Abstract
Infection with the bacteria Mycobacterium tuberculosis causes the disease tuberculosis. In Present research work we synthesized some N-alkylated indole chalcone derivatives and determined their biological activity against the H37Rv strain of Mycobacterium tuberculosis. Inside this library of compounds, (E)-1-(4-bromo-2-hydroxyphenyl)-3-(1-butyl-1H-indol-3-yl)prop-2-en-1-one (S1R8) and (E)-1-(4-bromo-2-hydroxyphenyl)-3-(1-pentyl-1H-indol-3-yl)prop-2-en-1-one (S1R16) displayed anti-tubercular activity with 04 µg/mL and 08 µg/mL MIC values, respectively. The in vitro antibacterial activity of all compounds was calculated against Gram-positive and Gram-negative bacteria. N-alkylated Indole Chalcone Derivatives were designed, synthesized and measured by ESI-MS and spectroscopic (FT-IR and 13CNMR) techniques.
Keywords
Mycobacterium Tuberculosis, Indole chalcones, Antimicrobial Agents, Antituberclar Agents.
Introduction
Mycobacterium tuberculosis, which causes the chronic infectious disease known as tuberculosis (TB), continues to be a major global health issue.1 Each year, TB continues to kill millions of people. There were over 1.5 million fatalities from TB in 2018. According to the WHO, 186,772 people acquired rifampicin-resistant TB in 2017 and an estimated 500,000 people globally developed MDR-TB.2 While first and second line drugs that are typically toxic for a longer period of time are required for the treatment of MDR- and XDR-TB, drug susceptible TB requires multiple antibiotics to be taken daily for at least 6 months.3 Due to the length of the treatment and the toxicity of second-line medications, patients frequently discontinue their treatment or do not strictly follow the regimen. To reduce the length of the therapy period and effectively treat MDR/XDR-TB, new medicines with novel mechanisms of action or the repurposing of FDA-approved medications are urgently needed.4 Today, the study of heterocyclic compounds is a hot topic because they make up the majority of natural compounds. In the field of pharmaceuticals, molecules with an indole base are among the heterocyclic with the most potential.5 Tryptophan, serotonin, reserpine, and indole 3-acetic acid are examples of naturally occurring alkaloids that contain the key bioactive molecule indole heterocyclic as their essential skeleton.6 Furthermore, indole alkaloids from bacteria and the ocean exhibit anti-cancer, antiviral, antibacterial, and anti-HIV properties.7 In addition, indole derivatives have a long history of use as antibacterial, antiviral, insecticidal, analgesic, anti-inflammatory, anti-depressant, anti-tubercular, antineoplastic, antihypertensive, antioxidant, and anti-diabetic medicines.8 The Food and Drug Administration (FDA) has even released a database showing the significance of N-containing heterocyclic compounds in 2015. With 17 indole-containing medications on the market, indole derivatives rank ninth among the top 25 FDA-approved drugs.9
Experimental
General procedure for synthesis of intermediate
The microwave reaction was accomplished in a solution of Indole-3-carboxaldehyde (435mg, 3mmol), different alkylating agents (4.5mmol), KOH (673mg, 12mmol), and anhydrous K2CO3 (1.65g, 12mmol) in 3ml of solvent (DMF) in a Pyrex sample vial at 130-145ºC. After 40-45 minutes of irradiation, again for the next five min., the chemical compound was left to cool to ambient temperature. For the reaction’s completion, the compound was rinsed with cold water to eliminate any polar contaminants. After being washed with water, the product was collected by filtration before being dissolved in a hot solvent (ethanol). Once the product was entirely dissolved, it was kept at room temperature until the temperature approached ambient, and then it was kept at ice-cold water or 0°C. The product crystals appeared when the solvent was placed in an ice bath. It was then filtered before being washed in chilled ethanol. The reaction completion was monitored by TLC. Ethyl acetate: Pet. ether was used as solvent system. (10)
