Abstract
Carbazole moiety is the key structural motif of many biologically active compounds including synthetic and natural products. Over the past several years, a large number of research highlighting the significance of carbazole derivatives has been reported in the literature. The present review focuses on the recent synthetic process, and their various biological properties of classical, tricyclic carbazole derivatives.
Keywords
Carbazoles, Vibrant, Medicinal, Synthetic
Introduction
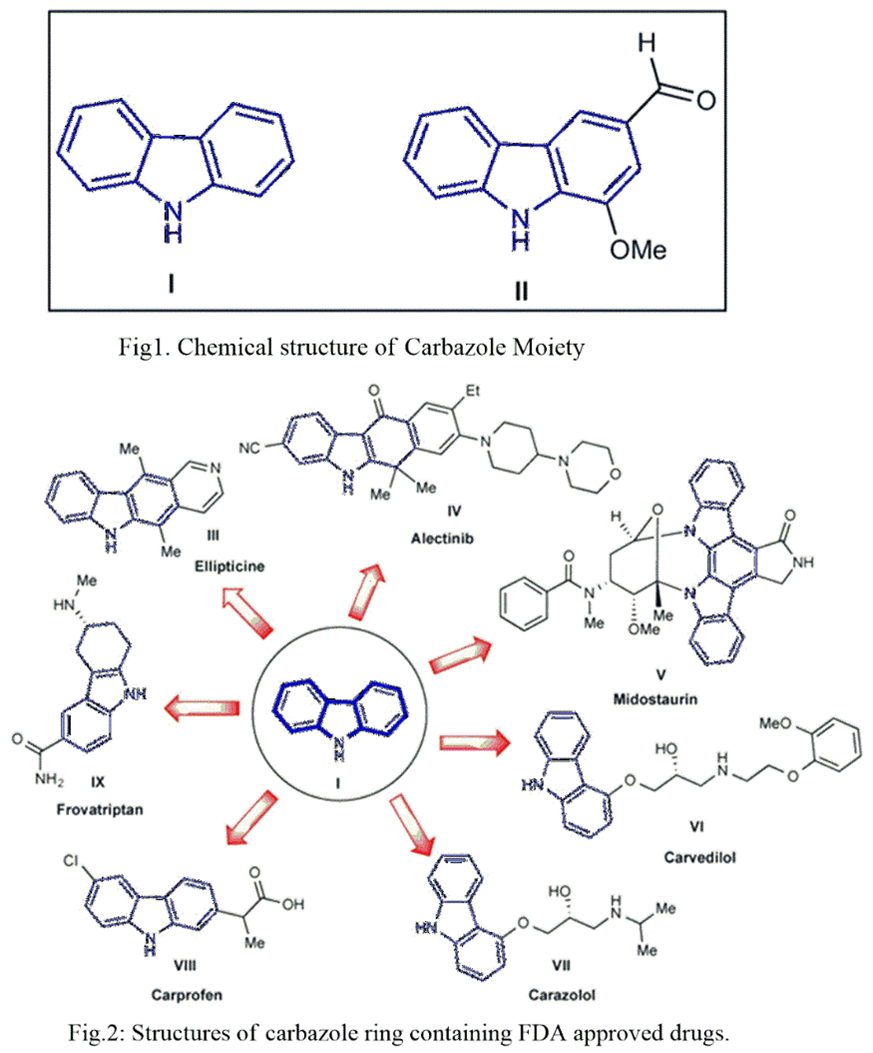
9H-carbazole (Fig.1) is an aromatic molecule that is tricyclic in nature, with two benzene rings fused onto a 5-membered pyrrole ring. Obtained from natural sources or by synthetic routes, this scaffold has gained much interest due to its wide range of biological activity upon modifications, including antibacterial, antimalarial, anticancer, and anti-Alzheimer properties. It is a tricyclic compound with the carbon skeleton of fluorene, occurring in coal tar. Due to its fluorescent properties, carbazole ring system is a structural element of many compounds used in electronics to produce electroluminescent materials [1-4]. The Carbazole ring is present in a variety of naturally occurring medicinally active substances viz. carbazomycins and murrayafoline A. Various carbazole derivatives such as benzocarbazoles, oxazinocarbazoles, tetrahydrocarbazoles, furo-carbazoles, pyridocarbazoles, pyrrolocarbazoles, indolocarbazoles, oxazolinylcarbazoles, thienocarbazoles, imidazocarbazoles, thiazolocarbazoles , benzopyrano-carbazoles, benzofurano-carbazoles and N-substituted carbazoles have been synthesized and are well known for their pharmacological activities. Several research studies are focused on carbazole nucleus due to their wide applicability. Carbazole derivatives are promising drug candidate for the treatment of various diseases [5-6]. Carbazole (I) was first isolated by Graebe and Glazer in 1872 from coal tar (Fig.1). The carbazole analog, murrayanine (1-methoxy-3-formylcarbazole) (II), was isolated from the stem-back of Murraya koenigii Spreng by Chakraborty, Barman, and Bose in 1965. Therapeutically important natural carbazoles have inspired synthetic medicinal chemists to design and develop novel (semi)synthetic carbazole derivatives. The carbazole moiety is present in several important commercially available drug molecules such as ellipticine (III), alectinib (IV), midostaurin (V), carvedilol (VI), carazolol (VII), carprofen (VIII), and frovatriptan (IX). The naturally occurring alkaloid ellipticine (III) was first discovered in 1959 as an anticancer agent. It was extracted from the leaves of Ochrosia elliptica and is one of the initial leading therapeutic carbazole analogues for the treatment of cancer. Alectinib (IV) was approved by the FDA in 2015 for the treatment of advanced non-small cell lung cancer (NSCLC). Midostaurin (V) was permitted by the FDA in 2017 for the treatment of newly diagnosed acute myeloid leukemia (AML) and for advanced systemic mastocytosis (SM) [7-11] (Fig.2).
2. Synthetic Strategies of Carbazoles
