This study aimed to develop stable room-temperature granules of Lactobacillus acidophilus (NCIM 2285) probiotics while optimizing granule formulation to maximize viable cell count after 2 hours of incubation in an acidic medium. Spray drying and tray drying techniques were employed to prepare probiotic powders, offering cost-effective alternatives to the expensive freeze-drying method. The granules were evaluated for antioxidant activity and antimicrobial properties. Antioxidant activity was assessed through in vitro methods, including the 2, 2-diphenyl-1-picrylhydrazyl (DPPH) free radical scavenging assay, superoxide scavenging assay and resistance to hydrogen peroxide. Additionally, in vivo antioxidant effects were examined in oxidative stress-induced male Wistar rats by measuring the activities of Superoxide Dismutase (SOD) and Catalase enzymes. This study highlights the potential of L. acidophilus NCIM 2285 as a multifunctional probiotic with notable antioxidant and antimicrobial properties. These findings support the use of alternative drying methods for large-scale production of stable probiotic formulations and emphasize the therapeutic applications of L. acidophilus in managing oxidative stress and promoting antimicrobial effect.
Probiotics, Lactobacillus acidophilus, Spray drying, tray drying, Antioxidant and Antimicrobial effect.
Microorganisms have long been a source of medicine, and the search for new microbial products remains a promising approach, particularly for probiotics. Common probiotics like Lactobacillus and Bifidobacterium are being developed as Live Biotherapeutic Products (LBPs) under FDA guidelines, which define LBPs as biological products containing live microorganisms used to prevent, treat, or cure diseases in humans. Probiotics, meaning "for life," confer health benefits when administered in adequate amounts, a concept first introduced by Nobel laureate Elie Mutchnick off, who recognized their potential to enhance human health. The popularity of probiotics continues to grow for managing and preventing various diseases1. Lactobacillus acidophilus (L. acidophilus) is a key probiotic in the gastrointestinal tract with health benefits such as immune stimulation, anti-carcinogenic, and antioxidant effects. A major species of lactic acid bacteria (LAB), it is used in food and agricultural fermentation and naturally inhabits the human intestine and vagina. L. acidophilus ferments sugars into lactic acid, thrives at low pH (below 5.0), and grows optimally at 33–37°C. Despite its presence in dairy products and ability to lower serum cholesterol and inhibit certain cancers, its survival in stomach acids and bile limits its effectiveness in reaching the intestines, posing a challenge for therapeutic use2. Microorganisms possess antioxidative systems to regulate free radicals and prevent cellular damage. Lactic acid bacteria (LAB), including Lactobacillus acidophilus and Bifidobacterium longum, exhibit antioxidant properties by scavenging reactive oxygen species (ROS), degrading superoxide anion and hydrogen peroxide, and modulating oxidative stress. Their metabolic activities include enzyme inhibition, iron chelation, and neutralizing free radicals, enhancing antioxidative enzymes like superoxide dismutase and catalase. LAB strains have shown to inactivate ROS through enzymatic systems and inhibit lipid peroxidation by 28%–48%. These properties highlight LAB's potential to prevent oxidative stress and protect against carcinogen-induced damage3. Probiotics, such as Lactobacillus acidophilus, help control intestinal infections by competitively excluding pathogens through mechanisms like organic acid production, bacteriocin synthesis, and nutrient competition. Organic acids lower intracellular pH in pathogens, while bacteriocins, like lactacin B and acidophilin, inhibit pathogen growth by pore formation or cell wall synthesis. L. acidophilus also produces antimicrobial compounds, acidifies intestinal contents, and affects toxin receptor modification, enhancing immunity against harmful bacteria like E. coli, Salmonella, and Staphylococcus aureus. Additionally, it restores normal intestinal flora during antibiotic therapy and neutralizes dietary carcinogens. Unlike synthetic antibiotics, probiotics are non-invasive, have fewer side effects, and reduce the risk of antibiotic resistance, making them a superior preventive option4. Probiotics, classified as Generally Recognized as Safe (GRAS) by FDA and FAO/WHO, offer health benefits with minimal side effects, though ongoing research monitors rare adverse effects5. Lactobacillus acidophilus plays a significant role as both an antioxidant and antimicrobial agent. Its antioxidant properties are demonstrated through its ability to scavenge free radicals, such as DPPH, superoxide anions, and hydrogen peroxide, which helps in reducing oxidative stress and protecting cells from damage. In terms of antimicrobial activity, Lactobacillus acidophilus exhibits inhibitory effects against various pathogenic microorganisms, including Pseudomonas aeruginosa, Streptococcus pneumonia, and Shigella dysentriae, by producing antimicrobial substances that suppress microbial growth. These dual properties make Lactobacillus acidophilus a promising candidate for therapeutic applications, contributing to enhanced immunity and providing a natural alternative to synthetic drugs. The development of a novel probiotic formulation using Lactobacillus acidophilus is crucial due to the growing demand for natural, multifunctional health products. Beyond its well-known role in supporting gut health, Lactobacillus acidophilus has demonstrated significant antioxidant and antimicrobial effects, which are valuable in combating oxidative stress and microbial infections—two major contributors to gastrointestinal disorders, inflammation, and immune system dysfunction. By leveraging these additional therapeutic properties, a novel probiotic formulation could provide a holistic approach to health, offering natural alternatives to synthetic antioxidants and antibiotics. This formulation has the potential to improve gut health while also addressing broader health concerns, making it a promising candidate for both clinical use and consumer markets focused on wellness and disease prevention. This study is aimed to develop a novel probiotic formulation incorporating Lactobacillus acidophilus for enhanced stability, and therapeutic efficacy, to evaluate the physicochemical properties to investigate the in-vitro and in-vivo effects of the novel probiotic formulation to assess the safety and tolerability of the novel probiotic formulation through toxicity studies.