General procedure for synthesis of S1R1-S1R8 derivatives
To a washed, cleaned, and dried 30 ml Pyrex microwave glass vial, (80.85mg, 0.4mmol) of 1-butyl-1H-indole-3-carbaldehyde (S1I1) was dissolved in 3.27 ml of ethanol. In the other dried glass vial (3.75ml) of 40% NaOH was taken and (0.4mmol) of different acetophenones was added drop wise. Then, a vial filled with base and different acetophenones was transferred to the Pyrex microwave glass vial. Then, the reaction vial was kept in Monowave. The reaction was monitored with the help of TLC. The final product was neutralized with the help of 0.1 N HCl. The compound was dried and triturated with pet ether to remove non-polar impurities.
(E)-3-(1-butyl-1H-indol-3-yl)-1-(4-fluorophenyl)prop-2-en-1-one (S1R1): M.p. 215-220°C, IR cm-1:3108.49 (Ar C-H) (str.), 1741.09(C=O), 1647.11(C=C), 1339.78(C-N) (str.), 13C NMR: (500MHz, CDCl3) ? 14.5 , 20.1 , 29.4 , 50.1 , 109.7 , 112.0 , 115.4 , 118.7 , 121.4 , 125.1 , 128.2 , 128.3-128.5 , 132.5 , 133.1 , 135.5 , 136.7 , 162.5 , 188.9 , LC/MS (ESI) m/z 322.1 (M+).
(E)-1-(4-bromophenyl)-3-(1-butyl-1H-indol-3-yl)prop-2-en-1-one (S1R2): M.p. 220-225°C IR cm-1:3110.53(Ar C-H) (str.), 1740.18(C=O), 1650.15(C=C), 1325.01(C-N) (str.), 13C NMR: (500MHz, CDCl3) ? 14.0 , 21.8 , 29.9 , 53.5 , 110.8 , 114.0 , 118.9 , 121.9 , 122.3 , 125.2 , 128.2 , 128.3-128.6 , 129.9 , 131.7 , 133.1 , 136.5 , 136.7 , 189.9 , LC/MS (ESI) m/z 383.23 (M+1).
(E)-3-(1-butyl-1H-indol-3-yl)-1-(4-hydroxyphenyl)prop-2-en-1-one (SIR3):M.p. 205-210°C IR cm-1:3110.83 (Ar C-H) (str.), 1742.05 (C=O), 1648.10 (C=C), 1330.97 (C-N) (str.), 13C NMR: (500MHz, CDCl3) ? 15.3 , 20.8 , 30.2 , 52.5 , 108.5 , 111.5 , 115.7 , 118.9 , 121.5 , 125.2 , 128.3 , 128.3-128.5 , 130.7 , 133.2 , 135.6 , 136.7 , 157.4 , 187.9 , LC/MS (ESI) m/z 320.50 (M+).
(E)-3-(1-butyl-1H-indol-3-yl)-1-(2,4-dichlorophenyl)prop-2-en-1-one (SIR4): M.p. 215-220°C IR cm-1:3103.84 (Ar C-H) (str.), 1740.81(C=O), 1646.49.15(C=C), 1315.51(C-N) (str.), 13C NMR: (500MHz, CDCl3) ? 16.1 , 22.1 , 30.5 , 52.1 , 107.7 , 112.5 , 115.8 , 118.8 , 121.3 , 125.5 , 128.2 , 128.3-128.5 , 130.8 , 133.2 , 135.6 , 136.9 , 157.8 , 190.1 , LC/MS (ESI) m/z 377.21 (M+1).
(E)-1-(3-bromophenyl)-3-(1-butyl-1H-indol-3-yl)prop-2-en-1-one (SIR5): M.p. 200-205°C, IR cm-1:3107.17(Ar C-H) (str.), 1741.08(C=O), 1647.26(C=C), 1338.21(C-N) (str.), 13C NMR: (500MHz, CDCl3) ? 14.5 , 23.2 , 29.6 , 49.2 , 111.8 , 111.5 , 118.4 , 119.9 , 122.9 , 126.5 , 128.2 , 128.3-128.5 , 129.0 , 130.0-130.2 , 132.0 , 133.1 , 136.7 , 140.2 , 193.9 , LC/MS (ESI) m/z 385.00 (M+).
(E)-3-(1-butyl-1H-indol-3-yl)-1-(2,4-dihydroxyphenyl)prop-2-en-1-one(SIR6): M.p. 230-235°C, IR cm-1 :3108.34 (Ar C-H) (str.), 1738.25(C=O), 1655.82(C=C), 1340.01(C-N) (str.), 13C NMR: (500MHz, CDCl3) ? 16.0 , 21.1 , 28.4 , 54.1 , 107.8 , 115.0 , 118.5 , 121.7 , 122.4 , 127.1 , 128.2 , 128.3-128.7 , 129.0 , 130.1-130.2 , 132.5 , 135.2 , 136.8 , 140.2 , 190.5 , LC/MS (ESI) m/z 336.20 (M+).
(E)-1-(2-bromophenyl)-3-(1-butyl-1H-indol-3-yl)prop-2-en-1-one (SIR7): M.p. 210-215°C, IR cm-1:3100.53 (Ar C-H) (str.), 1741.18(C=O), 1647.15(C=C), 1335.01(C-N) (str.), 13C NMR: (500MHz, CDCl3) ? 14.5 , 25.4 , 30.4 , 55.1 , 109.7 , 111.1 , 119.4 , 119.9 , 123.8 , 126.5 , 128.5 , 128.3-128.5 , 130.5 , 130.0-130.2 , 132.0 , 133.1 , 136.7 , 142.2 , 188.9 , LC/MS (ESI) m/z 384.30 (M+1).