Carbazole is an aromatic heterocyclic organic compound. It has a tricyclic structure, consisting of two six-membered benzene rings fused on either side of a five-membered nitrogen-containing ring, with a large, aromatic system and a central nitrogen atom showing extensive electron delocalization. The Borsche–Drechsel cyclization (Fig.3) is a chemical used to synthesize tetrahydrocarbazoles by the acid-catalyzed cyclization of cyclohexanone arylhydrazones. Here, the acid-catalyzed proton transfer first converts the cyclohexanone phenylhydrazone 1 to the intermediate 2. Subsequently, a heat-induced sigma tropic reaction occurs to produce 3, which is protonated and cyclizes into 4. Elimination of ammonia then leads to the final product, the tetrahydrocarbazoles 5. Another method for the synthesis of carbazole is the Graebe–Ullmann reaction (Fig. 4). In the first step, an N-phenyl-1,2-diaminobenzene (N-phenyl-o-phenylenediamine) 6 is converted into a diazoniumsalt 7 which instantaneously forms a 1,2,3-triazole. The triazole is unstable and at elevated temperatures, nitrogen is released and the carbazole 8 is formed [12]. A palladium-catalyzed reaction sequence consisting of an intermolecular amination and an intramolecular direct arylation enabled highly regioselective syntheses of functionalized indoles or carbazoles (Fig.5) and proved to be amenable to the use of inexpensive 1,2-dichloroarenes as electrophiles [13]. Ultrasmall nanoclusters offer a high surface area and unsaturated active sites. A copper nanocluster-based catalyst enables C-N bond-forming reactions of aryl chlorides under visible-light irradiation at room temperature. A range of N-heterocyclic nucleophiles and electronically and sterically diverse aryl/hetero chlorides react to provide C-N coupling products in good yields (Fig.6) [14]. NH4I promotes an efficient indole-to-carbazole strategy under metal-free conditions. The reaction offers high regioselectivity through formal [2 + 2 + 2] annulation of indoles, ketones, and nitroolefins and enables the assembly of many diversified carbazoles with good functional group tolerance (Fig.7) [15]. An efficient palladium-catalyzed tandem reaction for the one-pot synthesis of 9H-carbazoles under microwave irradiation is developed. This approach involves a sequential Buchwald–Hartwig amination and a direct arylation from affordable and inexpensive anilines and 1,2-dihaloarenes. For the development of this purpose, a novel and magnetically recoverable palladium nano catalyst supported on a green biochar under ligand-free conditions is used. Compared to other existing palladium-based protocols, the present synthetic methodology shows a drastic reduction in reaction times and excellent compatibility with different functional groups allowing to obtain a small library of 9H-carbazoles in high yields and with good regioselectivity. This procedure represents the first example in the direct synthesis of carbazoles using a heterogeneous palladium nano catalyst from commercial precursors. (Fig.8) [16]. An efficient Cu-catalyzed synthesis of carbazole derivatives is reported, which proceeds by double C–N coupling reactions of 2,2?-dibromobiphenyl and amines in the presence of air. The reaction is robust, proceeds in high yields, and tolerates a series of amines including neutral, electron-rich, electron-deficient aromatic amines and aliphatic amines (Fig.9) [17].

3.Pharmacological Implication