Materials and methods
Lactobacillus acidophilus was sourced from NCIM, Pune, and maintained as glass bead cultures at -20°C in Man, Rogosa, and Sharpe (MRS) broth from Siffin Pharma, Germany. Buffalo milk was pasteurized to eliminate other microbes, and Lactobacillus strains from MRS media were transferred aseptically to the milk. The inoculated flasks were incubated at 33°C to 37°C. The semi-solid masses, formed after 32 hours of incubation, were broken down into a liquid state by homogenization at 1200 rpm. A deep-frozen culture bead of Lactobacillus acidophilus was transferred to MRS broth, incubated overnight, and used to inoculate a final broth, which was incubated for 24 hours at 37°C. Cells were harvested, washed with PBS buffer, and re-suspended in a carrier solution. The microbes were diluted in saline or 5?xtrose solution (1:100, 1:1000, 1:10000) to maintain cell viability and osmosis, ensuring proper cell counting for plating and avoiding overlapping colonies6.
Plating method: After serial dilution, MRS media was sterilized in an autoclave at 120°C, transferred to sterilized plates, and inoculated with diluted microbial culture. The plates were incubated at 33°C to 37°C, and colonies formed as colony-forming units (CFU) 7.
Gram staining: For Gram staining, a colony is emulsified in a drop of sterile water on a glass slide, dried, and fixed. The slide is flooded with crystal violet for 1 minute, rinsed, and decolorized with 95% alcohol/acetone. After rinsing, the slide is counterstained with Safranin for 10 seconds, rinsed, and dried. A drop of immersion oil is added, and the slide is examined under oil immersion (100x) using coarse and fine adjustment knobs8.
Motility test: A clean cavity glass slide and cover slide were used; the microbe sample was mounted on the cover slide, edges sealed with paraffin, and the slide was positioned to observe motility under a microscope9.
Catalase test: A single colony was streaked on a glass slide, and a drop of 3% hydrogen peroxide was added; effervescence of oxygen indicated a positive catalase test10.
pH survival studies: A single colony of Lactobacillus acidophilus was sub-cultured in MRS broth with pH adjusted to 4.0, 5.0, 6.0, 7.0, and 8.0 using NaOH or HCl, and incubated at 37°C for 24 hours to assess growth under different pH conditions11.
Tray drying: The technique involves adding 5% maltodextrin to the broth, drying in a tray drier at 40°C for 48 hours with silver foil, and evacuating the chamber at 100°C using concentrated alcohol12.
Spray drying: The spray-drying of Lactobacillus acidophilus was performed using a JISL mini-spray drier, with feed solution atomized via a two-fluid nozzle at a constant flow rate (5-20 ml/min). The outlet temperature was set between 100°C and 110°C, and dried powder was collected in a cyclone separator. Excipients like starch and maltodextrin were tested in various combinations (1:1, 1:2, 2:1) to optimize viability outcomes13.
Formulating lactobacillus acidophilus as granules: Wet granulation was used to prepare Lactobacillus acidophilus NCIM 2285 granules by blending microcrystalline cellulose, lactose monohydrate, corn starch, and povidone binder. The mixture was blended with an edible broth medium containing L. acidophilus and skim milk, then screened through a 1.70 mm sieve, and dried at 38°C for 18 hours. The dried granules were sieved again, and those within the 0.71–1.00 mm size range were used for capsule preparation. Six formulations were developed and evaluated for optimization, including those made from tray-dried and spray-dried powders14,15.
Characterization of formulations: The granules were characterized by bulk density, tapped density, loss on drying, compressibility index, flowability, Hausner’s ratio, angle of repose, and viable count, with each experiment repeated five times.
