Abstract
These research aims to study the usage , formulation and preparation of transdermal patch. Research also includes the study regarding UV spectroscopy, extraction methods , concepts of thin layer chromatography & Fourier Transform Infrared Spectroscopy which is helpful in evaluation of transdermal patch. Research contains the monograph study of Liquorice , focus ,Thuja , Aloevera ,pipeline and Piper mint .
Keywords
Transdermal patch, Transdermal Drug Delivery System
Introduction
Topically administered medicaments in the form of patches or semisolids (gels) that deliver drugs for systemic effects at a predetermined and controlled rate are known as Transdermal Drug Delivery System (TDDS). In comparison to other routes of drug delivery, the transdermal route has attracted greater attention because of its flexibility and convenience, and it is one of the most acceptable, practical, safe, and cost-effective ways to administer drugs. [1] The advantages of a transdermal drug delivery system over traditional means of drug administration include a controlled rate of drug release, avoidance of hepatic metabolism, ease of termination, and a long duration of effect. A drug is applied to the inside of a patch that is worn on the skin for an extended period of time in reasonably high dosage. The drug enters the bloodstream immediately through the skin through a diffusion process. Because the patch has a high concentration and the blood has a low concentration, the drug will continue to diffuse into the blood for a long time, keeping a consistent drug concentration in the blood flow. [1] Wound healing is the process of repairing the skin and other soft tissues after an injury. An inflammatory reaction happens after an injury, and cells below the dermis (deepest skin layer) begin to produce more collagen (connective tissue). The epithelial tissue (outer skin) regenerates later. Wound healing is divided into three stages: inflammation, proliferation, and remodeling. Angiogenesis, collagen deposition, granulation tissue development, epithelialization, and wound contraction characterize the proliferative phase. Angiogenesis is the formation of new blood vessels by endothelial cells. Fibroblasts expel collagen and fibronectin to generate a new, provisional extracellular matrix during fibroplasia and granulation tissue development. Following that, epithelial cells crawl across the wound bed to cover it, and myofibroblasts contract the wound. [2] As herbal products are much more cost-efficient with fewer side effects, three of the herbals- Glycyyrhetinic acid, Thuja, and Ficus are used in this current research work which are known to have significant wound-healing action.