(E)-1-(4-bromo-2-hydroxyphenyl)-3-(1-butyl-1H-indol-3-yl)prop-2-en-1-one (SIR8): M.p. 235-240°C IR cm-1:3111.01(Ar C-H) (str.), 1741.05(C=O), 1646.52(C=C), 1334.44(C-N) (str.), 13C NMR: (500MHz, CDCl3) ? 14.0 , 29.1 , 29.4 , 52.1 , 106.8 , 112.0 , 118.4 , 118.7 , 121.4 , 125.1 , 128.2 , 128.3-128.5 , 129.0 , 130.1-130.2 , 133.0 , 133.4 , 136.9 , 139.5 , 191.9 , LC/MS (ESI) m/z 412.12 (M+).
General procedure for synthesis of S1R9-S1R16 derivatives
To a washed, cleaned, and dried 30 ml Pyrex microwave glass vial, (86.86mg, 0.4mmol) of 1-pentyl-1H-indole-3-carbaldehyde (S1I2) was dissolved in 3.27 ml of ethanol. In the other dried glass vial (3.75ml) of 40% NaOH was taken and (0.4mmol) different acetophenones was added drop wise. Then, a vial filled with base and different acetophenones was transferred to the Pyrex microwave glass vial. Then, the reaction vial was kept in MW. The reaction was monitored with the help of TLC (Ethyl acetate: pet ether/hexane). The product was neutralized with the help of 0.1 N HCl. The compound was dried and triturated with pet ether to remove non-polar impurities.
(E)-1-(4-fluorophenyl)-3-(1-pentyl-1H-indol-3-yl)prop-2-en-1-one (SIR9): M.p. 240-245°C IR cm-1:3115.53 (Ar C-H) (str.), 1745.18 (C=O), 1652.15 (C=C), 1320.01 (C-N) (str.), 13C NMR: (500MHz, CDCl3) ? 14.9 , 23.6 , 27.6 , 29.8 , 53.1 , 108.7 , 112.5 , 115.4 , 118.7 , 121.4 , 125.1 , 128.2 , 128.3-128.6 , 132.5 , 133.4 , 135.8 , 136.7 , 162.5 , 187.5 , LC/MS (ESI) m/z 340.12 (M+).
(E)-1-(4-bromophenyl)-3-(1-pentyl-1H-indol-3-yl)prop-2-en-1-one (SIR10): M.p. 235-240°C IR cm-1 :3110.53 (Ar C-H) (str.), 1740.18(C=O), 1641.65(C=C), 1340.50(C-N) (str.), 13C NMR: (500MHz, CDCl3) ? 15.5 , 22.8 , 28.2 , 29.7 , 52.1 , 110.7 , 112.6 , 118.7 , 121.4 , 122.3 , 125.1 , 128.2 , 128.3-128.5 , 129.9 , 131.7 , 133.1 , 135.5 , 136.7 , 191.5 , LC/MS (ESI) m/z 398.35 (M+1).
(E)-1-(4-hydroxyphenyl)-3-(1-pentyl-1H-indol-3-yl)prop-2-en-1-one (S1R11): M.p. 240-245°C IR cm-1 :3110.07 (Ar C-H) (str.), 1741.05(C=O), 1642.15(C=C), 1315.09(C-N) (str.), 13C NMR: (500MHz, CDCl3) ? 14.5 , 24.6 , 27.8 , 30.3 , 55.1 , 111.7 , 113.9 , 115.7 , 118.7 , 121.4 , 125.1 , 128.2 , 128.3-128.5 , 130.7 , 133.8 , 136.5 , 136.7 , 157.4 , 189.9 , LC/MS (ESI) m/z 347.43 (M+).
(E)-1-(2,4-dichlorophenyl)-3-(1-pentyl-1H-indol-3-yl)prop-2-en-1-one (SIR12): M.p. 215-220°C IR cm-1:3110.53 (Ar C-H) (str.), 1740.18(C=O), 1650.15(C=C), 1325.01(C-N) (str.), 13C NMR: (500MHz, CDCl3) ? 15.8 , 22.6 , 27.2 , 29.3 , 50.1 , 108.7 , 112.0 , 118.9 , 121.5 , 125.1 , 128.2 , 128.2-128.5 , 128.6-128.8 , 129.9 , 132.3 , 133.0-133.3 , 136.7-136.9 , 192.4 , LC/MS (ESI) m/z 389.35 (M+).
(E)-1-(3-bromophenyl)-3-(1-pentyl-1H-indol-3-yl)prop-2-en-1-one (SIR13): M.p. 220-225°C IR cm-1:3108.73(Ar C-H) (str.), 1742.01 (C=O), 1647.10(C=C), 1337.32 (C-N) (str.), 13C NMR: (500MHz, CDCl3) ? 14.2 , 22.6 , 27.2 , 29.3 , 51.1 , 109.7 , 112.0 , 118.4 , 118.7 , 121.4 , 125.1 , 128.2 , 128.3-128.5 , 129.0 , 130.0-130.2 , 132.0 , 133.1 (1C, s), 136.7 , 140.2 , 188.9 , LC/MS (ESI) m/z 396.00 (M+1).
(E)-1-(2,4-dihydroxyphenyl)-3-(1-pentyl-1H-indol-3-yl)prop-2-en-1-one (SIR14): M.p. 230-235°C, IR cm-1:3105.53 (Ar C-H) (str.), 1735.15(C=O), 1640.15(C=C), 1315.01(C-N) (str.), 13C NMR: (500MHz, CDCl3) ? 14.1 , 23.6 , 27.5 , 31.9 , 52.1 , 101.1 , 110.7 , 114.0 , 115.7 , 119.2 , 120.1 , 122.4 , 124.1 , 127.8 , 128.2 , 128.3-128.5 , 133.1 , 136.9 , 160.1 , 164.8 , 191.9 , LC/MS (ESI) m/z 354.47 (M+).