The carbazole moiety exhibits a wide range of pharmacological activities due to its high electron delocalization, resulting in favourable physicochemical properties. Derivatives containing the carbazole pharmacophore are associated with various therapeutic effects, including anti-Alzheimer, antioxidant, antidiabetic, anticancer, anticonvulsant, antimicrobial, and anti-inflammatory activities. These effects are achieved by specifically targeting molecular level proteins and factors such as RAS-MAPK, DAXX, ASK-1, AKT, and JNK, either through inhibition or activation via de-phosphorylation or phosphorylation. As a result of these significant activities, the carbazole nucleus has garnered attention from researchers in the quest for novel carbazole derivatives [18].
Anticancer Potential
Huang et al. explored new chemical entities containing carbazole scaffold as potential novel cytotoxic agents based on our developed three-component indole-to-carbazole reaction. Two series of carbazole derivatives were designed and synthesized, and their in vitro cytotoxic activities against three cell lines (A875, HepG2, and MARC145) were evaluated. The results indicated that some of these carbazole derivatives exhibited significantly good cytotoxic activities against tested cell lines compared with the control 5-fluorouracil (5-FU). Especially, carbazole acyl hydrazone compounds 9 and 10 displayed high inhibitory activity on cancer cells, but almost no activity on normal cells [19]. Based on the efficacy of EHop-016 as an inhibitor of migration and Rac1 activation, a new series of carbazole derivatives has been synthesized by Vlaar et.al. Preliminary investigations of their anticancer activity demonstrated that several compounds have moderate antiproliferative effects on cancer cell lines with GI50 values in the range of 13–50?µM. Furthermore, compound 11 inhibit migration activity of metastatic cell line MDA-MB-231 by 32% respectively. Compound 11 was shown to inhibit activation of the Rho GTPase Rac1 by 55% at 250?nM in both MDA-MB-231 and MDA-MB-435 cell lines. Compared with the IC50 of Rac1 inhibition by lead compound EHop-016 of 1.1?µM, compound 11 demonstrates 4X improved in vitro efficacy [20]. A series of N-substituted carbazoles synthesized by Akue-Gedu et al. have been studied for their antiproliferative activity. These N-substituted pyrrolocarbazoles 12, 13 were found to be most potent inhibitors for pim-kinase activity with IC50 in the nanomolar range (46–75 nM) and have demonstrated antiproliferative activities against three human cancer cell lines, PA1 (ovarian carcinoma) PC3 and DU145 (prostatic carcinoma) with MIC values in the range of 8–20 µM [21]. Giraud et al. [22] have synthesized N1-N10-bridged pyrrolo [2,3-a] carbazoles. The ability of these compounds to inhibit pim-kinases has been evaluated. The compounds 5,6-dihydro-4H-indolo[1,2,3-ef] pyrrolo[3,2,1-jk][1,5] benzodiazepine-1-carbaldehyde (14) and 5,6,7,8-tetrahydro-4H-indolo[1,2,3-gh] pyrrolo[3,2,1-lm][1,5] benzodiazonine-1-carbaldehyde (15) showed potent activity with nanomolar inhibitory potencies against the acute myeloid leukemia IPC-81 cell line, which is a good predictor of leukaemia therapy. The 1-carbazole-9-yl-2-(substituted phenyl)-1,4-dihydroimidazo [4,5] indole-1-yl-amino-ethanones synthesized by Kumar et al. [23] have been evaluated for antitumor potential for laryngeal carcinoma cell lines (HEP2) and Ehrlich’s Ascites Carcinoma (EAC) cells. The compounds 16 were found to be active against tumor cell lines. The activity may be attributed due to the presence of electron donating group which may increase the basicity of the compound.

