Abstract
Immunoglobulin (Ig) G1 antibodies motivate antibody dependent cell mediated cytotoxicity (ADCC). Cetuximab, an IgG1 isotype monoclonal antibody which is a possible treatment for mainly advanced or recurrent squamous cell carcinoma of the head and neck (SCCHN) and metastatic colorectal cancer (CRC). Proof regarding the clinical significance of cetuximab mediated ADCC and several immune activities and give a biological rationale regarding that cetuximab as an alternative way for immune checkpoint inhibitors (ICIs) and several developing immunotherapies. Cetuximab interferes ADCC activity in the cancer environment and leading adaptive and innate cell mediated immunity. There is a favorable rationale for combine ICIs with cetuximab for the treatment of advanced cancers, as targeting to CTLA-4, PD-1/PD-L1 may apparently control these immunosuppressive counter incidents in the tumor microenvironment. However, combined ICIs with cetuximab is an alternative strategy for enhancing immunity and response rates and also its durability. Cetuximab immune activity involving, ADCC gives a possible rationale for its combine ICIs or different immunotherapies to symbiotically and fully assemble the adaptive and innate immunity to cancer cells.
Keywords
IgG1, Cetuximab, CTLA-4, PD-1, PD-L1, Immunotherapy.
Introduction
In currently, arising mechanism for targeting cancer cells by the immune system have modified immunologists’ concentrate apart from cytotoxic chemotherapy. The all functions of the immune system can have therapeutic inferences, and can have already been broadly investigated in experimental models and mammals including humans [1]. The antibody dependent cell-mediated cytotoxicity (ADCC) emerges to be a promising field of study. Clinical and preclinical outcome have seen that immunoglobulin (IgG1) monoclonal antibodies have the higher capacity for stimulating ADCC compared with other isotypes (IgG2) and, since that ADCC arises in humans treated with IgG1-based immunotherapies [2]. In oncology, various commonly used therapeutic monoclonal antibodies have the IgG1 foundation and are seen to trigger ADCC, involving trastuzumab is an anti-human epidermal growth factor receptor (EGFR), antibody which is broadly used in breast cancer, necitumumab (an anti-EGFR) used in lung cancer, rituximab (an anti-cluster of differentiation) used in non-Hodgkin lymphoma and chronic lymphocytic leukemia [3], and cetuximab (an anti-EGFR) used in RAS wild-type metastatic colorectal cancer (mCRC) and locally advanced and recurrent and/or metastatic squamous cell carcinoma of the head and neck (R/M SCCHN). These antibodies have the IgG1 foundation and are consider to mature part of their anticancer activity to regulation of immune cells, basically when covering immunologically ‘‘hot” cancers [4]. Present immunotherapies have made possible a new approach to combination therapy with IgG1 isotype antibodies like cetuximab, the balancing of ADCC and several capable immune reactions with further immunomodulatory treatments [5]. With the evolution of immune checkpoint inhibitors (ICIs) targeting programmed death ligand 1 (PD-L1), its receptor PD-1, and cytotoxic T-lymphocyte associated protein 4 (CTLA-4) along with several immunotherapies [6]. The abilities for combine different immunostimulatory medications are now being examined in clinical trials. ICIs and several immunotherapies have been evolved as well as are being employed in many demonstrations [7]. Although, in SCCHN and CRC, ICI monotherapy shows linked with relatively low overall response rates (18 % SCCHN and 0 % CRC) and a dramatic responses absence in several patients compared with the further imposing ORRs of up to 57 % in other advanced or pretreated signs, like melanoma and non-small cell lung cancer [8]. Combined immunotherapy indicates a positive path to enhance anticancer activity in indications like CRC and SCCHN as well as further indications right for immunomodulatory therapy. As cetuximab is already fixed a quality of care in both SCCHN and CRC. A cetuximab as an IgG1 therapy with clinically important ADCC and linked immunomodulatory activities in order to examine its capacity for combine with immunotherapies like ICIs [9]. Present report that elaborated mechanisms for cetuximab driven immune actions and outline the available proof for these effects in CRC and SCCHN. Additionally, supply the scientific understanding for combining ICIs or several immunotherapies with cetuximab to symbiotically assemble the adaptive and innate immune systems against cancer cells [10], so that probably enhancing upon stable responsiveness and patient survival in challenging symptoms like SCCHN and CRC (Fig. 1). These assumptions of combined immuno-stimulatory therapies are possible interest in signs beyond CRC and SCCHN [11].
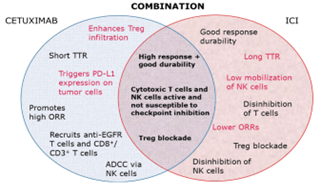
Fig. 1. Interdependent and symbiotic actions of combined therapy
IgG1 isotype directed immune mechanism
ADCC is a bioprocess that presents to the targeting and killing of antibody-faced cells through immune cells and is activated through IgG1 isotype antibodies in the presence of natural killer (NK) cells. Cetuximab has powerful immunomodulatory activity, beside to blocking of the EGFR intracellular signaling pathway [12]. Summarily, cetuximab stimulates ADCC when it’s constant region, attaches to a receptor expressed on NK cells (Fc receptor CD16), and leading in NK cell activation. Active NK cells may perform their own lytic activity on cancer cells, and active NK cell alone may simultaneously lyse numerous target cells [13]. Fundamentally, several immune activities come from the activation of NK cells by the interaction with the Fc region of an IgG1 isotype antibodies. NK cells arise to use interferon C and different cytokines to assist crosstalk with dendritic cells (DCs) and other immune cells such as macrophages [14]. Activated NK cells that lyse cancer cells result to the cancer antigens liberate, which may be cross expressed through DCs to cytotoxic T-cells, supporting them for extra cancer cell killing activity. Hence, the binding of an IgG1 isotype antibodies to its target and CD16 receptor on NK cells may stimulate the supporting and activation of both cells of the innate and adaptive immune systems [15]. In addition, cytokine mediated crosstalk with macrophages and other immune cells is important for assisting into the intra-tumoral space additional active, cytotoxic T-cells may then conduct lytic activity on cancer cells and hence produce extra cancer antigens and stimulate a several long-term immune responses [16]. Hence, cetuximab leads immunogenic cancer cell death, including several cytotoxic immune cells (Fig. 2) and present clinical and preclinical proof for cetuximab driven ADCC and other mechanisms is investigated (Tables 1 and 2) [17].