Table 1 : Pharmacogenetic Scheme of Plants:
But most of the drugs suffer from poor bioavailability and thus remain subtherapeutic because a considerable portion of a dose never enters the plasma or exerts its pharmacological action unless and until very large doses are delivered. Increasing drug bioavailability is therapeutically significant since it exerts a direct impact on plasma concentrations and therapeutic efficacy following the drug administration. Bioenhancers are one of the most used methods for improving drug bioavailability. Therefore, two bioenhancers – Piperine and Aloe vera gel are used in this present work for enhancing the bioavailability and wound-healing action of the polyherbal transdermal patch. [3]
OBJECTIVES
- The present work is aimed to develop a polyherbal transdermal patch containing Ficus, Glycyyrhetinic acid, and Thuja with natural bioenhancers (Piperine and Aloe vera gel) to assess the wound healing potency.
- To study the effect of varying concentrations of bioenhancers on the permeation of the drugs from transdermal patch.
- To Perform Phytochemical screening ests for all the drug extracts.
- To Perform the Qualitative Analysis on the Extracts including- Preliminary chemical tests, UV-Vis Analysis, FTIR Analysis, and Thin Layer Chromatography.
- To perform the Evaluation of the formulated polyherbal transdermal patch on the parameters based on drug release, stability studies, tensile strength, and many more.
PLAN OF WORK
Selection of Plant:
- Liquorice
- Ficus
- Thuja
- Aloe Vera
- Piperine
- Peppermint
COLLECTION
Liquorice stolons, Aloe vera gel, Menthol crystals will be procured from Dagdu Teli Chandwadkar, Raviwar Karanja, Panchavati, Nashik. Thuja and Ficus will be collected from the botanical Garden of Gokhale Education Society’s, Sir. Dr. M.S Gosavi College of Pharmaceutical Education and Research, Nashik, Maharashtra, India.
Authentication of Plants:
The authenticity of the plants used (Ficus and Thuja) will be certified by the Botanist of R.Y.K Science College, Nashik, Maharashtra, India.
Extraction:
- Liquorice (Glycyyrhetinic Acid)
- Ficus
- Thuja
- Piper niger (Piperine)
Formulation:
- Synthesis of Gel Base:
Polymer part of the transdermal patch was synthesized using Carbopol and other excipients.
B. Polyherbal Transdermal Patch containing bioenhancers is to be formulated.
Evaluation:
- Preliminary Screening: Phytochemical Tests.
- Physical Characterization.
- Thin Layer Chromatography.
- UV-Visible Spectroscopy.
- Fourier Transform Infrared Spectroscopy.
- Evaluation of Patch.
EXPERIMENTAL WORK:
Material:
- Liquorice stolons, Aloe vera gel, Menthol crystals were procured from Dagdu Teli Chandwadkar, Raviwar Karanja, Panchavati, Nashik. Thuja and Ficus were collected from the botanical Garden of Gokhale Education Society’s, Sir. Dr. M.S Gosavi College of Pharmaceutical Education and Research, Nashik, Maharashtra, India.
- The authenticity of the Plants used (Ficus and Thuja) was certified by the Botanist of R.Y.K Science College, Nashik, Maharashtra, India.
Extraction method:
- Glycyrrhenitic Acid:
Solvent Treatment Method: For 6 hours, a weighted amount of liquorice stolons were soaked in a 5 % H2SO4 solution in 500 mL distilled water. (Fraction A) The mixture was filtered. 500 mL alkali was added to the residual cake (Strong ammonia solution). The mixture was filtered after 2 hours (Fraction B). Alkali (Strong ammonia solution) was used to neutralize fractions 'A' and 'B.' By running the mixture through a charcoal column, it was decolourized. On a rotary evaporator, the mixture was concentrated and crystallized with ethanol. The component was then purified using column chromatography and tested to confirm its identity. [4]
- Ficus:
Dried leaf powder of Ficus racemosa was soaked in 30 ml of alcohol for 24 hr and then subjected to hot extraction by using a Soxhlet extracting apparatus. The powder was then defatted with petroleum ether at least two to three times. Further, extraction was proceeded with methanol, and the resultant extract of methanol was centrifuged at 10,000 rpm for 15 min; after which the centrifugation supernatant of methanol extract was collected in a flask. The collected supernatant was dried and used for the identification of active phytoconstituents. [4]
- Thuja:
The plant material was dried for about 10 days at room temperature. The dried leaves were powdered using a mechanical grinder and sieved to obtain a particle size of 50-150 mm and were stored in dry polythene bags. Accurately weighed 34gm of the powder was placed in the thimble and was extracted using solvents 70% methanol and ethyl acetate: chloroform: ethanol in the ratio 40:30:30 respectively in a Soxhlet extractor for 2 days. The extracts were concentrated using a hot plate. [5]
d. Piperine:
Powdered drug (20 g) with 250 ml of ethanol (95%) was extracted in a Soxhlet apparatus for 3 hours. The solution was filtered and concentrated under vacuum on a water-bath at 60°C. 20 ml of 10 per cent alcoholic potassium hydroxide was added with constant stirring to the concentrated extract and filter. The alcoholic solution was allowed to stand overnight where upon needles of piperine separate out. The yield of yellow needle shaped crystals of piperine is approximately 2.5 per cent w/w. [6]
3.3 Formulation: formula of patch [7]