Antifungal Activity
Fungal infections (mycosis) are increasing throughout the world. Although there are several reasons for this higher increase in mycosis, immune modulation of the host is one of the foremost risk factors for intrusive mycosis. Tang et al. tested the antifungal activity of all the synthesized compounds against different fungal strains and found that compound 17 (2-(9H-carbazol-9-yl)-N- (4,5-dihydro-5-(thiophen-2-yl)-1,3,4-thiadiazol-2-yl) acetamide) and 18 (2-(1-chloro-9H-carbazol-9-yl)- N-(4,5-dihydro-5-p-tolyl-1,3,4-thiadiazol-2-yl)acetamide) exhibited the most potent inhibitory activity against C. wilt having inhibition rates 72.40% and 67.65% [24]. Shaikh et al. synthesized two series of carbazole analogues. First one is 8-methoxy-N-substituted9H-carbazole-3-carboxamides, and the second one is carbazolyl-substituted rhodamines. Various substituents, nitrogen-containing heterocyclic systems like pyrimidinyl and piperidinyl on carbazole nucleus exerted a significant antifungal activity in the first series of compounds. 2-Methyl piperidinyl carboxamide derivative compound (8-Methoxy-9H-carbazol-3-yl) (2-methylpiperidin-1-yl) methanone found as potent antifungal and antibacterial agent. In second series substitution at 3 positions of rhodamines which is conjugated to -methyl carbazole through an acrylidine linkage, the result showed the efect of bioisosteres coumarin present in compound 19 [(5Z)-3-(4-methyl-2-oxo-2H-chromen-7-yl)-5-((9-methyl9H-carbazol-6-yl) methylene)-2-thioxothiazolidin-4-one] were shown potent inhibitory activity. It was concluded that electron donating groups such as hydroxy, alkoxy, and alkyl groups on the aromatic ring greatly contributed toward the antifungal and antibacterial activity [25]. Novel carbazole derivatives of 2-(9H-carbazol-9-yl)-N-(5-phenyl-1,3,4-thiadiazol-2-yl) acetamide were designed, synthesized, and evaluated for their antifungal activities against Pellicularia sasakii (P.sasakii), Fusarium oxysporum (F. oxysporum), Gibberella zeae (G.zeae), Phytophthora infestans (P.infestans), Cytospora mandshurica (C. mandshurica) and Capsicum wilt (C. wilt). The results of antifungal activity tests indicated that the inhibitory rates of compound 2-(2-chloro-9H-carbazol-9-yl)-N-(5-(thiophen-2-yl)-1,3,4-thiadiazol-2-yl) acetamide against P. sasakii was 74.29%, which displayed better bioactivity compared with commercial fungicides hymexazol (53.09%) and carbendazim (69.65%) [26]. Xue et al. synthesized novel carbazole derivatives containing an aminoguanidine, dihydrotriazine, thiosemicarbazide, semicarbazide and isonicotinic moiety and evaluated for their antimicrobial activities. Structure–activity relationship analyses and docking studies implicated the dihydrotriazine group in increasing the antimicrobial potency and reducing the toxicity of the carbazole compounds. In vitro enzyme activity assays suggested that the compounds binding with dihydrofolate reductase might account for the antimicrobial effect [27].