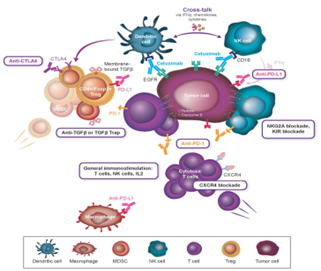
Fig. 2. Immune mechanism of cetuximab-based activity
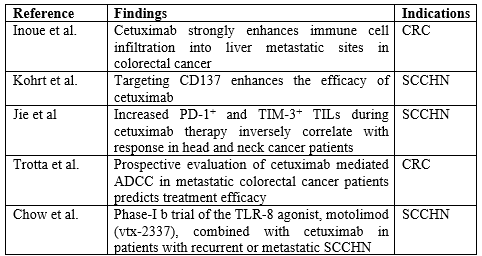
Table 1. Preclinical proof for cetuximab-mediated immune mechanisms

Table 2. Clinical and in-human, ex-vivo findings for cetuximab-mediated immune mechanisms
Cetuximab evoke cancer cell apoptosis using EGFR blocking and further cancer cell death mediated through the different antibody dependent immune events and mechanisms particular to its IgG1 backbone. The presence of an IgG2 isotype anti-EGFR monoclonal antibody, panitumumab, which does not trigger NK cell mediated ADCC, has provided immunologists the special chance to compare the effects of antibodies mediated EGFR inhibition and the assign of NK cell stimulation [18]. The immunologic difference between these two monoclonal antibodies has been conclusively illustrated ex-vivo, when further conditions are equal and developed. An IgG1 anti-EGFR antibodies (cetuximab) stimulates NK cell mediated ADCC. Hence, rises immune mediated cancer cell death to a higher level than does an IgG2 anti-EGFR monoclonal antibodies [19]. This variation in activity can account for the different efficiency of the two monoclonal antibodies sometimes reported in human patients. For instance, in clinical trials, cetuximab has measurable anticancer activity (leading to overall survival advantages) in SCCHN in combined with radiotherapy or other chemotherapy, whilst panitumumab was unable to illustrate a statistically positive variation [20]. Tumor antigen binding to EGFR and ADCC stimulation are interconnected processes. A phenomenon that can elaborate why ADCC shows highly suitable for anticancer efficiency in SCCHN lead to very high cancer EGFR expression [21]. Furthermore, there are populations of patients with mCRC who can advantage more from an IgG1-based therapy compare to an IgG2, certainly because of raised sensitivity to immune stimulation, involving the mechanism of ADCC. In fact, this can be the case for other symptoms with high cancer EGFR expression [22] (like lung cancer), or for a patient with cancer who has risen basal ADCC activity. Practically, ex-vivo and in-vitro assays with patients’ purified lymphocyte populations from the cancer environment. The peripheral blood is employed to directly notice NK cell activation and lytic activity [23]. Indirect estimations are conducted using markers on circulating and cancer infiltrating T-cells, NK cells, DCs, and cytokine levels in the plasma, involving expression of activating receptors like CD16, CD107a, CD137, NK cell (NKp46 and NKG2D) receptors [24]. Moreover, perforin and granzyme B (NK cell’s cytotoxic proteins which responsible for cell lytic activity) expression shows increase in cancer cell killing, and their reduction may cause to the eventual decreasing in lytic activity [25]. Contrarily, raised levels of transforming growth factor or interleukin 10 in plasma, raised CTLA-4 and PD-1 expression on T-cells, PD-L1 expression on cancer/immune cells, or NK receptor (NKG2A) expression on NK cells are evaluated indicators of immunosuppression [26]. Eventually, raised frequency of CD4+ or FoxP3+ regulatory T-cells, mainly in the cancer environment, is correlated with suppressed NK lytic activity and decrease of the immune response markers introduced earlier, likewise suppressing ADCC activity. The plenty of regulatory mechanisms marks the relevance that ADCC and further cetuximab mediated immune activity have in cancer control and eradication [27]. In specific through ‘‘priming” innate and adaptive immunity, and through introducing a tumor microenvironment that is well perfect to inhibition of ICIs or its elimination conduct dysfunctional lymphocytes to stimulate preferable adaptive and T-cell mediated immunity [28]. In general, particular patients’ basal ADCC activity, high NKp46 expression, and overall raised ADCC mediated killing have all been displaying to connect with positive clinical results, involving longer free survival recurrence, developed possibility of response to therapy, and prolonged overall survival [29]. Generally, these instigations strongly support the conclusion that ADCC is main component of cetuximab’s anticancer activity. Basic investigations recommend that ADCC measurement, monitoring, and targeting to clinical importance during cancer treatment of specific patients with IgG1 isotype monoclonal antibodies [30].