(E)-1-(2-bromophenyl)-3-(1-pentyl-1H-indol-3-yl)prop-2-en-1-one (S1R15): M.p. 225-230°C, IR cm-1:3112.55(Ar C-H) (str.), 1740.18(C=O), 1645.12(C=C), 1325.01(C-N) (str.), 13C NMR: (500MHz, CDCl3) ? 15.5 , 22.6 , 28.3 , 30.5 , 50.1 , 111.8 , 112.1 , 118.6-118.8 , 121.4 , 125.2 , 128.1-128.3 , 128.3-128.5 , 128.6 , 132.6 , 133.1 , 136.7 , 141.0 , 183.6 , LC/MS (ESI) m/z 398.25 (M+).
(E)-1-(4-bromo-2-hydroxyphenyl)-3-(1-pentyl-1H-indol-3-yl)prop-2-en-1-one (SIR16): M.p. 245-250°C, IR cm-1 :3111.39(Ar C-H) (str.), 1741.06 (C=O), 1647.55(C=C), 1345.01 (C-N) (str.), 13C NMR: (500MHz, CDCl3) ? 14.6 , 22.8 , 27.2 , 29.3 , 50.9 , 111.9 , 112.9 , 118.1 , 118.7 , 120.1 , 121.4 , 122.7 , 125.1 , 127.8 , 128.9 , 128.3-128.5 , 132.7 , 134.1 , 140.7 , 160.0 , 193.9 , LC/MS (ESI) m/z 410.33 (M+1).
General procedure for synthesis of S1R17-S1R24 derivatives
To a washed, cleaned, and dried 30 ml Pyrex microwave glass vial, (86.06mg, 0.4mmol) of 1-(3-methylbut-2-en-1-yl)-1H-indole-3-carbaldehyde (S1I3) was dissolved in 3.27 ml of ethanol. In the other dried glass vial (3.75ml) of 40% NaOH was taken and (0.049ml, 0.4mmol) of 4-fluoroacetophenone was added drop wise. Then, a vial filled with base and different acetophenones was transferred to the Pyrex microwave glass vial. Then, the reaction vial was kept in MW. The reaction was monitored with the help of TLC (Ethyl acetate: pet ether/hexane). The product was neutralized with the help of 0.1 N HCl.The compound was dried and triturated with pet ether to remove non-polar impurities.
(E)-1-(4-fluorophenyl)-3-(1-(3-methylbut-2-en-1-yl)-1H-indol-3-yl)prop-2-en-1-one (S1R17): M.p. 235-240°C, IR cm-1:3109.71 (Ar C-H) (str.), 1741.01 (C=O), 1648.30 (C=C), 1338.63 (C-N) (str.), 13C NMR: (500MHz, CDCl3) ? 17.9 , 25.8 , 49.0 , 109.7 , 112.0 , 115.4 , 118.7 , 120.9 , 121.4 , 125.1 , 128.2 , 128.3-128.5 , 132.5 , 133.1 , 135.5 , 136.7 , 137.1 , 162.5 , 188.9 , LC/MS (ESI) m/z 334.21 (M+).
(E)-1-(4-bromophenyl)-3-(1-(3-methylbut-2-en-1-yl)-1H-indol-3-yl)prop-2-en-1-one (SIR18): M.p. 245-250°C, IR cm-1:3108.57 (Ar C-H) (str.), 1745.01 (C=O), 1650.15 (C=C), 1339.01 (C-N) (str.), 13C NMR: (500MHz, CDCl3) ? 18.5 , 26.9 , 49.5 , 110.8 , 113.1 , 119.7 , 120.5 , 121.4 , 122.3 , 125.2 , 128.1 , 128.3-128.5 , 129.9 , 131.7 , 133.5 , 135.6 , 134.7 , 137.1 , 186.9 , LC/MS (ESI) m/z 398.1 (M+).
(E)-1-(4-hydroxyphenyl)-3-(1-(3-methylbut-2-en-1-yl)-1H-indol-3-yl)prop-2-en-1 one (SIR19): M.p. 230-235°C, IR cm-1:3112.53 (Ar C-H) (str.), 1741.10 (C=O), 1646.90 (C=C), 1325.05 (C-N) (str.), 13C NMR: (500MHz, CDCl3) ? 16.9 , 25.5 , 49.9 , 111.7 , 114.0 , 115.7 , 119.3 , 121.3 , 121.4 , 125.9 , 128.2 , 128.3-128.5 , 130.7 , 133.1 , 135.5 , 137.1 , 137.3 , 157.4 , 188.9 , LC/MS (ESI) m/z 331.42 (M+).
(E)-1-(2,4-dichlorophenyl)-3-(1-(3-methylbut-2-en-1-yl)-1H-indol-3-yl)prop-2-en-1-one (SIR20): M.p. 235-240°C, IR cm-1 :3106.53 (Ar C-H) (str.), 1735.19 (C=O), 1640.15 (C=C), 1336.73(C-N) (str.), 13C NMR: (500MHz, CDCl3) ? 17.9 , 25.8 , 49.0 , 109.9 , 113.0 , 118.5 , 120.9 , 121.6 , 125.6 , 128.1 , 128.3-128.5 , 128.6-128.8 , 130.9 , 132.3 , 133.0-133.3 , 136.7-136.9 , 137.1 , 192.4 , LC/MS (ESI) m/z 386.41 (M+).
(E)-1-(3-bromophenyl)-3-(1-(3-methylbut-2-en-1-yl)-1H-indol-3-yl)prop-2-en-1-one (SIR21): M.p. 230-235°C, IR cm-1:3105.43(Ar C-H) (str.), 1749.10 (C=O), 1635.15 (C=C), 1315.25 (C-N) (str.), 13C NMR: (500MHz, CDCl3) ? 17.1 , 25.1 , 53.0 , 110.7 , 112.5 , 118.4 , 118.9 , 120.9 , 121.4 , 125.1 , 128.2 , 128.3-128.5 , 129.0 , 130.0-130.2 , 132.0 , 133.1 , 136.7 , 137.6 , 140.2 , 192.9 , LC/MS (ESI) m/z 396.31 (M+).