Table 2 : Formulation of gel base

Table 3 : Formulation of Transdermal patch
*for 10 ml transdermal patch
EVALUATION:
Preliminary Screening:
- Chemical Tests
Glycyrrhetinic acid: Sulphuric acid (80 % W/V) is added to a thick section of the drug or powder. [8]
Ficus:
- Shinoda test. [5]
- Ferric chloride test. [5]
2. Thuja:
- Ferric chloride test. [9]
- Gelatin test. [9]
- Iodine test. [9]
3. Aloe vera:
A. Borax test. [10]
4. Piperine:
A. Mayer’s test. [11]
B. Wagner’s test [11]
4. Peppermint:
- A few drops of peppermint crystals are mixed with 5 ml of nnitric acid solution and heated on water bath. [12]
- Physical Characterization:
Glycyrrhetinic acid:
- Physical characterization
Of all salts was investigated, which included determining their physical state.[4]
- Organoleptic characterization:
All salts were studied for colour, odour, and taste. [4]
- Melting point and loss on drying:
The melting point is an important factor in identifying a compound since it represents solubility properties, component purity, and crystalline habit (crystalline or amorphous). Capillary melting was used to estimate the melting point of glycyrrhetinic acid ammonium. To begin, L-ascorbic acid AR and sodium carbonate AR were used to calibrate the melting point apparatus. The average melting point was calculated after a little amount of glycyrrhetinic acid ammonium was placed in a capillary tube and placed in the digital melting apparatus. [4]
A precisely weighed 10 g of the compound was placed in a hot air oven that had been preheated to 105°C. Weigh the sample every 1 hour until you have two consistent weight readings. [4]
- Determination of PH:
The pH of the 1 % aqueous chemical solution was evaluated using a standardized glass electrode pre-calibrated at pH 4, 7, and 9. [16]
Ficus:
- Physical characterization of leaves and fruits of ficus were investigated, which included determining their physical state. [13]
- Organoleptic characterization: Fresh dried leaf extract was spread on butter paper and various organoleptic properties were studied. Like colour, odour, taste etc. [14]
- Melting point and loss on drying: Leaf extract was freeze-dried using a refrigerator for 48 hr. After that, the extract was evaporated under vacuum at around 40-45c. The compound in pure crystalline form was obtained with a 0.2 % loss in weight. [15]
- Determination of PH: The PH of 1% aqueous solution was evaluated by using a standardized glass electrode which was pre-calibrated at PH 4,7, and 9. [16]
Thuja:
- Thuja contains volatile & essential oils, tannins, flavone, resin, and sugar. [17]
- pH determination.
- Total ash, water soluble ash, acid-insoluble ash, and sulphated ash, as well as loss on drying and volatile oil content, were determined [18].
Piperine:
- Melting point: Melting point of piperine alkaloid 1230C, which was found to be similar to piperine 1250C in the literature. [19]
- pH: The pH of piperine was revealed to be 7.9.
- Thin Layer Chromatography:
Ficus:
A. Mobile phase: Chloroform –Toluene- Acetic acid (5:4:1)
B. Stationary phase: Silica gel
C. Visualizing agent: UV – lamp
D. Standard Rf value: 0.05 [5]
Thuja:
A. Mobile phase: Chloroform and methanol solvent system (8:2, 9:1, 7:3, 6:4)
B. Stationary phase: Silica gel
C. Visualizing agent: UV lamp
D. Standard Rf value: 0.5 [20]
Piperine:
A. Mobile phase: Ethanol and hexane (7:3)
B. Stationary phase: Silica gel
C. Visualizing agent: Dragendorff’s reagent
D. Standard Rf value: 0.25 [21]
4. UV-Visible Spectroscopy:
Glycyyrhetinic acid:
- Selection of media:
The 0.1N HCl was used to prepare the calibration curve by UV spectrophotometer.
- Stock solution preparation:
In 0.1N HCl, a stock solution of 1 mg/ml was produced in a 100 ml volumetric flask. As a blank/reference, 0.1N HCl was employed. Using a UV spectrophotometer, the sample was scanned to find the ?max (Shimadzu 1700S). To measure absorbance, the dilutions (1-10 g/ml) were prepared and scanned at ?max. Finally, a calibration curve for glycyrrhetinic acid ammonium was created, as well as the equation of line was established. The ?max of glycyrrhetinic acid ammonium was found to be 251-252 nm in 0.1N HCl. [4]
Ficus:
UV–Visible spectra of Ficus racemosa in 0.5M H2SO4 were recorded. The spectra were taken out in two different situations i.e., the acidic solution of inhibitor in which the mild steel specimens were not immersed and the solution in which the steel specimens were immersed for 24 h at 298 K. [22]
Piperine:
Stock solution of piperine was prepared by dissolving 10 mg of piperine in 100 ml of methanol. Standard solutions of piperine were prepared from stock solution in the concentration range of 2- 20µg/ml in 100 ml volumetric flask using methanol as solvent. The absorbance of piperine standard solutions was measured at 342 nm against methanol as blank. [23
- Fourier Transform Infrared Spectroscopy
Glycyyrhetinic acid:
The FTIR spectra were recorded in the M.G.V College of Pharmacy, Nashik. The product was analyzed. In a mortar pestle, the thoroughly dried glycyrrhetinic acid ammonium (1 mg) was combined with potassium bromide KBr powder (10 mg). The prepared mixture was then compacted into a fine disc using a KBr press at 15,000 Psi pressure. To determine the various bonds and groups present in the chemical, the prepared disc was put on the window of an IR spectrometer. [24]
Ficus:
Fourier Transformation Infrared Spectra of active phytoconstituents isolated was recorded by using a spectrophotometer for studying the functional groups. The sample was loaded in potassium bromide (KBr) disc and then the spectrum was measured at wave numbers ranging from (600 – to 4000 cm-1). The various bands of wavelength were identified relating to their wave numbers. [5]
- Evaluation of Patch:
For determination of durability of the film, a specific area of the film was taken out and it was repeatedly folded till it breaks. The time required for the breaking of the film gave the durability of the film. [25]
From each film of extract three strips were taken from a specific area I. e. Left side, centre and right side respectively. Then length and breadth were measured and constriction was concluded. [25]
The pH of the polyherbal transdermal patch solution was determined by the Systronics- pH meter ? pH system.
The melting point of the polysaccharide based dermal patch was determined in open capillaries.
The tensile strength of the patch was determined by using the Tensiometer. It has two pullies upper one was movable and lower one was fixed load. Sample was placed in between these pullies and force was applied which gave the elongation capability of the patch. [25]
specified area of the patch was socked in 100 ml of methanol and shaken continuously for 24 hr on a magnetic stirrer. Then the solution was ultrasonicated for 15 min. The solution after that filtered using filter paper and then the filtrate was spectroscopically studied at 273 to 287 nm for the different drug extracts. [25]
the film of the patch was pre-weighed and then put in desiccator at room temperature containing potassium chloride maintaining the RH Value at 84%. After 24 hr the patch was re-weighed and moisture content was calculated. [26]
The thickness of the fil was measured by using a digital micrometre screw gauge from three places. [25]
From the formulated patch strip of (2x2) cm2 was placed on egg membrane which was previously treated with concentrated hydrochloric acid and it was soaked overnight in the phosphate buffer 7.4. Then the solution was put into the Franz diffusion cell such that the cell’s drug releasing surface remained towards the receptor compartment; which contained 50 ml of phosphate buffer pH 7.4 at room temperature the cell was placed on a magnetic stirrer, and the solution in the receptor compartment was continuously stirred using magnetic bead at 50 rpm at normal body temperature and then 5 ml solution was withdrawn at predefined time intervals and changed with the same amount of phosphate buffer pH 7.4. Then the solution was extracted with 5 ml chloroform. The chloroform layer was evaporated on a water bath and the residue was dissolved in 5 ml of methanol. Finally, the test solution was quantified for glycyrrhetinic acid, piperine, thuja, and ficus at a maximum wavelength of 250 nm and 343.17 nm using a simultaneous UV method against the standard solution containing a placebo patch. [27]
stability of the patch was performed as per International conference of harmonization Q1A(R2) guidelines by storing the prepared patch formulation at different atmospheric conditions of 25°C (60 ± 5 % RH), 30°C (65 ± 5 % RH) and 40°C (75 ± 5 % RH) in stability chamber for 15 days. Then the sample was taken out and examined for physical properties, drug quantity and drug release. [25]
Sensory analysis for the transdermal patch was performed. 20 individuals of various age groups were selected. They were told to observe the colour, appearance, clarity, and odour of the patch.
RESULT