ADCC biomarkers and immunological responses
Biological variations in cancers may be darken through the specific inter variableness shown between patients with a given cancer type based on characteristics like disease stage, age, genetic markers, and cancer molecular biomarker expression [31]. Particular ADCC activity and CD16 receptor allelomorph can be anticipating for clinical results in response to IgG1-based anticancer immunotherapy [32]. Moreover, the additive presence of rise levels of baseline ADCC and EGFR expression may have a positive relation with the complete responses rate in patients with LA SCCHN who are induced with cetuximab and radiotherapy [33]. Desirable effect on baseline ADCC activity is KRAS mutations, presence of disease, and polymorphisms in the CD32A and CD16 Fc receptors. Due to raised capability to mount an ADCC response manages to correspond with prolonged overall survival, it is necessary that these variations be acknowledged and employed to possibly guide personalized treatment conclusions [34]. Such details are principally critical in the first line, when the immune system can be leading balanced to mount an anticancer response. The CD16 polymorphism is the best investigated biomarker for ADCC [35]. While CD16 is not needed for endogenous NK cell mediated cancer cell lysis, it is important for IgG1-mediated ADCC, and investigation recommend that rising the binding affinity of the Fc region to CD16 can higher NK cell cytotoxic activity. A CD16 Fc receptor that has a valine (V) at codon 158 has a much higher binding affinity for monoclonal antibodies [36]. Hence, patients with the V/V polymorphism are more immunologically active to IgG1 isotype antibody-based therapy (cetuximab, rituximab, trastuzumab) than patients with the F/F polymorphism. The V/F variant arises to an affinity phenotype intermediate between V/V and F/F or equal to V/V [37]. Downstream of CD16 activation, CD137 [removed]stimulates EGFR specific cytotoxic T-cells) coordinated with clinical response. Fascinating, in an evaluation of 107 patients with SCCHN who administered cetuximab, no predictive value for CD16 codon 158 polymorphism was reported for anti-EGFR therapy efficiency (while 13 patients had the V/V variant) [38]. Further investigation with KRAS (49 patients) wild type mCRC recorded a main variation in results between patients with various genetic variants of CD16. An extra polymorphism related with cetuximab immune activity is showed on codon 131 of the CD32A/Fcc RIIa receptor on DCs and neutrophils [39]. This polymorphism helps restore cancer immune surveillance and stimulates downstream immunogenic response. The disease control rate (DCR: 6 months) was rise in patients treated with cetuximab (CRC: 47) and bearing the H/H and H/R variants (67 % and 50 %) vs the R/R variant (17 %), with all patients having a mutation in KRAS, NRAS, BRAF, or PI3K [40]. Moreover, 31 patients showing the V/V or V/F variant on CD16 (overall study population: 70 %) had a combination 6-month DCR (52 % vs 23 %) in patients showing the F/F variant (n = 13). Likewise, in patients with mCRC introduced with cetuximab plus chemotherapy, overall survival was vitally prolonged in patients conducting a 158 V genotype [41]. The investigating meta-analysis of anti-EGFR antibody-based therapy in CRC observed that neither CD32A nor CD16 polymorphisms are anticipate of response between therapies. It should be reported that IgG2-driven immune activity can be linked with polymorphisms on CD32A. In fact, some CD32A polymorphisms effect shows on baseline ADCC activity is because of linkage dis-equilibrium instead of direct interaction [42]. Several research is needed to fully identify the anticipating value of CD16/32 receptor polymorphisms between immunomodulatory therapies. Treg and further immunosuppressive reactions are activated in the intra-tumoral environment as to resist cytotoxic cancer cell lysis. These negative regulatory mechanisms, could become extra therapeutic targets when design combined treatments with cetuximab [43].
T-cells immune signaling and indications for cetuximab therapy
Treg activity is one of the most commanding immunosuppressive mechanisms in the tumor microenvironment (Fig. 3). In comparison, cancer patients’ peripheral blood and tumor-infiltrating lymphocytes are improved Treg, certainly because of conversion from FoxP3- to FoxP3+ in response to raised TGF-? signaling. Treg produce suppressive cytokines and display membrane bound TGF-?, leads to blocking the cytolytic activity of T-cells and NK cells, and the maturing DCs [44].
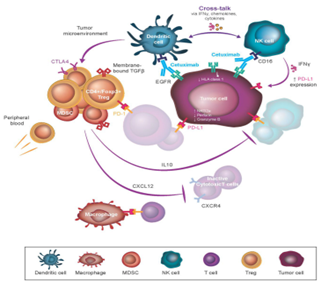
Fig. 3. Immunosuppressive mechanisms and diminishing of cetuximab-based immune activity
Moreover, highly immunosuppressive FoxP3+/CTLA-4+ or PD-L1+ Treg are show to be more vigorous in the tumor microenvironment than peripheral blood. Contrarily, raised CD4+/CD25hi/FoxP3+ Treg populations in the intra-tumoral space, NK cells have reduced expression of molecular markers presence of ADCC activity, like perforin, granzyme B and CD16 [45]. In-vitro and ex-vivo outcomes illustrate that the CD4+/CD25hi/FoxP3+ addition Treg suppress cetuximab-driven NK-mediated ADCC in patients with SCCHN using produced cytokines and TGF-?. TGF-? blockers are enough to inhibit this Treg mediated immune suppression in-vitro [46]. Necessarily, in-vitro and a phase I-a clinical trial recommend that decreasing the CD4+/Foxp3+ Treg population may boost NK cell cytotoxic activity. Moreover, it is believable that Treg mediated suppression of cetuximab driven immune activity may be an important prognostic factor in patients ongoing treatment with cetuximab for LA SCCHN. Cetuximab with chemotherapy combine treatment in patients with LA SCCHN (n = 22) showed to a notable rise in the frequency of CD4+/Foxp3+ Treg in lymphocyte populations in the peripheral blood and tumor microenvironment [47]. Moreover, cetuximab monotherapy (4 weeks, n = 18 patients) developed to rise the efficacy of intra-tumoral CD4+/FoxP3+ Treg displaying immunosuppression markers like CTLA-4, CD39, and TGF-?. Peripheral CD4+/FoxP3+ Treg were importantly improved in CTLA-4, feasibly reporting a response to cetuximab driven immune stimulation and through increase the conversion of an immunologically cancer phenotype [48]. When understanding the Treg efficacy in the periphery and the intra-tumoral space in clinical respondent to cetuximab with that of none. While investigations reported that respondent have steady Treg populations, non-respondent have important raises in CTLA-4+ Treg in the peripheral blood and tumor-infiltrating lymphocyte populations. Related examinations regarding the relation in Treg recruitment to the cancer microenvironment and reduced patient survival have been made across several cancers [49]. Fascinating, particularly in CRC, tumor-infiltrating Treg may have suppressive and non-suppressive FoxP3 expression, and the existence of the latter can be a positive prognostic marker of immune response. Overall, it develops that cetuximab driven ADCC and further immune activity starts a negative feedback loop of immunosuppression using immune checkpoints [50]. Hence, blocking of suppressive Treg by ICI treatment is a rational therapeutic strategy to implement in combined cetuximab with SCCHN and CRC [51]. Additionally, clinical report highlights the role of the PD-1/PD-L1 blocking in the hindrance of the peripherally introduced Treg causing to cell population reduction into the tumor microenvironment. This significant investigation demonstrated a reverse relationship in NK cell activation and the expansion of the Treg population [52].