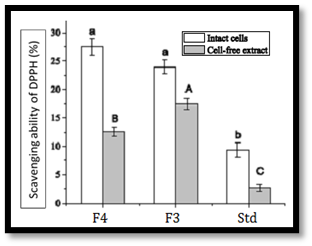
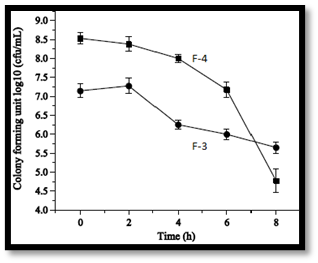
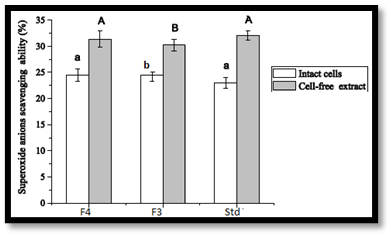
In vitro antioxidant activity screening: Cells of Lactobacillus were harvested by centrifugation at 4°C for 30 minutes and washed with 20 mM sodium phosphate buffer (SPB, pH 7.4), then re-suspended in SPB. The washed cell suspension was disrupted using an ultrasonic disrupter and filtered, with cell debris removed by centrifugation. The total cell count was adjusted to 1 mg/mL or 10^9 CFU/mL. For antioxidant activity, cells or intracellular extracts were mixed with a DPPH solution and incubated for 30 minutes, with the decrease in absorbance measured at 517 nm to assess radical scavenging ability. For hydrogen peroxide resistance, cells were suspended in SPB and incubated with hydrogen peroxide, with viable counts measured over time. The resistance to superoxide anions was quantified by measuring absorbance at 325 nm after incubating with a mixture containing lactobacilli and 1,2,3-Benzenetriol. All experiments were conducted in triplicate16,17.
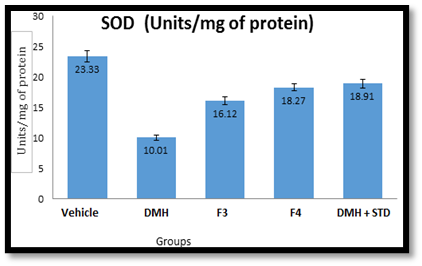
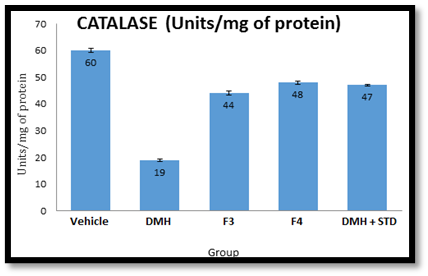
In-vivo antioxidant screening: Male Wistar rats (6 weeks old, weighing 150-200g) were administered 1, 2-dimethyl hydrazine (20 mg/kg body weight, S.C.) dissolved in 1mM EDTA once a week for 6 weeks. Drug dosing was done using formulation F3 (500 mg/kg/day orally) and F4 (500 mg/kg/day orally) alongside Capecitabine (150 mg/kg/day, I.V.) for 10 weeks. After 6 weeks, the rats were fasted overnight with ad libitum drinking water and sacrificed the following day. Superoxide dismutase and catalase activities in the liver were estimated using the methods described by McCord and Fridovich (1969) and Abei and Bergmeyer (1983), respectively18,19,20,21.
Anti-microbial screening by well assay method: MRS agar plates were inoculated with a 24-hour culture of P. aeruginosa using a swab. Wells were punched in the agar, and 80 mg of L. acidophilus granules were added to the wells. The plates were incubated at 37°C for 24 hours. Separate plates with S. pneumoniae and S. dysentriae were prepared, and similar wells were punched in the agar using a sterile gel puncher with a 5 mm diameter 22,23.
Acute toxicity study: An acute toxicity study of Lactobacillus acidophilus powder was conducted using rats, with doses of 500, 1000, 2000, and 5000 mg/kg administered orally. No mortality or behavioral changes were observed up to 7 days post-treatment, indicating that Lactobacillus acidophilus powder is safe at doses up to 5000 mg/kg 24,25.
Stability study: The granules were stored in polythene bags under two conditions for three months to assess their stability and viability: (a) at room temperature with exposure to room light, and (b) at room temperature in the dark 25.
Statistical analysis: The data were expressed as mean ± SEM, and statistical significance was determined using Dunnett’s test with p<0>
Results and discussions
Lactobacillus Acidophilus: Lactobacillus acidophilus NCIM 2285, obtained from NCIM Pune, was stored as glass bead cultures at -20°C for long-term maintenance, with a white color and cotton-covered tip.
Thriving of the lactobacillus acidophilus: The CFU count of Lactobacillus acidophilus was found to be 107 CFU/ml in buffalo milk, with pure white colonies and no contamination, after incubation at 34°C for less than 24 hours.
Homogenization: Homogenization at 1200 rpm for 20 minutes using a REMI mini homogenizer effectively reduced the size of semi-solid beads, resulting in a liquid form suitable for spray drying, preventing clogging of the nozzle.
Preparation of bacterial suspension: The deep-frozen bead of Lactobacillus acidophilus was transferred to MRS broth, incubated overnight at 37°C, and diluted in saline (1:100 to 1:10^12). CFUs appeared as unique white tiny dots on MRS agar, confirming pure culture without contamination.


 Nurzeba M. Bepari*
Nurzeba M. Bepari*
 Ketura Kambampaty
Ketura Kambampaty

 10.5281/zenodo.14640687
10.5281/zenodo.14640687