Preliminary Screening:
- Chemical tests
Glycyyrhetinic acid: Deep yellow colour is produced.
Ficus:
- Shinoda test: pink, orange or red colour were observed.
- Ferric chloride test: Violet colour was observed.
Thuja:
- Ferric chloride test: Green or blue colour formed which indicates the presence of phenol;
- Gelatin test: precipitate was formed which shows the presence of tannins and phenolic compounds.
- Iodine test: Appearance of transient red colour indicated the presence of tannins and phenolic compounds.
Aloe:
- Borax test: Green coloured fluorescence was produced indicating the presence of aloe-emodin anthranol.
Piperine:
- Mayer’s test: Yellowish white precipitate was formed.
- Wagner’s test: Brown precipitate was formed.
Peppermint:
Within 5 minutes, liquid developed blue colour, which on further heating deepened and showed copper colours fluorescene and then eventually became golden yellow.
4.2Physical Characterization:
Glycyyrhetinic acid:
- The extract was found to be crystalline whereas the % yield was obtained as 6.9 % Organoleptic characterization: The colour was found to be white and the compound was found to be odourless. The taste was revealed to be characteristic.
- Melting point and Loss on drying: The M.P was found to be 292+- 0.5 degrees and the loss on drying was found to be 0.1 %.
- Determination of pH: The 1% solution of glycyrrhetinic acid ammonium exhibited a pH of 4.1
Ficus:
- The obtained percentage yield of extract was 7 %.
- Organoleptic characterization: The colour of the extract was found to be dark greenish and odourless. The drug extract was tasteless.
- Loss on drying: The compound in pure crystalline form was obtained with a 0.2 % loss in weight.
- The PH of 1 per cent solution was found to be 7.4.
Thuja:
- The PH of 1 per cent solution was found to be 7.3.
![The physicochemical parameters of thuja orientalis.. [18].png](https://www.ijpsjournal.com/uploads/createUrl/createUrl-20240402132129-17.png)
Table 4- The physicochemical parameters of thuja orientalis.. [18]
Thin Layer Chromatography:
Ficus: The Rf value was found to be 0.06

Fig. 1- TLC of ficus
- Thuja: The Rf value was found to be 0.5.
- Piperine: Piperine has a conventional Rf value of 0.25 in the literature. The refined Rf value of the TLC yielded 0.23 per piperine
- 4.3UV-Visible Spectroscopy: Glycyyrhetinic acid:
- Ficus: The spectrum of Ficus religiosa extract shows two adsorption peaks at 271.31 nm and 351.26 n

Fig 2: UV Spectra of GA.

Fig 3: UV Spectra of Ficus racemose extract after and before immersion of mild

Fig 4: U.V Spectra of Piperine
Fourier Transform Infrared Spectroscopy:
Glycyyrhetinic acid:
At a frequency of 400.1299 MHz, the product's Fourier transform infrared spectroscopy revealed a significant variation in bands around 617, 837, 980, 1110, 1384, 1623, 2071, 2117, 2372, 3413 nm.