Enhancing mechanism with cetuximab and immunotherapy
Cetuximab has illustrated clinically important activity in SCCHN and RAS wild type mCRC. It is a key component of the level of care for both symptoms in the metastatic setting, and it bears appreciative results in clinical trials and in the real-world setting [53]. Moreover, cetuximab encourages high response rates as proofed through its addition to earlier standard-of-care treatments (chemotherapy and radiotherapy), which has showed to boosted ORRs and prolonged survival [54]. Additionally, to the advantages related with EGFR blocking, cetuximab-mediated ADCC and the recruitment and cytotoxic T-cells drilling to the intra-tumoral space are effective credits [55]. Moreover, immune-stimulation is undoubtably related with negative feedback loops (Treg, MDSCs, and checkpoint receptors like PD-1, PD-L1, and CTLA-4). Hence, co-targeting of these immunosuppressive activities, and the possible collaboration between the various mechanisms of cetuximab action and ICIs, grasps the possible to enhance patient results in SCCHN and CRC [56]. For instance, CTLA-4 or PD-1/PD-L1 inhibition has the probable to reduce MDSC or Treg mediated blocking on T-cells and NK cells. Although replacing cytotoxic activity and fully steading the adaptive and innate immunity to cancer cells, due to further immunosuppressive actions affect upon negative regulation of T-cells and NK cells using PD-1/PD-L1, or CTLA-4 (Fig. 4) [57].
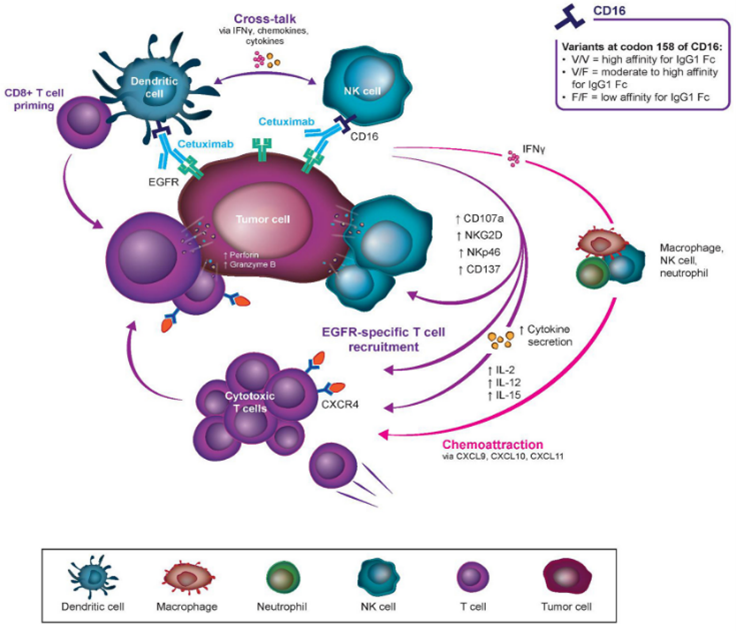
Fig. 4. Co-action between cetuximab and other immunotherapies (or ICIs)
Several proof in approval of this combined of drugs, cetuximab recruits new immune cells to the tumor microenvironment, while ICIs disinhibit cells earlier present. Hence, cetuximab and ICIs complement could assist to the immune system in preparation for (or T-cell and NK cell reduction) ICI therapy [58], increasing the probability of true symbiotic activity using interdependent activation of the innate and adaptive immune systems and arrangement of several types of immune cells. While the investigated protection outlines of cetuximab and ICIs don’t emerge to overlap, nominal protection and efficiency report are presently available from trials of cetuximab and combined ICI [59]. Investigations evaluating acute and delayed toxicities of cetuximab and ICI combines are presently ongoing. Besides, compounding of toxicities has been showed in ICI plus ICI combined treatments, with which additional immune-related adverse events may be critical and can prohibit the broadly use of combined ICI immunotherapy [60]. Patients with SCCHN are favorable candidates for powerful immunostimulatory therapy, since such cancers’ practicable beginning procedures are linked with an immunologically ‘‘hot” phenotype [61]. The combined treatment of cetuximab and avelumab (anti-PD-L1 IgG1 isotype) is of enough valuable because of both agents’ capacity to affect ADCC, since the use of 2 ADCC introducing monoclonal antibodies could possibly developed a significant immune effect through primarily and activating NK cells symbiotically [62]. While CRC has commonly been compared an immunoresistant cancer, prognostic constituent like high basal ADCC activity and the presence of tumor-infiltrating T-cells recommend this precondition is not accurate [63]. Although independent cancer molecular subtypes can be differently liable to ICI monotherapy, and hence far only microsatellite unsteady cancers have seen responses to such a possible way due to their potency to develop neoantigens [64]. Hence, research into ICIs combined therapy with an agent that earlier shown activity in mCRC (cetuximab) is beneficiary to examine whether such a combined regimen would possess activity in non-microsatellite unsteady cancers [65]. While CRC and SCCHN are very differently conditions, ICIs with cetuximab can stationarily result in additional activity in CRC cancers through primarily them for immunotherapy through introducing PD-1/PD-L1 expression on immune cells and recruiting its effector cells to the cancer [66]. Cetuximab may mediate raised immune activity in the cancer environment (trigger crosstalk between NK cells, DCs and enroll killer T-cells), hence primarily the immune system to be more reactive to ICI therapy [67]. Complementary, in-vitro investigation on this combined recommend that ICIs added to cetuximab may control cetuximab resistance, like that led through mutations in RAS and several genes [68]. Cetuximab mediated immune action assist crosstalk with a kind of immune cell types and its mechanisms, and hence it carries the possible for combine with several immunotherapy classes [69]. From ex-vivo investigations in CRC, combined treatment of cetuximab with cytokines (IL-2 or IL-15) was adequate to restore the patient lytic activity derived NK cells to levels compare to healthy ones. likewise, in SCCHN, treatment involve cetuximab with urelumab in a phase I-b trial showed to raised granzyme B and NKp46 levels on NK cells, whilst there were unchanged in IFNc, PD-1, CD107a, CD16 and NKG2D [70]. CD137 is an important biomarker for clinical response, and urelumab treatment has been reported to raise in IFNc-driven gene expression, cytokine production and also overall promoted immunologic activity. Moreover, cetuximab in combine with cytokines or urelumab was capable to apply immune activity on EGFR-expressing CRC cell lines or xenograft models [71]. Same examinations have been made for cetuximab in breast cancer xenografts and KRAS mutant cell lines. Extra non-ICI presently in combine clinical trials with cetuximab involve monalizumab (antiNKG2A) that inhibits this inhibitory receptor on NK cells. This combined would affect ADCC, block the EGFR, and continuously disinhibit NK cells suppressed using TGF-? or IL-10 [72]. TLR-8 is being trialed in combine with cetuximab for patients and has possible outcomes showing a DCR of 54 % and rises in circulating cytokines. Combination TLR-8 with cetuximab and/or nivolumab is now being trialed in patients with LA SCCHN (NCT02124850) [73]. Several combines involve structural immunomodulation using whole killed mycobacteria (IMM-101, NCT03009058), DNA demethylation using valproic acid (NCT02624128) that has been seen to carries anticancer effects in several actions including neutrophil growth stimulation and activity with granulocyte colony stimulating factor (NCT02124148) [74]. Therefore, further clinical trials are demonstrating ex-vivo grown and activated immune cells, involving NK, CD4+ and CD8+ T-cells in combined with cetuximab (NCT02028455, NCT02507154) across symptoms (Table 3). Combined therapies will possibly construct the next wave for extra novel targets, like CXCR4 activation, MDSC inhibition, and TGF-? traps [75].