(E)-1-(2,4-dihydroxyphenyl)-3-(1-(3-methylbut-2-en-1-yl)-1H-indol-3-yl)prop-2-en-1-one (SIR22): M.p. 235-240°C, IR cm-1:3109.59 (Ar C-H) (str.), 1741.0 (C=O), 1646.45(C=C), 1318.24 (C-N) (str.), 13C NMR: (500MHz, CDCl3) ? 17.9 , 25.8 , 49.0 , 101.1 , 108.8 , 113.0 , 115.7 , 118.7 , 120.1 , 121.5 , 121.4 , 126.7 , 127.8 , 128.2 , 128.3-128.5 , 133.1 , 136.9 , 137.1 , 160.1 , 164.8 , 191.3 , LC/MS (ESI) m/z 341.40 (M+).
(E)-1-(2-bromophenyl)-3-(1-(3-methylbut-2-en-1-yl)-1H-indol-3-yl)prop-2-en-1-one (SIR23): M.p. 225-230°C, IR cm-1:3100.53(Ar C-H) (str.), 1738.18 (C=O), 1650.74 (C=C), 1330.01 (C-N) (str.), 13C NMR: (500MHz, CDCl3) ? 18.4 , 25.5 , 53.5 , 107.7 , 112.1 , 118.6-118.8 , 120.9 , 122.4 , 125.1 , 128.1-128.3 , 128.3-128.5 , 128.6 , 132.6 , 134.2 , 136.7 , 136.1 , 141.0 , 183.6 , LC/MS (ESI) m/z 393.11 (M+).
(E)-1-(4-bromo-2-hydroxyphenyl)-3-(1-(3-methylbut-2-en-1-yl)-1H-indol-3-yl)prop-2-en-1-one (SIR24): M.p. 225-230°C, IR cm-1:3108.07 (Ar C-H) (str.), 1741.18 (C=O), 1647.15 (C=C), 1339.93 (C-N) (str.), 13C NMR: (500MHz, CDCl3) ? 17.9 , 25.8 , 49.0 , 109.7 , 112.0 , 118.1 , 118.7 , 120.1 , 120.9 , 121.4 , 122.7 , 125.1 , 127.8 , 128.2 , 128.3-128.5 , 131.7 , 133.1 , 136.7 , 137.5 , 160.0 , 191.9 , LC/MS (ESI) m/z 412.12 (M+).
Result and Discussion
Based on the computational studies, N-alkylated indole chalcone derivatives were successfully synthesized, characterized, and tested for in vitro antibacterial and antitubercular action against the H37Rv strain of Mtb. All the synthesized compounds were screened for in vitro antimicrobial activity against Salmonella bongori (MTCC no. 1167) and Bacillus subtilis (MTCC no. 441) growth in culture using well diffusion method (Reference Ciprofloxacin) at Scan Research laboratory, Bhopal Madhya Pradesh. The results of antimicrobial activity are summarized in table 2. In this research work, out of 24 compounds, 12 have shown good results against gram-negative bacteria as well as gram-positive bacteria. Compound S1R8 at 10 ?g/ml and 30 ?g/ml concentrations shows the best results of 19±0.25 mm and 24±1.99 mm, respectively, against gram-negative bacteria. In 20 ?g/ml concentration, compound SIR11 gives a maximum 24±1.99 mm zone of inhibition, which is higher than the results of standard compounds. For gram-positive bacteria, many compounds from scheme-1 show encouraging activity, Compound S1R8 shows promising zones of inhibition of 16±01mm,19±0.12mm, and 23±0.11mm against gram-positive bacteria at all three concentrations (10?g/ml,20?g/ml and 30?g/ml) respectively. The substitution of alkylating agents at the first position of indole chalcone increases the microbial inhibition. At the CSIR-Central Drug Research Institute in Lucknow, Uttar Pradesh, India, the synthesized N-alkylated indole chalcone derivative was tested for in vitro antitubercular activity against the H37Rv ATCC 27294 strain of Mycobacterium TB. The MABA technique was utilized to measure the antitubercular activity of N-alkylated indole chalcone derivatives, with isoniazid and ethambutol serving as the reference drugs. Table 3 provides an overview of the antitubercular activity results. The inclusion of various substituents in conjugation with N-alkylated indole chalcones affected the activity. With MIC values ranging from 04 to 26 g/mL, the various heterocyclic substituents demonstrated moderate to good efficacy with 60%-90% inhibition. In present work, in the structure of SIR8, 4-bromo-2-hydroxyacetophenone, substituted on position 3 of the phenyl indole chalcone ring, is responsible for antitubercular activity. The OH group forms a hydrogen bond with the target residue, which