (2017).** "Exploring the potential of endophytic fungi in enhancing bioactive metabolite production from Withaniasomnifera (L.) Dunal." *Frontiers in Microbiology,8*,1469.
- Raham, M.A., Begum, S., &Islam., M.R. (2019). "Endophytic fungi associated with turmeric (Curcuma longa L.) plant: Diversity and antimicrobial activity against human pathogens." Microbial Pathogenesis, 127, 16-20.
- T. Sutjaritvorakul, "Antimicrobial activity from endophytic fungi isolated from plant leaves in Dipterocarpous forest at Viengsa district Nan province, Thailand,” Journal of Agri cultural Technology, Volume 7, Issue 1, 2011.
- C. Perez,“Antibiotic assay by agar-well diffusion method,” Acta Biology Medicine Experimental, vol. 15, pp. 113–115, 1990.


 Sahil Agrawal*
Sahil Agrawal*
 Bhushan Potdar
Bhushan Potdar




























 10.5281/zenodo.12749440
10.5281/zenodo.12749440