Anti-Inflammatory Potential
Inflammation is a result of a complex biological response that includes inflammatory mediators, sensors, inflammation-inducing factors, and affected target tissues. Inflammatory pathways are mediated by some inflammatory mediators like cyclooxygenase (COX), chemokines, vasoactive amines nitric oxide (NO), etc. Bandgar et al. designated a novel series of carbazoles and tested against the anti-inflammatory activity. Compound 20 via hydrophobic interactions potently binds at the site of COX-II. The oxygen atom of the methoxy group on compound 20 formed two hydrogen bonds with the Asp B:225 and Nag C:671 and the nitrogen atom present in the pyrazoline ring on compound 20 formed two hydrogen bonds with the Leu A:131. Carbazole derivatives inhibit the lipopolysaccharide-induced inflammatory mediator production in macrophages via suppression of p38 MAPK. Carbazoles inhibit the formation of TNF-? (tumor necrosis factor ?), PGE2 (prostaglandin E2), and nitric oxide (NO) induced by Lipopolysaccharide [28]. Chemical investigation of the traditional Chinese medicine, Murraya kwangsiensis, led to the isolation of undescribed biscarbazole alkaloids, kwangsines, two undescribed natural products, (+/?)-bispyrayafoline C, and known monomeric analogues. (±)-Bispyrayafoline C and (±)-kwangsines 21-24 are four pairs of biscarbazole atropisomers, and they were separated by chiral HPLC to obtain the optically pure compounds. The structures of the undescribed compounds were elucidated based on HRESIMS and NMR data analysis. Their absolute configurations were assigned via comparison of the specific rotation, ECD exciton coupling method, as well as comparison of experimental and calculated ECD data. A compound showed significant inhibition on NO production in lipopolysaccharide-stimulated BV-2 microglial cells, and four compounds exhibited moderate cytotoxicities against HepG2 cells, with IC50 values less than 20??M [29].

Anti-Diabetic Potential
Adib et al. synthesized carbazole–imidazoles complex and screened as new ?-glucosidase inhibitors. All the synthesized fused carbazole-imidazoles were found to be more active than acarbose (IC50 = 750.0 ± 1.5 µM) against yeast ?-glucosidase with IC50 values in the range of 74.0 ± 0.7–298.3 ± 0.9 µM. Kinetic study of the most potent compound 25 demonstrated that this is a competitive inhibitor for ?-glucosidase (Ki = 75 µM). Furthermore, the in-silico studies of the most potent compounds 25 confirmed that these compounds interacted with the key residues in the active site of ?-glucosidase [30]. Accordingly, in 2016, Wang et al. developed a small series of triazines, used as different starting substitute carbazoles, to identify novel ?-glucosidase inhibitors. A number of the newly synthesized compounds showed noteworthy activity against the enzyme. Compound 26 (1-((5,6-di(furan-2-yl) -1,2,4-triazin-3-yl) thio)-3-(3,6-dibromo-9H-carbazol-9-yl) propan-2-ol)) was found to be the most promising, with IC50 values of 4.27 ± 0.07 µM, significantly higher than the control drug acarbose (IC50 = 995.55 ± 2.71 µM) [31]. Zhang et al., in 2018, developed a new series of tetrahydrocarbazoles and tested them in an in vitro assay on human hepatoma cell lines (HepG2) to assess their hypoglycemic activity. Several of the compounds tested exhibited significant activity, with carboxyl (27), 6-(benzyloxy)-9-(chlorobenzoyl)-2,3,4,9-tetrahydro-1H-carbazole-3-carboxylic acid being the most promising [32]. Identification of compound (S)-3-(4-(2-(9H-carbazol-9-yl)-ethoxy) phenyl)-2-ethoxypropanoic acid (28), which showed dual activity on both PPAR isoforms ? and ?. The results of an oral glucose tolerance test (OGTT) showed that treatment with compound 28 promoted an improvement in insulin sensitivity, greater than that seen with both pioglitazone and rosiglitazone. Compound 28 was also able to lower plasma concentrations of triglycerides and cholesterol in rats fed high cholesterol, whereas treatment with other PPAR? agonists did not have the same results [33].