has an impact on antitubercular activity. The bromo group is an electron-withdrawing group and based on resonance, we found that the electron-withdrawing groups in the substituted phenyl rings of N-alkylated indole chalcones increased the inhibition of microbes and mycobacteria. Additionally, it may be inferred that the size of the substituents has had an impact on activity based on the diminishing inhibitory effects of halogen-substituted molecules. By using the inductive effect, all of the halogens can draw electrons from other atoms, which causes the complex to have a dipole moment. This may improve the drug's solubility in water and allow it to interact with the biologically active site. In the first position of indole chalcone derivatives, we have substituted butyl, pentyl, and 3, 3-dimethylallyl bromide alkylating agents. Butyl and pentyl substituted compounds were found to be more active than 3, 3-dimethylallyl bromide. The activity is highest when R1 is butyl and pentyl. Interestingly, reducing the chain size or expansion leads to the derivatives with lower antitubercular activity. SIR16's structural feature is pentyl substitution at the first position of the indole chalcone ring, which is less potent than the butyl chain. The third position has been replaced with 4-bromo-2-hydroxyacetophenone, which has antitubercular activity. S1R2 and S1R10 are bromine-containing indole chalcone compounds with butyl and pentyl substitutions at the first positions, respectively. Both compounds have shown moderate activity against Mycobacterium tuberculosis. S1R1 and S1R9 are N-alkylated indole chalcone derivatives substituted with fluorine groups. In compounds S1R1 and S1R9, the first position is substituted with a butyl and a pentyl chain, respectively. Compound S1R3 are phenyl OH substituted indole chalcone derivatives and OH group has ability to form H-bonds, the OH group in S1R3 has an electron-donating group that can be associated with the target. As well as the Butyl substitution at the first position of compound S1R3 is required for anti-tubercular activity. The S1R11 has shown a 15 µg/mL MIC value against tuberculosis, it contains a bromo substituted phenyl ring in the third position, which alters the antitubercular activity. The pentyl substituted first position of compound SIR11 has a minor effect on TB activity. The S1R24 has shown a 14 µg/mL MIC value. It is substituted with 4-bromo-2-hydroxyacetophenone, substituted on position 3 of the phenyl indole chalcone ring, and is responsible for antitubercular activity. The OH group forms a hydrogen bond with the target residue, which has an impact on antitubercular activity. The bromine group is an electron-withdrawing group responsible for resonance. S1R17 and SIR18 are substituted with fluorine and bromine groups at the para position of the phenyl ring in the third position of indole chalcone derivatives, which is responsible for anti-tubercular activity, but at the first position, they contain substituted allyl bromides, which have a lower response against Mtb. The additional objectives, such as preventing the undesired reaction at the nitrogen itself, acidic oligomerization, and avoiding the addition reaction at the 2, 3 double bonds of the indole system, are achieved by a different phenacyl bromide substitution at the first position. The antitubercular action of derivatives of synthesized indole chalcones is significantly influenced by N-alkylation. We found that the Mycobacterium TB reacts inconsistently to indole chalcone derivatives with a substituted phenyl ring in the first position (N-alkylation). In all the schemes, we designed 24 compounds, and based on the computational results, synthesized only 24 compounds, and evaluated them against tuberculosis activity. Compound S1R8 has shown good activity (MIC value 04µg/mL) against the mycobacterium tuberculosis. The MIC value of S1R8 is not as high as that of isoniazid, but when compared to other reference drugs, we discovered that the compound had a similar MIC value to ethambutol.
