This study is the first to evaluate the cytotoxic effects of the chloroform extract and its fractions from Tecoma stans (L.) Kunth leaves on cancer cells. The extract demonstrated significantly enhanced cytotoxic activity, and its antioxidant potential was also assessed. These findings provide valuable evidence supporting the potential use of Tecoma stans leaves in managing oxidative stress and inhibiting cancer progression.
Silver Nanoparticle, Tecoma Stans Leaf Extract, Anticancer Effects.
Medicinal plants have played a vital role in human healthcare, with around 80% of the global population relying on traditional medicine derived from natural sources. Systems like Ayurveda, Siddha, Unani, and folk medicine have used plants to treat a wide range of ailments for centuries. In the last two decades, extensive scientific efforts have been dedicated to validating the efficacy of plant-based treatments used in traditional healthcare systems.
In this study, we investigate the anticancer potential of the chloroform extract from Tecoma stans leaves and flowers. Our evaluation includes conventional cancer treatment methods and photodynamic therapy (PDT), leveraging the synergistic effect of light, oxygen, and a photosensitizer. Flowers were specifically chosen due to their rich phytochemical composition with diverse bioactivities. The study aims to explore the cytotoxic impact of the extract on lung and breast cancer cell lines while identifying promising phytocompounds for cancer therapy.
Plant profile
Plant Profile: Tecoma stans
Botanical Name: Tecoma stans (L.) Juss. ex Kunth

Family: Bignoniaceae
Common Names: Yellow Trumpetbush, Yellow Bells, Ginger-Thomas
Origin: native American plant( central America, southern United States, north Argentina)
Plant properties:
Tecoma stans (L.) Kunth, is a fast-growing herb of the Bignoniaceae family. It is cultivated for its ornamental value and is widely recognized for its medicinal properties. Reports indicate that different parts of the plant—such as leaves, roots, flowers, seeds, fruit, and bark—possess therapeutic properties. Traditionally, Tecoma stans has been used as a diuretic, vermifuge, and tonic, and to manage conditions like diabetes, digestive issues, and yeast infections. Phytochemical analysis of the plant has identified tannins, flavonoids, alkaloids, quinones, and trace amounts of saponins and amino acids, which contribute to its diverse pharmacological activities, including cytotoxic, antibacterial, antifungal, anti-inflammatory, and wound-healing effects.
MATERIALS AND METHODS
- Plant Selection
The selection of the plant was carried out following an extensive literature review. It was chosen for its proven in vitro antioxidant and anticancer properties, specifically against NCI-H460 cell lines. The plant was sourced and cultivated from the region surrounding the Tirumala hills.
- Plant Authentication
The Tecoma stans plant was authenticated by the Department of Botany at Sri Venkateswara University, Tirupati (517502), Andhra Pradesh. The authentication was conducted in line with the SVU Herbarium specimens, under the supervision of Dr. K. Madhava Chetty, Assistant Professor at the Department of Botany.
3 Extraction of the Plants
3.1. Materials and Methods:
Leaves of Tecoma stans (L.) Kunth were sourced from the Medicinal Garden at Sree Vidyanikethan College of Pharmacy, located in A. Rangampet, Chittoor district, Andhra Pradesh, India. The collected fresh leaves were identified and authenticated by Dr. K. Madhav Chetty, Assistant Professor of the Department of Botany, Sree Venkateshwara University. The leaves were then dried in the shade for 20 days. After drying, the leaves were ground into a powder and stored in a bottle at room temperature until further analysis.
3.2 Maceration
Maceration is a traditional and cost-effective method used to extract essential oils and active compounds from plants. In this process, coarsely ground crude drugs are mixed with a solvent (menstruum) to increase surface area for better extraction. The solvent is then strained, and the solid residue (marc) is pressed to recover the remaining liquid. The combined liquid is filtered to remove impurities. Frequent agitation during maceration aids in promoting diffusion and separating the concentrated solution.
Preparation of extract
• Fresh Tecoma stans leaves were collected and air-dried for 20 days at room temperature.
• The dried leaves were ground into fine powder using an electric grinder.
• The powdered extract was stored in a sealed bottle at room temperature.
• 10 grams of the powder were mixed with 100 ml of chloroform.
• The mixture was allowed to macerate for 2 days.
• After 48 hours, the mixture was filtered using Whatman filter paper.
• Pressure was applied to the residue to extract the remaining solvent.
• The final extract was stored in a tightly covered container for phytochemical screening
Preparation of AgNPs
To prepare AgNPs, a crude sample was made by dissolving 10 mg/ml of the substance in distilled water. Then, 2 ml of this solution was diluted to 5 ml with distilled water. 10 ml of 1M Silver Nitrate (AgNO?) solution was added to the extract, and the mixture was heated in a water bath at 70-80°C for about 30 minutes to allow the reduction reaction. A colour change indicated the successful synthesis of AgNPs, along with a peak observation.