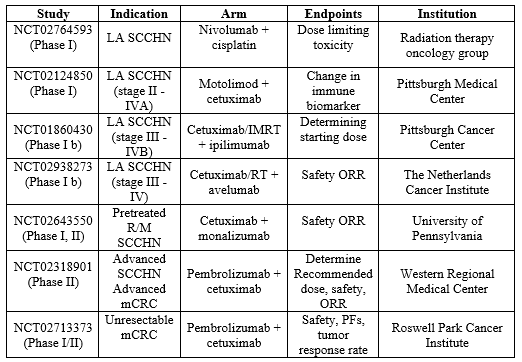
Table 3. Ongoing major clinical trials of cetuximab with several immunotherapy
FUTURE PROSPECTUS
Recent perspective investigations will demonstrate that, the case of cetuximab with avelumab whether using 2 ADCC introducing monoclonal antibodies will develop an important immune effect through priming and activating NK cells symbiotically. Basically, through complementary and fully steadying the adaptive and innate immune systems to cancer cells [76]. Cetuximab with combined ICIs or several immunotherapies could stay the major to increasing ORRs and long-lasting of response in problematic situations like SCCHN and CRC [77]. Experimental data from present clinical trials that are investigating this hypothesis is enthusiastically predicted. Current investigations will examine the clinical incidents of these combine trials and their immune effect in CRC and SCCHN and also in several mechanisms [78].
CONCLUSION
ICI monotherapy is a modern and compelling treatment option, but response rates are quite in several symptoms, involving SCCHN and CRC. There is a favorable scientific understanding for combined ICIs and the being standard-of-care monoclonal antibody cetuximab for the treatment of advanced SCCHN and CRC. Additionally, to EGFR blocking, cetuximab assists clinically related ADCC and several immune activities in the intra-cancerous environment, which is linked with cancer cell destroying through components of the innate and adaptive immune systems. Cetuximab may promote the immune system for ICI therapy through recruiting cytotoxic cell effectors of the innate and adaptive immune systems to the intra-cancerous environment. In addition, related negative feedback loops caused to CTLA-4, PD-1 and/or PD-L1 mediated immunosuppression of active cytotoxic cells, an issue that apparently could be control surely using ICIs combined therapy. Several incidents like non-small cell lung cancer, it has been seen that strong PD-L1 expression is related with satisfied results when administered with anti-PD-L1.
ACKNOWLEDGEMENTS
The author thankful to Visvesvaraya National Institute of Technology, Nagpur to encouraged and motivated for research work.
AUTHOR CONTRIBUTION
The author has alone responsible for research work and writing this article.
FUNDING SOURCE
None
CONFLICT OF INTEREST
The authors have no conflict of interest.
REFERENCES
- Ferris RL, Lenz H-J, Trotta AM, Jesus G-F, Schulten J, Audhuy F, et al. Rationale for combination of therapeutic antibodies targeting tumor cells and immune checkpoint receptors: Harnessing innate and adaptive immunity through IgG1 isotype immune effector stimulation. Cancer Treat Rev 2018; 63:48–60.
- Chow LQ, Morishima C, Eaton KD, Baik CS, Goulart BH, Anderson LN, et al. Phase 1b trial of the toll-like receptor 8 agonist, motolimod (VTX-2337), combined with cetuximab in patients with recurrent or metastatic SCCHN. Clin Cancer Res 2017; 23:2442–50.
- Rizvi NA, Hellmann MD, Snyder A, Kvistborg P, Makarov V, Havel JJ, et al. Cancer immunology. Mutational landscape determines sensitivity to PD-1 blockade in non-small cell lung cancer. Science 2015; 348:124–8.
- Trotta AM, Ottaiano A, Romano C, Nasti G, Nappi A, De Divitiis C, et al. Prospective evaluation of cetuximab-mediated antibody-dependent cell cytotoxicity in metastatic colorectal cancer patients predicts treatment efficacy. Cancer Immunol Res 2016; 4:366–74.
- Mellor JD, Brown MP, Irving HR, Zalcberg JR, Dobrovic A. A critical review of the role of Fc gamma receptor polymorphisms in the response to monoclonal antibodies in cancer. J Hematol Oncol 2013; 6:1–10.
- Pahl JH, Ruslan SE, Buddingh EP, Santos SJ, Szuhai K, Serra M, et al. Anti-EGFR antibody cetuximab enhances the cytolytic activity of natural killer cells toward osteosarcoma. Clin Cancer Res 2012; 18:432–41.
- McLaughlin P, Grillo-Lopez AJ, Link BK, Levy R, Czuczman MS, Williams ME, et al. Rituximab chimeric anti-CD20 monoclonal antibody therapy for relapsed indolent lymphoma: half of patients respond to a four-dose treatment program. J Clin Oncol 1998; 16:2825–33.