Anti-alzheimer Potential
Mishra et al. explored multi-functional activity of carbazole derivatives, have been assessed by performing various in-vitro assays and these compounds appeared to be potent AChE inhibitors, A? aggregation inhibitors, anti-oxidant, and neuroprotective agents. Among the entire series, 29 and 30 were most potent multifunctional agents which displayed effective and selective AChE inhibition, A? disaggregation, anti-oxidant, and metal chelation action [34].

FUTURE ASPECTS
Carbazole moiety itself is responsible for various types of pharmacological activities; due to high electron delocalization, it shows better physicochemical properties. Derivatives having carbazole pharmacophore are responsible for various therapeutic activities like anti-Alzheimer, antidiabetic, anticancer, antimicrobial, and anti-inflammatory, etc., by specifically acting on potential molecular level proteins and factors such as RAS-MAPK, DAXX, ASK-1, AKT, and JNK, either by inhibiting or by activating them by de-phosphorylation or phosphorylation. Due to all these important activities, the carbazole nucleus has attracted the attention of researchers in the discovery of other novel derivatives of carbazole. Overall, this review underscores the significance of carbazole derivatives as a class of compounds with tremendous potential for diverse applications. We hope that this review will motivate and inspire researchers to explore new avenues for the synthesis and application of carbazole derivatives, thus contributing to the development of innovative materials with novel properties and functionalities.
CONFLICTS OF INTEREST
The authors declare no conflict of interest.
REFERENCES
- K.R.J. Thomas, J.T. Lin, Y.-T. Tao, C.-W. Ko, J. Am. Chem. Soc. 123 (2001) 9404-9411.
- A. Van Dijken, J.J.A.M. Bastiaansen, N.M.M. Kiggen, B.M.W. Langeveld, C. Rothe, A. Monkman, I. Bach, P. Stossel, K. Brunner, J. Am. Chem. Soc. 126 (2004) 7718-7727.
- H.-y. Fu, H.-r. Wu, X.-y. Hou, F. Xiao, B.-x. Shao, Synth. Met. 156 (2006) 809-814.
- A. Ryan, B. Tuffy, S. Horn, W.J. Blau, M.O. Senge, Tetrahedron 67 (2011) 8248-8254.
- Gluszynska.A, Biological potential of carbazole derivativs, European Journal of Medicinal Chemistry, 2015,94, 405-426
- Chaudhary, Bashir.M, Bano.A, Recent Developments and Biological Activities of N-Substituted Carbazole Derivatives: A Review, Molecules, 2015, 20, 13496-13517
- Patil, S. A., Patil, S. A., Ble-González, E. A., Isbel, S. R., Hampton, S. M., & Bugarin, A. (2022). Carbazole derivatives as potential antimicrobial agents. Molecules, 27(19), 6575.
- Garbett N.C., Graves D.E. Extending nature’s leads: The anticancer agent ellipticine. Curr. Med. Chem. Anticancer Agents. 2004; 4:149–172.
- Ruiz-Ceja K.A., Chirino Y.I. Current FDA-approved treatments for non-small cell lung cancer and potential biomarkers for its detection. Biomed. Pharmacother. 2017; 90:24–37
- Chakraborty D.P., Barman B.K., Bose P.K. On the constitution of murrayanine, a carbazole derivative isolated from Murraya koenigii Spreng. Tetrahedron. 1965; 21:681–685.