Figure 2: Maceration for chloroform extract of Tecoma stans

Figure 3: Chloroform extract of Tecoma stans (L.) Kunth.

Figure 4: Samples for cell line studies
Phytochemical Screening
- Alkaloid Test (Dragendorff’s Method):
To 1 ml of the extract, 1 ml of Dragendorff’s reagent (potassium bismuth iodide) is added. The presence of alkaloids is confirmed by the formation of an orange or red precipitate.
- Tannin Test (Gelatin Precipitation Method):
A few drops of gelatin solution are added to 2-3 ml of the extract. The presence of tannins is indicated by the formation of a white precipitate.
- Flavonoid Test:
1 ml of the extract is mixed with a few drops of dilute ammonium solution and concentrated hydrochloric acid. A yellow color indicates the presence of flavonoids.
- Test for Saponins:
Mix 1 ml of extract with 5 ml distilled water. Froth formation indicates saponins.
- Test for Phenolics:
Combine 1 ml of extract with 1 ml lead acetate solution. Precipitate formation shows phenols.
- Test for Glycosides (Legal’s Test):
Hydrolyze a small amount of extract with HCl for 5 minutes. Add 1 ml pyridine, sodium nitroprusside, and make it alkaline with NaOH. A color change indicates glycosides
- Test for Phytosteroids (Salkowski Test):
Dissolve extract in chloroform, add concentrated sulfuric acid. A brown ring suggests phytosteroids.
- Test for Proteins (Ninhydrin Test):
Heat 3 ml test solution with 3 drops of 5% Ninhydrin solution in a water bath. A purple or blue color indicates proteins.
- Test for Amino Acids (Biuret Test):
Add 4% NaOH and 1% CuSO4 to the test solution. A violet or pink color shows amino acids.
- Test for Carbohydrates (Fehling’s Test):
Mix 1 ml of Fehling’s A and B, boil, then add the test solution. A yellow or red precipitate indicates carbohydrates.