- Hodi FS, O’Day SJ, McDermott DF, Weber RW, Sosman JA, Haanen JB, et al. Improved survival with ipilimumab in patients with metastatic melanoma. N Engl J Med 2010; 363:711–23.
- Gotwals P, Cameron S, Cipolletta D, Cremasco V, Crystal A, Hewes B, et al. Prospects for combining targeted and conventional cancer therapy with immunotherapy. Nat Rev Cancer 2017; 17:286–301.
- Toh JW, de Souza P, Lim SH, Singh P, Chua W, Ng W, et al. The potential value of immunotherapy in colorectal cancers: review of the evidence for programmed death-1 inhibitor therapy. Clin Colorectal Cancer 2016; 15:285–91.
- Ferris RL, Blumenschein Jr G, Fayette J, Guigay J, Colevas AD, Licitra L, et al. Nivolumab for recurrent squamous-cell carcinoma of the head and neck. N Engl J Med 2016; 375:1856–67.
- Chen DS, Mellman I. Elements of cancer immunity and the cancer-immune set point. Nature 2017; 541:321–30.
- Daud AI, Wolchok JD, Robert C, Hwu WJ, Weber JS, Ribas A, et al. Programmed death-ligand 1 expression and response to the anti-programmed death 1 antibody pembrolizumab in melanoma. J Clin Oncol 2016; 34:4102–9.
- Borghaei H, Paz-Ares L, Horn L, Spigel DR, Steins M, Ready NE, et al. Nivolumab versus docetaxel in advanced nonsquamous non-small-cell lung cancer. N Engl J Med 2015; 373:1627–39.
- Li S, Schmitz KR, Jeffrey PD, Wiltzius JJ, Kussie P, Ferguson KM. Structural basis for inhibition of the epidermal growth factor receptor by cetuximab. Cancer Cell 2005; 7:301–11.
- Mehra R, Cohen RB, Burtness BA. The role of cetuximab for the treatment of squamous cell carcinoma of the head and neck. Clin Adv Hematol Oncol 2008; 6:742–50.
- Trivedi S, Srivastava RM, Concha-Benavente F, Ferrone S, Garcia-Bates TM, Li J, et al. Anti-EGFR targeted monoclonal antibody isotype influences anti-tumor cellular immunity in head and neck cancer patients. Clin Cancer Res 2016; 22:5229–37.
- Goodwin JF, Kothari V, Drake JM, Zhao S, Dylgjeri E, Dean JL, et al. DNA-PKcs mediated transcriptional regulation drives prostate cancer progression and metastasis. Cancer Cell 2015; 28:97–113.
- Groom JR, Luster AD. CXCR3 ligands: redundant, collaborative and antagonistic functions. Immunol Cell Biol 2011; 89:207–15.
- Lee SC, Srivastava RM, Lopez-Albaitero A, Ferrone S, Ferris RL. Natural killer (NK): dendritic cell (DC) cross talk induced by therapeutic monoclonal antibody triggers tumor antigen-specific T-cell immunity. Immunol Res 2011; 50:248–54.
- Srivastava RM, Lee SC, Andrade Filho PA, Lord CA, Jie HB, Davidson HC, et al. Cetuximab-activated natural killer and dendritic cells collaborate to trigger tumor antigen-specific T-cell immunity in head and neck cancer patients. Clin Cancer Res 2013; 19:1858–72.
- Inoue Y, Hazama S, Suzuki N, Tokumitsu Y, Kanekiyo S, Tomochika S, et al. Cetuximab strongly enhances immune cell infiltration into liver metastatic sites in colorectal cancer. Cancer Sci 2017; 108:455–60.
- Kroemer G, Galluzzi L, Kepp O, Zitvogel L. Immunogenic cell death in cancer therapy. Annu Rev Immunol 2013; 31:51–72.
- Pozzi C, Cuomo A, Spadoni I, Magni E, Silvola A, Conte A, et al. The EGFR-specific antibody cetuximab combined with chemotherapy triggers immunogenic cell death. Nat Med 2016; 22:624–31.
- Monteverde M, Milano G, Strola G, Maffi M, Lattanzio L, Vivenza D, et al. The relevance of ADCC for EGFR targeting: a review of the literature and a clinically-applicable method of assessment in patients. Crit Rev Oncol Hematol 2015; 95:179–90.
- Jochems C, Hodge JW, Fantini M, Fujii R, Morillon 2nd YM, Greiner JW, et al. An NK cell line (haNK) expressing high levels of granzyme and engineered to express the high affinity CD16 allele. Oncotarget 2016; 7:86359–73.
- Argiris A. EGFR inhibition for recurrent or metastatic HNSCC. Lancet Oncol 2015;16:488–9.
- Vermorken JB, Mesia R, Rivera F, Remenar E, Kawecki A, Rottey S, et al. Platinum-based chemotherapy plus cetuximab in head and neck cancer. N Engl J Med 2008; 359:1116–27.
- Bonner JA, Harari PM, Giralt J, Azarnia N, Shin DM, Cohen RB, et al. Radiotherapy plus cetuximab for squamous-cell carcinoma of the head and neck. N Engl J Med 2006; 354:567–78.
- Douillard JY, Pirker R, O’Byrne KJ, Kerr KM, Storkel S, von Heydebreck A, et al. Relationship between EGFR expression, EGFR mutation status, and the efficacy of chemotherapy plus cetuximab in FLEX study patients with advanced non-small-cell lung cancer. J Thorac Oncol 2014; 9:717–24.
- Lattanzio L, Denaro N, Vivenza D, Varamo C, Strola G, Fortunato M, et al. Elevated basal antibody-dependent cell-mediated cytotoxicity (ADCC) and high epidermal growth factor receptor (EGFR) expression predict favorable outcome in patients with locally advanced head and neck cancer treated with cetuximab and radiotherapy. Cancer Immunol Immunother 2017; 66:573–9.
- Fassy J, Tsalkitzi K, Salavagione E, Hamouda-Tekaya N, Braud VM. A real-time digital bio-imaging system to quantify cellular cytotoxicity as an alternative to the standard chromium-51 release assay. Immunology 2017; 150:489–94.