Fig 5: FTIR spectra of Glycyyrhetinic acid
Ficus:

Fig 6: FTIR spectra of ficus
Evaluation of the patch:
Evaluation parameters of transdermal patch:

Table 5-Evaluation of transdermal patch
Permeation of the drug:

Table 6: Cumulative amount of drug released
Drug release studies of formulation
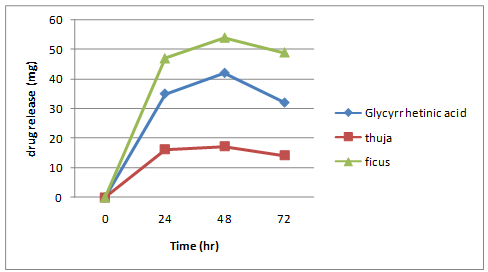
Fig. 7- Formulation F1

Fig.8- Formulation F2

Fig.9- Formulation F3

Fig.10- Formulation F4

Fig.11- Formulation F5

Fig.12- Formulation F6
Stability of the patch:

Table 7- stability studies of transdermal patch
Sensory analysis:

Table 8- Sensory analysis of transdermal patch

Fig 13- Sensory analysis of transdermal patch
DISCUSSION
- Herbal drugs- Glycyrrhetinic acid, Ficus, and Thuja were incorporated into a novel drug delivery system, i.e. a transdermal patch with bioenhancers for the wound-healing action.
- The selection of phytoconstituents was based on therapeutic efficacy implying they decrease inflammation, heal wounds, possess analgesic and antimicrobial properties and have been proven to be safe and effective.
- In reservoir type patch, 4?rbopol 934 was used as polymer and the bioenhancers added were piperine and Aloe vera gel.
- The release of a drug from a reservoir type patches occurred by diffusion. Transport of Glycyrrhetinic acid, Ficus and Thuja from the transdermal film into the in-vitro study medium depended upon % of Carbopol gel base as well as % of bioavailability enhancers, piperine and aloe vera.
- The increase in rate of drug release was found to be: F4 > F3 > F6 > F5 > F2 > F1.
- All formulation showed good physicochemical properties like thickness, weight variation, drug content, flatness, folding endurance, moisture content and moisture uptake. The in-vitro release data showed that drug release from the patch formulation have been affected by types of polymer and concentration of polymer as well as the herbal drugs and the excipients.
- The physicochemical parameters of the formulated transdermal patch did not considerably vary on storage. The result indicated that the formulation was stable on the required storage condition
CONCLUSION
- Reservoir type F4 patch formulated using 0.2ml menthol, 2 ml alcohol, 50 ml carbopol934 gel base with active drugs- 180 mg Glycyrrhetinic acid, 90 mg thuja and 200 mg ficus and bioenhancers- 50 mg piperine, 0.3 ml aloe vera gel.
- The present study was one of the few attempts to incorporate phytoconstituents in the transdermal system and it needs to be further optimized and characterised.
- These types of herbal formulations may possess tremendous potential for the wound healing and analgesic action.
- The polyherbal transdermal patches are easy to formulate.
- All the evaluation parameters of the polyherbal transdermal patch gave good results.
- Effect of bioenhancers- piperine and aloe vera have been checked on in-vitro permeation of drug. These studies indicated that the optimum concentration of bioenhancers increases drug permeation.
FUTURE PERSPECTIVES OF TRANSDERMAL DRUG DELIVERY SYSTEM:
- In current scenario, transdermal route of drug delivery system (TDDS) in comparison with oral treatment as the most successful innovative research area in new drug delivery system, with around 40% of the drug delivery candidate products under clinical trials related to transdermal system.
- Transdermal drug delivery is known to work wonders on the wound and is known to heal it.
- However, more evidence - based research is required on the drug release study, palatability and patient convenience of the wound healing transdermal patch is required.
- This Polyherbal transdermal patch with natural bioenhancers is needed to be explored more for further studies including various qualitative and quantitative methods; and pharmacological screenings.
ACKNOWLEDGEMENT:-
Authors want to thank Mr. Karmarkar Ritesh , Department of pharmaceutics , Mahatma Gandhi Vidya Mandir college of pharmacy panchavati Nashik 3. For the guidance and support.
REFERENCES
- V. Ravichandiran and S. Manivannan, “WOUND HEALING POTENTIAL OF TRANSDERMAL PATCHES CONTAINING BIOACTIVE FRACTION FROM THE BARK OF FICUS RACEMOSA,” Int. J. Pharm. Pharm. Sci., vol. 7, no. 6, pp. 326–332, 2015.
- R. Raina and parwez sahid, “Medicinal plants and their role in wound healing,” vetscan, vol. 3, no. 1, 2008.
- javed Shamama, A. waquar, and K. kohali, “The Concept Of Bioenhancers In Bioavailability Enhancement Of Drugs -A Patent Review,” J. Sci. Lett., vol. 1, no. 3, pp. 143–165, 2016.
- S. Visht and G. Kulkarni, “A Comparison between different methods for extraction of glycyrrhetinic acid from liquorice stolons,” Int. J. Pharma Prof. Res., vol. 3, pp. 622–626, 2012.
- Dr. K. Vanegaon and R. Londonkar, “Isolation, purification and spectral characterization of flavonoid from fruits of ficus glomerata,” World J. Pharm. Res., vol. 3, pp. 2168–2177, Jul. 2014.
- C. K. Kokate, Practical Pharmacognosy, 5th ed. Mumbai: Vallabh Prakashan, 2014.
- A. A. Alsaad, “Formulation & evaluation of ?-glycyrrhetinic acid patches with natural bioenhancer,” Mater. Today Proc., 2021.
- “Liquorice - Pharmacognosy,” pharmacy180.com. http://www.pharmacy180.com/article/liquorice-184/ (accessed May 19, 2022).
- S. Shrivastava and S. J. Daharwal, “Extensive review on the analytical methods for the estimation of Thuja occidentalis homeopathic mother tincture,” Res. J. Pharm. Technol., vol. 12, no. 9, pp. 4523–4530, 2019.
- “Aloe - Pharmacognosy,” pharmacy180.com. http://www.pharmacy180.com/article/aloe-170/ (accessed May 19, 2022).
- F. F. Alyaseen, B. A. Hassan, and H. S. Abdulhussein, “Extraction, Isolation And Chemical Identification of Piperine Alkaloid from Black Pepper Seeds And Its Antibacterial Activity,” Plant Arch., vol. 18, no. 2, pp. 2171–2176, 2018.
- C. K. Kokate, A. P. Purohit, and S. B. Gokhale, Pharmacognosy, 55th ed. Mumbai: Nirali Publication.
- S. Bhalerao, D. Verma, N. Teli, V. Didwana, and S. Thakur, “Ficus racemosa Linn.?: A Comprehensive Review,” J. Appl. Chem., vol. 3, no. 4, pp. 1423–1431, Jun. 2014.
- F. Ahmed and A. Urooj, “Pharmacognostical studies on Ficus racemosa stem bark,” Pharmacogn. J., vol. 3, no. 19, pp. 19–24, Jan. 2011, doi: 10.5530/pj.2011.19.4.