CONCLUSION
The effectiveness of a library of N-alkylated indole chalcone derivatives against the H37Rv strain of Mycobacterium TB ATCC 27294 was docked, synthesized, and evaluated. Among them, two substances (E)-1-(4-bromo-2-hydroxyphenyl)-3-(1-butyl-1H-indol-3-yl)prop-2-en-1-one (S1R8) and (E)-1-(4-bromo-2-hydroxyphenyl)-3-(1-pentyl-1H-indol-3-yl)prop-2-en-1-one (S1R16) demonstrated anti-tubercular action. The activity is related to the capacity of N-alkylation indole chalcone derivatives to bind metal atoms necessary for mycobacterium as well as the presence of electron-withdrawing groups in these compounds. Compound S1R8 demonstrated binding mechanisms similar to those of FAS-II inhibitors like INH, according to docking experiments to comprehend how N-alkylated indole chalcones bind.
ACKNOWLEDGMENTS
The authors are thankful to the Department of Pharmacy, Shri G. S. Institute of Technology and Science, Indore, Madhya Pradesh, India, for providing required research facility, guidance, and support for the completion of the present research work.
REFERENCES
- Ruiz-Tagle C, Ugalde JA, Naves R, Araos R, García P, Balcells ME.: Reduced microbial diversity of the nasopharyngeal microbiome in household contacts with latent tuberculosis infection. Scientific Reports 2023; 13:7301-7305.
- Zhang C, Ouyang Q, Zhou X, Huang Y, Zeng Y, Deng L: In vitro activity of tetracycline analogs against multidrug-resistant and extensive drug resistance clinical isolates of Mycobacterium tuberculosis. Tuberculosis (Edinburgh, Scotland) 2023;140; 1023-36.
- Boshoff Helena I. M.: Drug-resistant Mycobacterium tuberculosis. Frontiers in Cellular and Infection Microbiology 2023: 13: 1-4.
- Jessy Lallungawi Khawbung: Drug resistant Tuberculosis: A review. Comparative Immunology, Microbiology and Infectious Diseases 2021: 74:18-28.
- Porwal A. : Indole Moiety in Organic Synthesis: A Comprehensive Review of Methods and Mechanisms. International Journal of Pharmaceutical Investigation 2024: 14 : 1052-1060.
- Sharma PK, Kumar M. :Synthesis of bioactive substituted pyrazolylbenzothiazinones. Research on Chemical Intermediates 2020: 41,6141-8.
- Sarkar D, Amin A, Qadir T, Sharma P. :Synthesis of Medicinally Important Indole Derivatives:. The Open Medicinal Chemistry Journal 2021:15:1-16.
- Shirude PS. :Azaindoles: Noncovalent DprE1 Inhibitors from Scaffold Morphing Efforts, Kill Mycobacterium tuberculosis and Are Efficacious in Vivo. Journal of Medicinal Chemistry 2022: 56 :9701-8.
- Vitaku E, Smith DT, Njardarson JT: Analysis of the Structural Diversity, Substitution Patterns, and Frequency of Nitrogen Heterocycles among U.S. FDA Approved Pharmaceuticals. Journal of Medicinal Chemistry 2021:57:10257-74
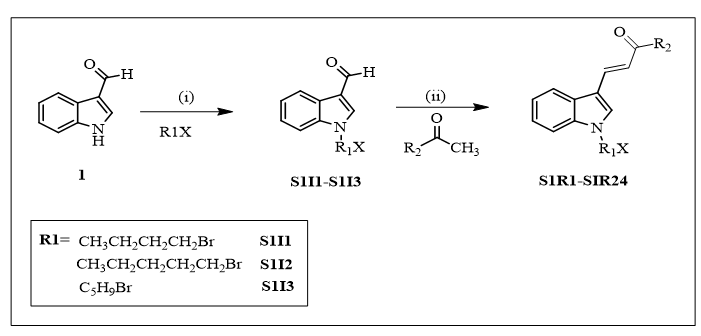
Scheme 1: Reagents and conditions: (i) alkylating agents, potassium carbonate, KOH, DMF, M.W. 130-145°C, 40-45Min. (ii) ethanol, sodium hydroxide 40% solution, acetophenones, 100-120°C 5-15Min/RT stirred for 2-6 hrs.
Reaction condition for intermediate S1I3, 30 min stirring at 0°C.
Scheme-1 R1 and R2 substitution with appearance of compound.
|
S.no
|
Compound code
|
R1
|
R2
|
Appearance
|
|
1
|
S1R1
|