- Jie HB, Schuler PJ, Lee SC, Srivastava RM, Argiris A, Ferrone S, et al. CTLA-4+ regulatory T-cells increased in cetuximab-treated head and neck cancer patients suppress NK cell cytotoxicity and correlate with poor prognosis. Cancer Res 2015; 75:2200–10.
- Srivastava RM, Trivedi S, Concha-Benavente F, Gibson SP, Reeder C, Ferrone S, et al. CD137 stimulation enhances cetuximab-induced natural killer: dendritic cell priming of antitumor T-cell immunity in patients with head and neck cancer. Clin Cancer Res 2017; 23:707–16.
- Strauss L, Bergmann C, Szczepanski M, Gooding W, Johnson JT, Whiteside TL. A unique subset of CD4+ CD25 high Foxp3+ T-cells secreting interleukin-10 and transforming growth factor-beta 1 mediates suppression in the tumor microenvironment. Clin Cancer Res 2007; 13:4345–54.
- Jie HB, Srivastava RM, Argiris A, Bauman JE, Kane LP, Ferris RL. Increased PD-1+ and TIM-3+ TILs during cetuximab therapy inversely correlate with response in head and neck cancer patients. Cancer Immunol Res 2017; 5:408–16.
- Jie HB, Gildener-Leapman N, Li J, Srivastava RM, Gibson SP, Whiteside TL, et al. Intratumoral regulatory T-cells upregulate immunosuppressive molecules in head and neck cancer patients. Br J Cancer 2013; 109:2629–35.
- Liu D, Tian Y, Sun D, Sun H, Jin Y, Dong M. The FCGR3A polymorphism predicts the response to rituximab-based therapy in patients with non-Hodgkin lymphoma: a meta-analysis. Ann Hematol 2016; 95:1483–90.
- Shimizu C, Mogushi K, Morioka MS, Yamamoto H, Tamura K, Fujiwara Y, et al. Fc-Gamma receptor polymorphism and gene expression of peripheral blood mononuclear cells in patients with HER2-positive metastatic breast cancer receiving single-agent trastuzumab. Breast Cancer 2016; 23:624–32.
- Gamerith G, Auer T, Amann A, Putzer D, Schenk B, Kircher B, et al. Increase in antibody-dependent cellular cytotoxicity (ADCC) in a patient with advanced colorectal carcinoma carrying a KRAS mutation under lenalidomide therapy. Cancer Biol Ther 2014; 15:266–70.
- Kasper S, Breitenbuecher F, Reis H, Brandau S, Worm K, Kohler J, et al. Oncogenic RAS simultaneously protects against anti-EGFR antibody-dependent cellular cytotoxicity and EGFR signaling blockade. Oncogene 2013; 32:2873–81.
- Congy-Jolivet N, Bolzec A, Ternant D, Ohresser M, Watier H, Thibault G. Fc gamma RIIIa expression is not increased on natural killer cells expressing the Fc gamma RIIIa-158V allotype. Cancer Res 2008; 68:976–80.
- Hatjiharissi E, Xu L, Santos DD, Hunter ZR, Ciccarelli BT, Verselis S, et al. Increased natural killer cell expression of CD16, augmented binding and ADCC activity to rituximab among individuals expressing the Fc{gamma} RIIIa-158 V/V and V/F polymorphism. Blood 2007; 110:2561–4.
- Ochoa MC, Minute L, Rodriguez I, Garasa S, Perez-Ruiz E, Inoges S, et al. Antibody-dependent cell cytotoxicity: immunotherapy strategies enhancing effector NK cells. Immunol Cell Biol 2017; 95:347–55.
- Romain G, Senyukov V, Rey-Villamizar N, Merouane A, Kelton W, Liadi I, et al. Antibody Fc engineering improves frequency and promotes kinetic boosting of serial killing mediated by NK cells. Blood 2014; 124:3241–9.
- Oboshi W, Watanabe T, Matsuyama Y, Kobara A, Yukimasa N, Ueno I, et al. The influence of NK cell-mediated ADCC: structure and expression of the CD16 molecule differ among FcgammaRIIIa-V158F genotypes in healthy Japanese subjects. Hum Immunol 2016; 77:165–71.
- Kohrt HE, Colevas AD, Houot R, Weiskopf K, Goldstein MJ, Lund P, et al. Targeting CD137 enhances the efficacy of cetuximab. J Clin Invest 2014; 124:2668–82.
- Calemma R, Ottaiano A, Trotta AM, Nasti G, Romano C, Napolitano M, et al. Fc gamma receptor IIIa polymorphisms in advanced colorectal cancer patients correlated with response to anti-EGFR antibodies and clinical outcome. J Transl Med 2012; 10:232–42.
- Rodriguez J, Zarate R, Bandres E, Boni V, Hernandez A, Sola JJ, et al. Fc gamma receptor polymorphisms as predictive markers of cetuximab efficacy in epidermal growth factor receptor downstream-mutated metastatic colorectal cancer. Eur J Cancer 2012; 48:1774–80.
- Schneider-Merck T, Lammerts van Bueren JJ, Berger S, Rossen K, van Berkel PH, Derer S, et al. Human IgG2 antibodies against epidermal growth factor receptor effectively trigger antibody-dependent cellular cytotoxicity but, in contrast to IgG1, only by cells of myeloid lineage. J Immunol 2010; 184:512–20.
- Coombes JL, Siddiqui KR, Arancibia-Carcamo CV, Hall J, Sun CM, Belkaid Y, et al. A functionally specialized population of mucosal CD103+ DCs induces Foxp3+ regulatory T-cells via a TGF-beta and retinoic acid-dependent mechanism. J Exp Med 2007; 204:1757–64.
- Ghiringhelli F, Menard C, Terme M, Flament C, Taieb J, Chaput N, et al. CD4+ CD25+ regulatory T-cells inhibit natural killer cell functions in a transforming growth factor-beta-dependent manner. J Exp Med 2005; 202:1075–85.
- Oida T, Xu L, Weiner HL, Kitani A, Strober W. TGF-beta-mediated suppression by CD4+ CD25+ T-cells is facilitated by CTLA-4 signaling. J Immunol 2006; 177:2331–9.