- S. Deraniyagala, R. Wijesundera, and J. Weerasena, “Antifungal activity of Ficus racemosa leaf extract and isolation of the active compound,” J. Natl. Sci. Found. Sri Lanka, vol. 26, Jul. 2011, doi: 10.4038/jnsfsr.v26i1.3081.
- V. N. Umeh, E. E. Ilodigwe, D. L. Ajaghaku, E. O. Erhirhie, G. E. Moke, and P. A. Akah, “Wound-healing Activity of the Aqueous Leaf Extract and Fractions of Ficus exasperata (Moraceae) and its Safety Evaluation on Albino Rats,” J. Tradit. Complement. Med., vol. 4, no. 4, pp. 246–252, Oct. 2014, doi: 10.4103/2225-4110.139105.
- Dr. N. Jasuja, S. Sharma, R. Saxena, J. Choudhary, R. Sharma, and S. Joshi, “Antibacterial, antioxidant and phytochemical investigation of Thuja orientalis leaves,” J. Med. Plant Res., vol. 725, pp. 1886–1893, Jul. 2013, doi: 10.5897/JMPR12.1323.
- Rajatrashmi, M. Sarkar, and Vikramaditya, “Pharmacognostic Studies of Thuja Occidentalis Linn. – A Good remedy for warts & tumours, used in Homeopathy,” Anc. Sci. Life, vol. 19, no. 1–2, pp. 52–58, 1999.
- S. Singh and S. K. Thukral, “Pharmacognostical standardization of leaves of Thuja orientalis (Linn.) Franco,” pp. 5, 2014.
- B. Singh, N. Haque, and N. Singh, “NOSOCOMIAL INFECTIONS: ANTI-MRSA ACTIVITY OF CANNABIS SATIVA AND THUJA ORIENTALIS ACTIVE COMPONENTS OBTAINED BY TLC,” Int. J. Recent Sci. Res., vol. 10, pp. 31933–31937, 2019, doi: 10.24327/ijrsr.2019.1004.3366.
- Samten, P. Wetwitayaklung, N. Kitcharoen, and U. Sotanaphun, “TLC image analysis for determination of the piperine content of the traditional medicinal preparations of Bhutan,” Acta Chromatogr., vol. 22, no. 2, pp. 227–236, 2010.
- R. Haldhar, D. Prasad, A. Saxena, and R. Kumar, “Experimental and theoretical studies of Ficus religiosa as green corrosion inhibitor for mild steel in 0.5 M H2SO4 solution,” Sustain. Chem. Pharm., vol. 9, pp. 95–105, 2018.
- N. K. Singh, P. Kumar, D. K. Gupta, S. Singh, and V. K. Singh, “UV-spectrophotometric method development for estimation of piperine in Chitrakadi Vati,” Pharm. Lett., vol. 3, no. 3, pp. 178–182, 2011.
- S. Ravi, P. Kabilar, S. Velmurugan, and R. A. K. and M. Gayathiri, “Spectroscopy Studies on the Status of Aloin in Aloe vera and Commercial Samples,” J. Exp. Sci., vol. 2, no. 8, pp. 10–13, 2011.
- S. T. Prajapati, C. G. Patel, and C. N. Patel, “Formulation and evaluation of transdermal patch of repaglinide,” Int. Sch. Res. Not., vol. 2011, 2011.
- R. P. Patel, G. Patel, H. Patel, and A. Baria, “Formulation and evaluation of transdermal patch of aceclofenac,” Res. J. Pharm. Dos. Forms Technol., vol. 1, no. 2, pp. 108–115, 2009.
- A. Vyas et al., “TLC Densitometric Method for the Estimation of Piperine in Ayurvedic Formulation Trikatu Churna,” Orient. J. Chem., vol. 27, no. 1, 2011.


 Shubham Bhaskar Tarle *
Shubham Bhaskar Tarle *
 Vaishnavi A.Chavan
Vaishnavi A.Chavan
 Akash Anil pawar
Akash Anil pawar
 Rushikesh s. Sonawane
Rushikesh s. Sonawane
 Siddhesh R. gavali
Siddhesh R. gavali
 Mangesh R.Bhadane
Mangesh R.Bhadane
 Manisha p. Padme
Manisha p. Padme




![The physicochemical parameters of thuja orientalis.. [18].png](https://www.ijpsjournal.com/uploads/createUrl/createUrl-20240402132129-17.png)

















 10.5281/zenodo.10906881
10.5281/zenodo.10906881