|

|
Yellow
|
|
2
|
S1R2
|

|

|
Yellow
|
|
3
|
S1R3
|

|

|
Yellow
|
|
4
|
S1R4
|

|

|
Brownish
|
|
5
|
S1R5
|

|

|
Yellow
|
|
6
|
S1R6
|

|

|
Brownish
|
|
7
|
S1R7
|

|

|
Yellow
|
|
8
|
S1R8
|

|

|
Red
|
|
9
|
S1R9
|

|

|
Yellow
|
|
10
|
S1R10
|

|

|
Yellow
|
|
11
|
S1R11
|

|

|
Yellow
|
|
12
|
S1R12
|

|

|
Yellow
|
|
13
|
S1R13
|

|

|
Yellow
|
|
14
|
S1R14
|

|

|
Brownish
|
|
15
|
S1R15
|

|

|
Yellow
|
|
16
|
S1R16
|

|

|
Red
|
|
17
|
S1R17
|

|

|
Yellow
|
|
18
|
S1R18
|

|

|
Yellow
|
|
19
|
S1R19
|

|

|
Yellow
|
|
20
|
S1R20
|

|

|
Yellow
|
|
21
|
S1R21
|

|

|
Yellow
|
|
22
|
S1R22
|

|

|
Brownish
|
|
23
|
S1R23
|

|

|
Yellow
|
|
24
|
S1R24
|

|

|
Red
|
Table 2: Antimicrobial activity of N-alkylated indole chalcone derivatives.
|
S.no
|
Sample code
|
Zone of Inhibition (mm)
|
|
Salmonella bongori
|
Bacillus subtilis
|
|
10 ?g/ml
|
20 ?g/ml
|
30 ?g/ml
|
10 ?g/ml
|
20 ?g/ml
|
30 ?g/ml
|
|
1
|
S1R1
|
18±0.17
|
24±0.01
|
25±0.95
|
13±0
|
18±0.23
|
21.3±0.24
|
|
2
|
S1R2
|
18±0.07
|
23±0.90
|
25±0.6
|
14±0
|
19±0
|
22±0
|
|
3
|
S1R3
|
17±0.6
|
22±0.23
|
25±0.66
|
13±0
|
18±0
|
21±0.66
|
|
4
|
S1R4
|
11±0
|
12±0
|
15.3±3.77
|
6±0
|
6±0
|
6±0
|
|
5
|
S1R5
|
10±0
|
12±0
|
14±0
|
8±0
|
10±0
|
11±0
|
|
6
|
S1R6
|
11±0
|
13±0
|
15±0
|
6±0
|
6±0
|
6±0
|
|
7
|
S1R7
|
10±0
|
12±0
|
13±0
|
10±0
|
12±0
|
13±0
|
|
8
|
S1R8
|
19±0.25
|
23±0.96
|
26±1.94
|
16±01
|
19±0.12
|
23±0.11
|
|
9
|
S1R9
|
18±0
|
23±0.96
|
25.6±1.49
|
13±0
|
18±0
|
21±0
|
|
10
|
S1R10
|
18±0
|
24±0
|
25±2.16
|
12±0.75
|
19±0
|
21±0.30
|
|
11
|
S1R11
|
18±0
|
24±1.99
|
26±1.05
|
15.6±3.29
|
17±1.63
|
21±2.16
|
|
12
|
S1R12
|
10±0
|
11±0
|
13±0
|
10±0
|
11±0
|
13±0
|
|
13
|
S1R13
|
19±0
|
23±0.99
|
24±0.99
|
19.3±2.62
|
17.3±1.24
|
20±0.50
|
|
14
|
S1R14
|
6±0
|
6±0
|
6±0
|
12±0
|
14±0
|
11±0
|
|
15
|
S1R15
|
8±0
|
12±0
|
12±0
|
9±0
|
10±0
|
13±0
|
|
16
|
S1R16
|
18±0.5
|
23±0.9
|
26±0.10
|
14±0
|
18±0
|
22.6±.22
|
|
17
|
S1R17
|
18±0
|
23±0.85
|
25±0.78
|
15±0
|
18±0
|
21.6±1.24
|
|
18
|
S1R18
|
18±0
|
24±0
|
25±0.75
|
14±0
|
17±0.78
|
22.3±1.24
|
|
19
|
S1R19
|
7±0
|
11±0
|
13±0
|
10±0
|
12±0
|
13±0
|
|
20
|
S1R20
|
7±0
|
12±0
|
14±0
|
10±0
|
11±0
|
13±0
|
|
21
|
S1R21
|
8±0
|
11.±0
|
13±0
|
10±0
|
11.±0
|
13±0
|
|
22
|
S1R22
|
17±0.11
|
23±1.23
|
25±0.35
|
13±0.3
|
18±0.12
|
21±0
|
|
23
|
S1R23
|
10±0
|
12±0
|
14±0
|
7±0
|
11.3±1.24
|
13±0
|
|
24
|
S1R24
|
18±0
|
24±0
|
25±0.51
|
15±0
|
18±0
|
21±0.22
|
|
25
|
STD (Cip.)
|
17±0.15
|
23±0.86
|
25±0.5
|
12±0.5
|
17±0.74
|
20±0.15
|
Table 3: Antitubercular activity of N-alkylated indole chalcone derivatives.
|
S.no
|
Compound codes
|
MIC value (µg/mL)
|
|
1
|
SIR1
|
12
|
|
2
|
S1R2
|
11
|
|
3
|
S1R3
|
12
|
|
4
|
S1R8
|
04
|
|
5
|
S1R9
|
14
|
|
6
|
S1R10
|
13
|
|
7
|
S1R11
|
15
|
|
8
|
S1R13
|
25
|
|
9
|
S1R16
|
06
|
|
10
|
S1R17
|
26
|
|
11
|
S1R18
|
25
|
|
12
|
S1R22
|
16
|
|
13
|
S1R24
|
14
|
|
14
|
INH
|
0.03
|
|
15
|
ETM
|
08
|


 Shivam Joshi *
Shivam Joshi *
 Neha Kawathekar
Neha Kawathekar

 10.5281/zenodo.14516472
10.5281/zenodo.14516472