- Terme M, Chaput N, Combadiere B, Ma A, Ohteki T, Zitvogel L. Regulatory T-cells control dendritic cell/NK cell cross-talk in lymph nodes at the steady state by inhibiting CD4+ self-reactive T-cells. J Immunol 2008; 180:4679–86.
- Hiraoka N, Onozato K, Kosuge T, Hirohashi S. Prevalence of FOXP3+ regulatory T-cells increases during the progression of pancreatic ductal adenocarcinoma and its premalignant lesions. Clin Cancer Res 2006; 12:5423–34.
- Ichihara F, Kono K, Takahashi A, Kawaida H, Sugai H, Fujii H. Increased populations of regulatory T-cells in peripheral blood and tumor-infiltrating lymphocytes in patients with gastric and esophageal cancers. Clin Cancer Res 2003; 9:4404–8.
- Kurose K, Ohue Y, Wada H, Iida S, Ishida T, Kojima T, et al. Phase Ia study of FoxP3+ CD4 Treg depletion by infusion of a humanized anti-CCR4 antibody, KW-0761, in cancer patients. Clin Cancer Res 2015; 21:4327–36.
- Smyth MJ, Teng MW, Swann J, Kyparissoudis K, Godfrey DI, Hayakawa Y. CD4+ CD25+ regulatory T-cells suppress NK cell-mediated immunotherapy of cancer. J Immunol 2006; 176:1582–7.
- Curiel TJ, Coukos G, Zou L, Alvarez X, Cheng P, Mottram P, et al. Specific recruitment of regulatory T-cells in ovarian carcinoma fosters immune privilege and predicts reduced survival. Nat Med 2004; 10:942–9.
- Saito T, Nishikawa H, Wada H, Nagano Y, Sugiyama D, Atarashi K, et al. Two FOXP3+ CD4+ T-cell subpopulations distinctly control the prognosis of colorectal cancers. Nat Med 2016; 22:679–84.
- Takeuchi Y, Nishikawa H. Roles of regulatory T-cells in cancer immunity. Int Immunol 2016; 28:401–9.
- Chen X, Fosco D, Kline DE, Meng L, Nishi S, Savage PA, et al. PD-1 regulates extrathymic regulatory T-cell differentiation. Eur J Immunol 2014; 44:2603–16.
- Solito S, Pinton L, Mandruzzato S. In brief: myeloid-derived suppressor cells in cancer. J Pathol 2017; 242:7–9.
- Pogoda K, Pyszniak M, Rybojad P, Tabarkiewicz J. Monocytic myeloid-derived suppressor cells as a potent suppressor of tumor immunity in non-small cell lung cancer. Oncol Lett 2016; 12:4785–94.
- Parker KH, Beury DW, Ostrand-Rosenberg S. Myeloid-derived suppressor cells: critical cells driving immune suppression in the tumor microenvironment. Adv Cancer Res 2015; 128:95–139.
- Trotta R, Dal Col J, Yu J, Ciarlariello D, Thomas B, Zhang X, et al. TGF-beta utilizes SMAD3 to inhibit CD16-mediated IFN-gamma production and antibody-dependent cellular cytotoxicity in human NK cells. J Immunol 2008; 181:3784–92.
- Seliger B. Molecular mechanisms of MHC class I abnormalities and APM components in human tumors. Cancer Immunol Immunother 2008; 57:1719–26.
- Ferris RL, Hunt JL, Ferrone S. Human leukocyte antigen (HLA) class I defects in head and neck cancer: molecular mechanisms and clinical significance. Immunol Res 2005; 33:113–33.
- Pollack BP, Sapkota B, Cartee TV. Epidermal growth factor receptor inhibition augments the expression of MHC class I and II genes. Clin Cancer Res 2011; 17:4400–13.
- Concha-Benavente F, Srivastava RM, Trivedi S, Lei Y, Chandran U, Seethala RR, et al. Identification of the cell intrinsic and extrinsic pathways downstream of EGFR and IFN gamma that induce PD-L1 expression in head and neck cancer. Cancer Res 2016; 76:1031–43.
- Van Cutsem E, Lenz HJ, Kohne CH, Heinemann V, Tejpar S, Melezinek I, et al. Fluorouracil, leucovorin, and irinotecan plus cetuximab treatment and RAS mutations in colorectal cancer. J Clin Oncol 2015; 33:692–700.
- Akiyama Y, Nonomura C, Kondou R, Miyata H, Ashizawa T, Maeda C, et al. Immunological effects of the anti-programmed death-1 antibody on human peripheral blood mononuclear cells. Int J Oncol 2016; 49:1099–107.
- Qiao G, Yang L, Li Z, Ying H, Hassen Y, Yin F, et al. Program death-1 regulates peripheral T-cell tolerance via an anergy-independent mechanism. Clin Immunol 2012; 143:128–33.
- Simpson TR, Li F, Montalvo-Ortiz W, Sepulveda MA, Bergerhoff K, Arce F, et al. Fc-dependent depletion of tumor-infiltrating regulatory T-cells co-defines the efficacy of anti-CTLA-4 therapy against melanoma. J Exp Med 2013; 210:1695–710.
- Tang Q, Boden EK, Henriksen KJ, Bour-Jordan H, Bi M, Bluestone JA. Distinct roles of CTLA-4 and TGF-beta in CD4+ CD25+ regulatory T-cell function. Eur J Immunol 2004; 34:2996–3005.
- D’Angelo SP, Larkin J, Sosman JA, Lebbe C, Brady B, Neyns B, et al. Efficacy and safety of nivolumab alone or in combination with ipilimumab in patients with mucosal melanoma: a pooled analysis. J Clin Oncol 2017; 35:226–35.
- Gajewski TF, Schreiber H, Fu YX. Innate and adaptive immune cells in the tumor microenvironment. Nat Immunol 2013; 14:1014–22.
- McGranahan N, Furness AJ, Rosenthal R, Ramskov S, Lyngaa R, Saini SK, et al. Clonal neoantigens elicit T-cell immunoreactivity and sensitivity to immune checkpoint blockade. Science 2016; 351:1463–9.


 Ashish S. Ramteke*
Ashish S. Ramteke*






 10.5281/zenodo.10937907
10.5281/zenodo.10937907