- Knölker H.-J., Reddy K.R. Isolation and synthesis of biologically active carbazole alkaloids. Chem. Rev. 2002; 102:4303–4428.
- Bremer, O. (1934). Über die Bedeutung der Graebe?Ullmannschen Carbazolsynthese und deren Übertragung auf N?substituierte Pyridino?triazole. Justus Liebigs Annalen der Chemie, 514(1), 279-291.
- Ackermann, L., Althammer, A., & Mayer, P. (2009). Palladium-catalyzed direct arylation-based domino synthesis of annulated N-heterocycles using alkenyl or (hetero) aryl 1, 2-dihalides. Synthesis, 2009(20), 3493-3503.
- Sagadevan, A., Ghosh, A., Maity, P., Mohammed, O. F., Bakr, O. M., & Rueping, M. (2022). Visible-light copper nanocluster catalysis for the C–N coupling of aryl chlorides at room temperature. Journal of the American Chemical Society, 144(27), 12052-12061.
- Chen, S., Li, Y., Ni, P., Huang, H., & Deng, G. J. (2016). Indole-to-carbazole strategy for the synthesis of substituted carbazoles under metal-free conditions. Organic letters, 18(20), 5384-5387.
- Steingruber, H. S., Mendioroz, P., Volpe, M. A., & Gerbino, D. C. (2021). Convenient One-Pot Synthesis of 9H-Carbazoles by Microwave Irradiation Employing a Green Palladium-Based Nanocatalyst. Synthesis, 53(21), 4048-4058.
- Do, H. N., Quan, N. M., Van Phuc, B., Van Tinh, D., Tien, N. Q., Nga, T. T. T., ... & Langer, P. (2021). Efficient Copper-Catalysed Synthesis of Carbazoles by Double N-Arylation of Primary Amines with 2, 2?-Dibromobiphenyl in the Presence of Air. Synlett, 32(06), 611-615.
- Tiwari, A., & Mishra, B. (2024). Diverse pharmacological actions of potential carbazole derivatives by influencing various pathways of molecular signaling. Future Journal of Pharmaceutical Sciences, 10(1), 77.
- Huang, W., Gao, Z., Zhang, Z., Fang, W., Wang, Z., Wan, Z., ... & Ke, S. (2021). Selective and effective anticancer agents: Synthesis, biological evaluation and structure–activity relationships of novel carbazole derivatives. Bioorganic Chemistry, 113, 104991.
- Vlaar, C. P., Castillo-Pichardo, L., Medina, J. I., Marrero-Serra, C. M., Vélez, E., Ramos, Z., & Hernández, E. (2018). Design, synthesis and biological evaluation of new carbazole derivatives as anti-cancer and anti-migratory agents. Bioorganic & medicinal chemistry, 26(4), 884-890.
- Akué-Gédu, R., Letribot, B., Saugues, E., Debiton, E., Anizon, F., & Moreau, P. (2012). Kinase inhibitory potencies and in vitro antiproliferative activities of N-10 substituted pyrrolo [2, 3-a] carbazole derivatives. Bioorganic & medicinal chemistry letters, 22(11), 3807-3809.
- Giraud, F., Bourhis, M., Nauton, L., Théry, V., Herfindal, L., Døskeland, S. O., ... & Moreau, P. (2014). New N-1, N-10-bridged pyrrolo [2, 3-a] carbazole-3-carbaldehydes: synthesis and biological activities. Bioorganic Chemistry, 57, 108-115.
- Kumar, N., Sharma, G. K., & Pathak, D. (2013). Microwave assisted and parallel synthesis of novel substituted carbazole derivatives of biological interest. Int. J. Pharm. Chem. Sci, 2, 273-282.