Figure 5: Phytochemical tests
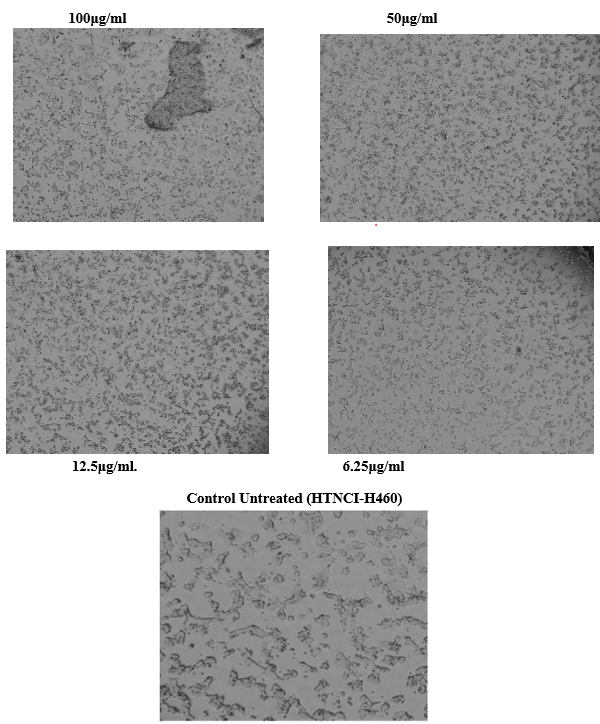
In Vitro Study of Tecoma Stans on NCI-H460 Cell Line Using MTT Assay
The MTT assay is a colorimetric technique used to assess cell proliferation and cytotoxicity. It works by converting the yellow tetrazolium dye, MTT, into insoluble purple formazan crystals through the action of mitochondrial lactate dehydrogenase in living cells. The intensity of the resulting purple color, measured at 570 nm, correlates with the number of viable cells (Alley et al., Mosmann et al.).
Equipment’s used in the study:
• Centrifuge (Remi: R-8oC).
• Pipettes: 2-10?l, 10-100?l, and 100-1000?l.
• Inverted binocular biological microscope
• Biosafety hood (Biobase, China)
• 37°C incubator witha humidified atmosphere of 5%
Assay Controls used in the study:
-
-
- Medium control (medium without cells)
- Negative control (medium with cells but without the Experimental drug/compound)
Materials used in the study:
-
-
-
- Cell lines:
- NCI-H460 cell line (ATCC, USA)
Cell culture medium:
- DMEM- High Glucose – (#AL111, Himedia)-For HMC-3 cells.
- Mc-Coy’s 5A growth media – (#AL0575, Himedia)-For SH-Sy5Y cells.
- Adjustable multichannel pipettes and a Pipette
- Fetal Bovine Serum (#RM10432, Himedia)
- MTT Reagent (5 mg/ml) (# 4060 Himedia)
- DMSO (#PHR1309, Sigma)
- D-PBS (#TL1006, Himedia)
- 96-well plate for culturing the cells (From Corning, USA)
- T25 flask (# 12556009, Biolite –Thermo)
- 50 ml centrifuge tubes (# 546043 TORSON)
- ml centrifuge tubes (TORSON)
- 10 ml serological pipettes (TORSON)
- 10 to 1000 µl tips (TORSON)
Maintenance of cell lines:
The NCI-H460 cell line was obtained from ATCC, USA. These cells were cultured in DMEM high glucose medium, while NCI-H460 cells were also cultured in McCoy’s 5A medium. Both media were supplemented with 10?S and 1% antibiotic-antimycotic solution. The cells were grown in an environment with 5% CO2, 18-20% O2, at 37°C inside a CO2 incubator, and were sub-cultured every two days.
Procedure:
- Seed 200?l of cell suspension (20,000 cells/well) in a 96-well plate; grow for 24 hours
- Add test agent at specified concentrations.
- Incubate for 24 hours at 37°C in a 5% CO2 atmosphere.
- Remove media, add MTT reagent (0.5 mg/mL), and cover plate with foil.
- Incubate for 3 hours.
- Remove MTT reagent, add 100?l of DMSO to dissolve formazan crystals.
- Stir gently or pipette to ensure full dissolution.
- Measure absorbance at 570nm (630nm as reference).
- Calculate ?ll viability: .
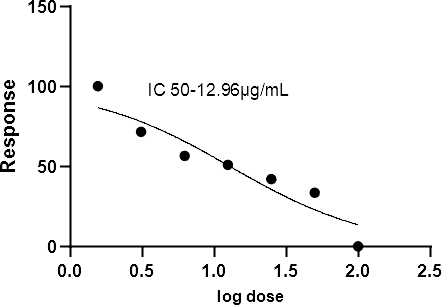
- Determine IC50 using linear regression (Y = Mx + C, where Y = 50).
RESULTS AND DISCUSSION
The phytochemical screening of Tecoma stans was conducted using a chloroform extract, following standard procedures. The results are presented in the table below.
|
Test
|
Chloroform extract of Tecoma Stans
|
|
Alkaloids
|
–
|
|
Flavonoids
|
+
|
|
Tannins
|
+
|
|
Steroids
|
+
|
|
Glycosides
|
+
|
|
Saponins
|
+
|
|
Carbohydrates
|
+
|
|
Amino acid
|
–
|
|
Proteins
|
–
|
|
Phenols
|
+
|
The determination of invitro proliferation and cytotoxicity Study of Tecoma stans on the NCI-H460 cell line by using MTT assay.
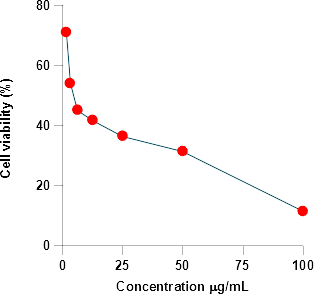
?ll viability is calculated using below formula:
?ll viability = [Mean abs of treated cells/Mean abs of Untreated
Cells] x 100
The IC50 value was determined by using linear regression Equation
i.e. Y =Mx + C. Here, Y= 50, M and C values were derived from the viability graph. The cell line NCI-H460 observation was mentioned in the below Table.
INCUBATION PERIOD: 48 Hrs
Sample ID : Cell Lines NCI-H460


 Pranav Alvakonda *
Pranav Alvakonda *





 10.5281/zenodo.14209167
10.5281/zenodo.14209167