- Tang, C., Chen, X., Yang, S., Guo, W., Yang, X., Li, P., & Wang, X. (2023). Discovery of novel carbazole derivatives containing a 1, 3, 4-thiadiazole moiety as antifungal candidates. Phosphorus, Sulfur, and Silicon and the Related Elements, 198(8), 627-631.
- Shaikh, M. S., Chandrasekaran, B., Palkar, M. B., Kanhed, A. M., Kajee, A., Mlisana, K. P., ... & Karpoormath, R. (2020). Synthesis and biological evaluation of novel carbazole hybrids as promising antimicrobial agents. Chemistry & Biodiversity, 17(5), e1900550.
- Tang, C., Chen, X., Yang, S., Guo, W., Yang, X., Li, P., & Wang, X. (2023). Discovery of novel carbazole derivatives containing a 1, 3, 4-thiadiazole moiety as antifungal candidates. Phosphorus, Sulfur, and Silicon and the Related Elements, 198(8), 627-631.
- Xue, Y. J., Li, M. Y., Jin, X. J., Zheng, C. J., & Piao, H. R. (2021). Design, synthesis and evaluation of carbazole derivatives as potential antimicrobial agents. Journal of Enzyme Inhibition and Medicinal Chemistry, 36(1), 296-307.
- Bandgar, B. P., Adsul, L. K., Chavan, H. V., Jalde, S. S., Shringare, S. N., Shaikh, R., ... & Masand, V. (2012). Synthesis, biological evaluation, and docking studies of 3-(substituted)-aryl-5-(9-methyl-3-carbazole)-1H-2-pyrazolines as potent anti-inflammatory and antioxidant agents. Bioorganic & medicinal chemistry letters, 22(18), 5839-5844.
- Chen, Y., Cao, N., Lv, H., Zeng, K., Yuan, J., Guo, X., ... & Jiang, Y. (2020). Anti-inflammatory and cytotoxic carbazole alkaloids from Murraya kwangsiensis. Phytochemistry, 170, 112186.
- Adib, M., Peytam, F., Shourgeshty, R., Mohammadi-Khanaposhtani, M., Jahani, M., Imanparast, S., ... & Mahdavi, M. (2019). Design and synthesis of new fused carbazole-imidazole derivatives as anti-diabetic agents: In vitro ?-glucosidase inhibition, kinetic, and in silico studies. Bioorganic & medicinal chemistry letters, 29(5), 713-718.
- Wang, G., Wang, J., He, D., Li, X., Li, J., & Peng, Z. (2016). Synthesis and biological evaluation of novel 1, 2, 4-triazine derivatives bearing carbazole moiety as potent ?-glucosidase inhibitors. Bioorganic & Medicinal Chemistry Letters, 26(12), 2806-2809.
- Zhang, J.Q.; Li, S.M.; Ma, X.; Zhong, G.; Chen, R.; Li, X.S.; Zhu, G.F.; Zhou, B.; Guo, B.; Wu, H.S.; et al. Discovery of Tetrahydrocarbazoles with Potent Hypoglycemic and Hypolipemic Activities. Eur. J. Med. Chem. 2018, 150, 102–112.
- Sauerberg, P.; Pettersson, I.; Jeppesen, L.; Bury, P.S.; Mogensen, J.P.; Wassermann, K.; Brand, C.L.; Sturis, J.; Wöldike, H.F.; Fleckner, J.; et al. Novel Tricyclic-Alpha-Alkyloxyphenylpropionic Acids: Dual PPAR Agonists with Hypolipidemic and Antidiabetic Activity. J. Med. Chem.
- Mishra, C. B., Gusain, S., Shalini, S., Kumari, S., Prakash, A., Kumari, N., ... & Tiwari, M. (2020). Development of novel carbazole derivatives with effective multifunctional action against Alzheimer’s diseases: design, synthesis, in silico, in vitro and in vivo investigation. Bioorganic Chemistry, 95, 103524.


 Subhajit makar*
Subhajit makar*

 10.5281/zenodo.13292980
10.5281/zenodo.13292980